Habitat Partitioning and Overlap by Large Lacertid Lizards in Southern Europe
Abstract
1. Introduction
2. Materials and Methods
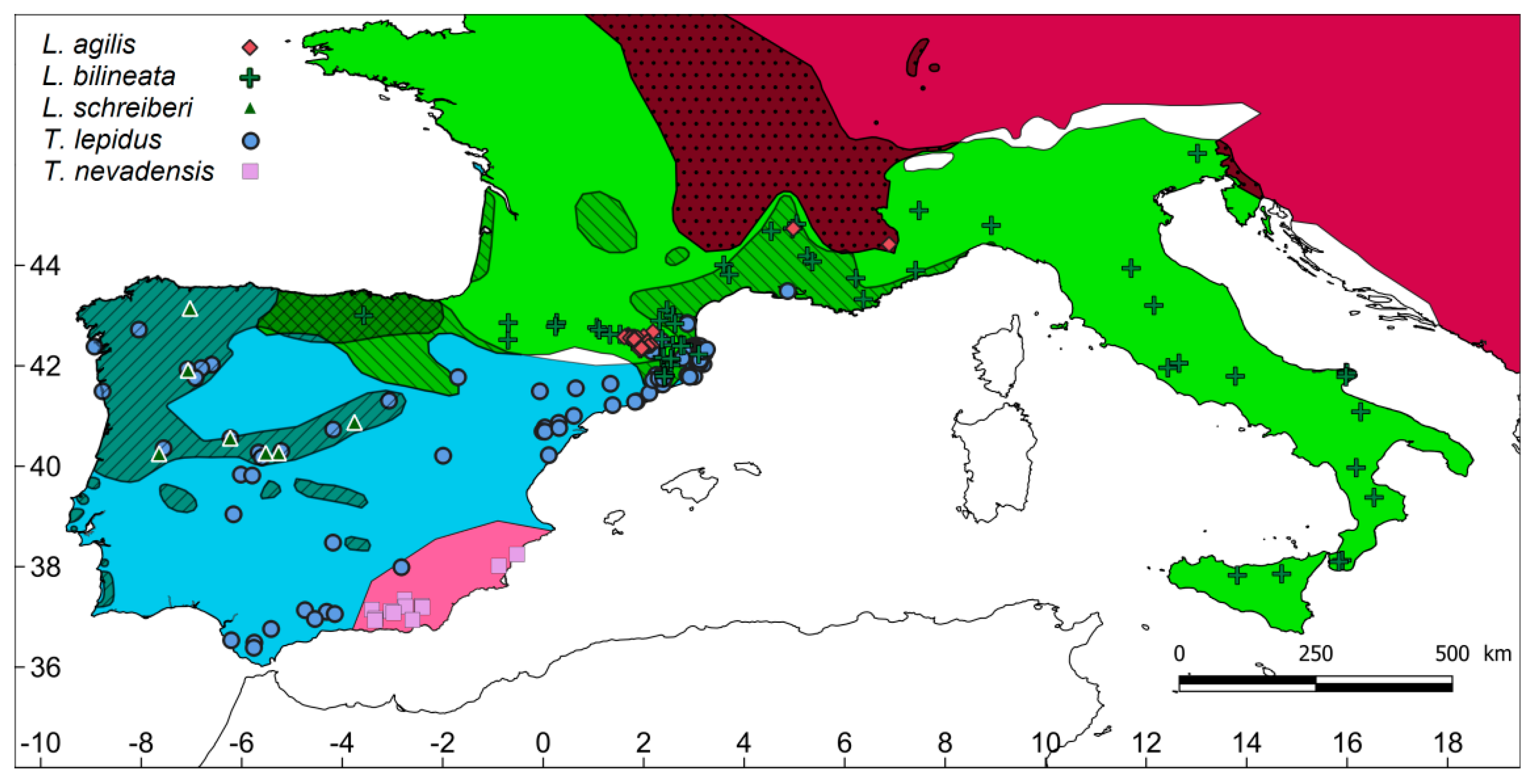
2.1. Study Region and Surveys
2.2. Environmental Data
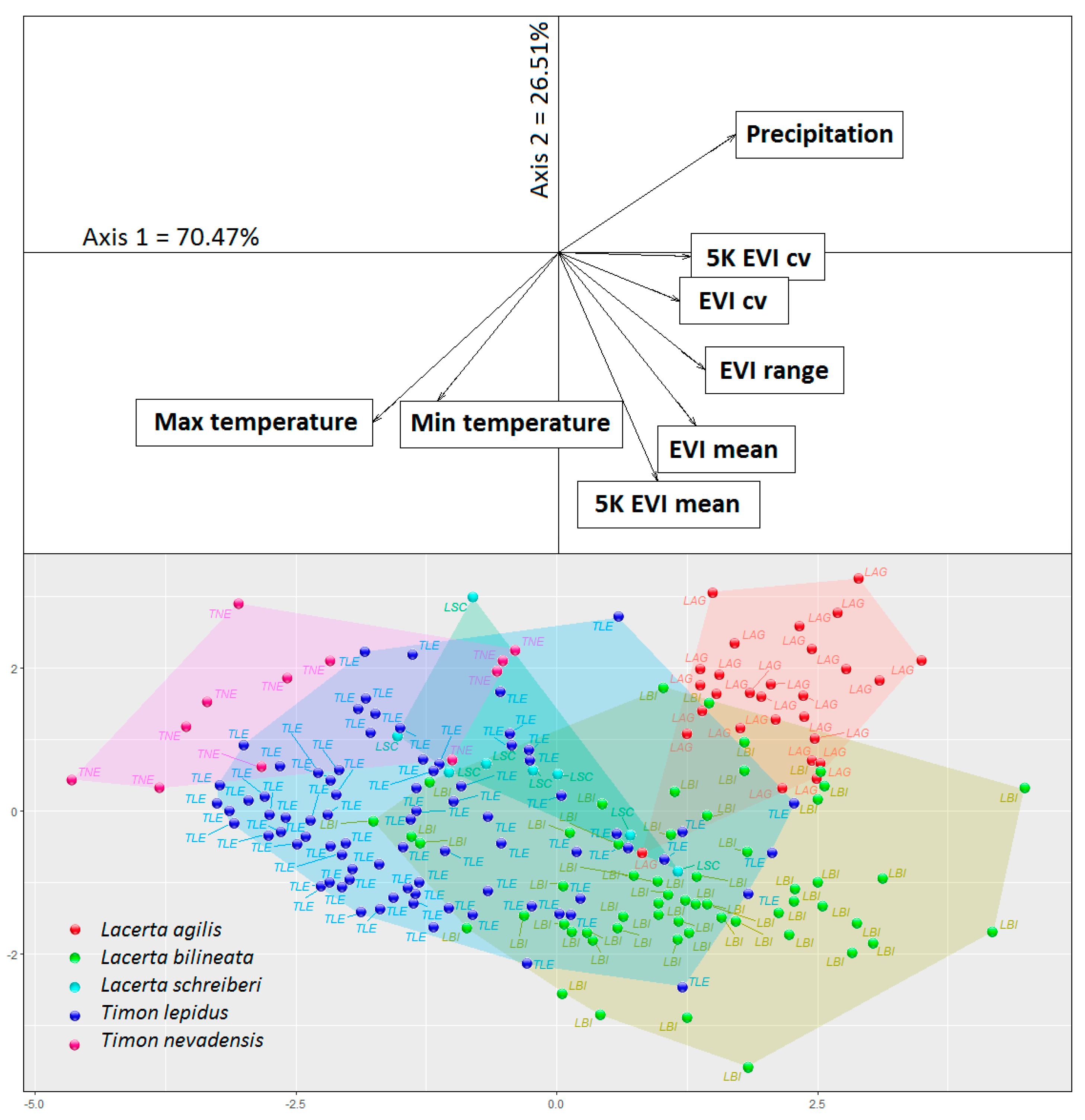
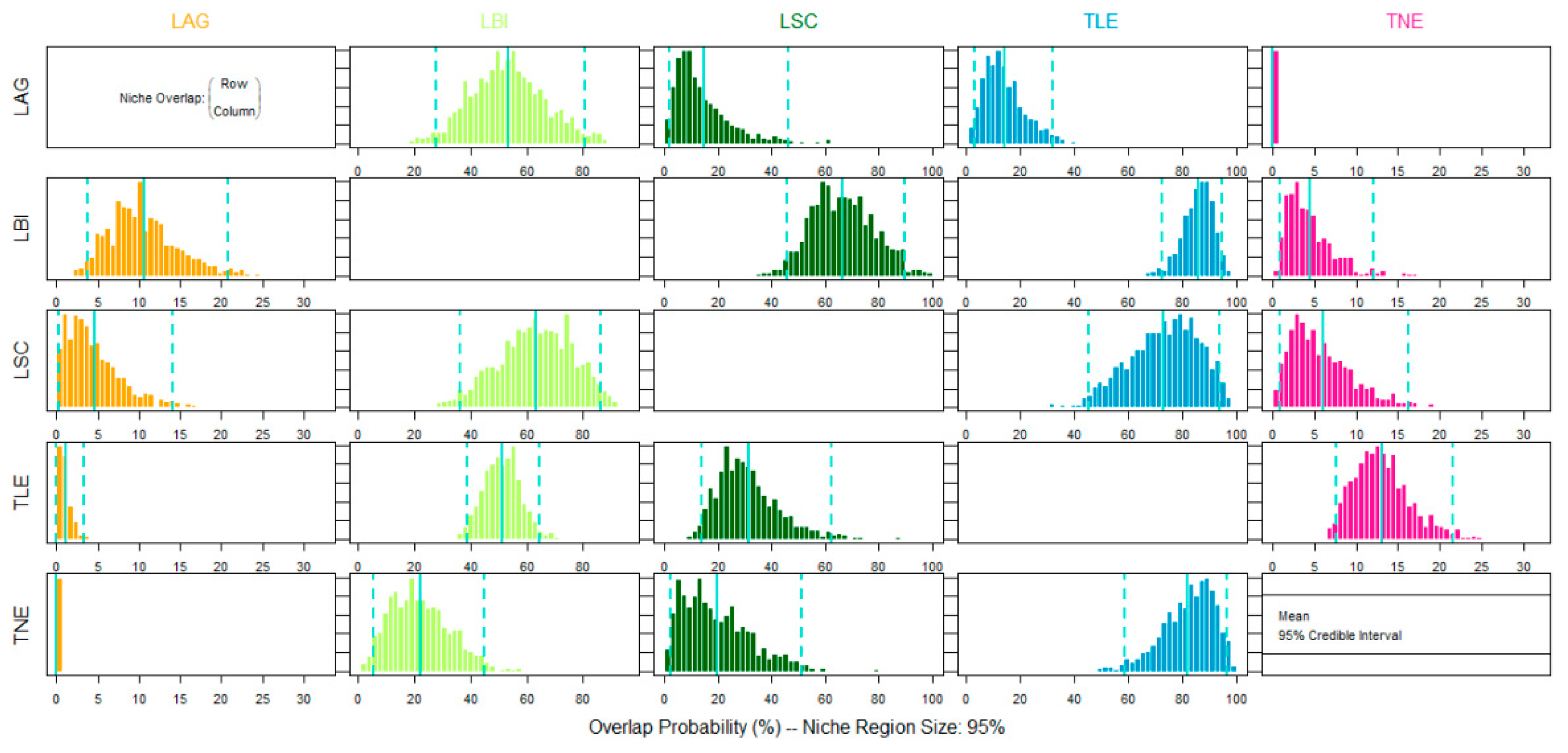
2.3. Data Analysis
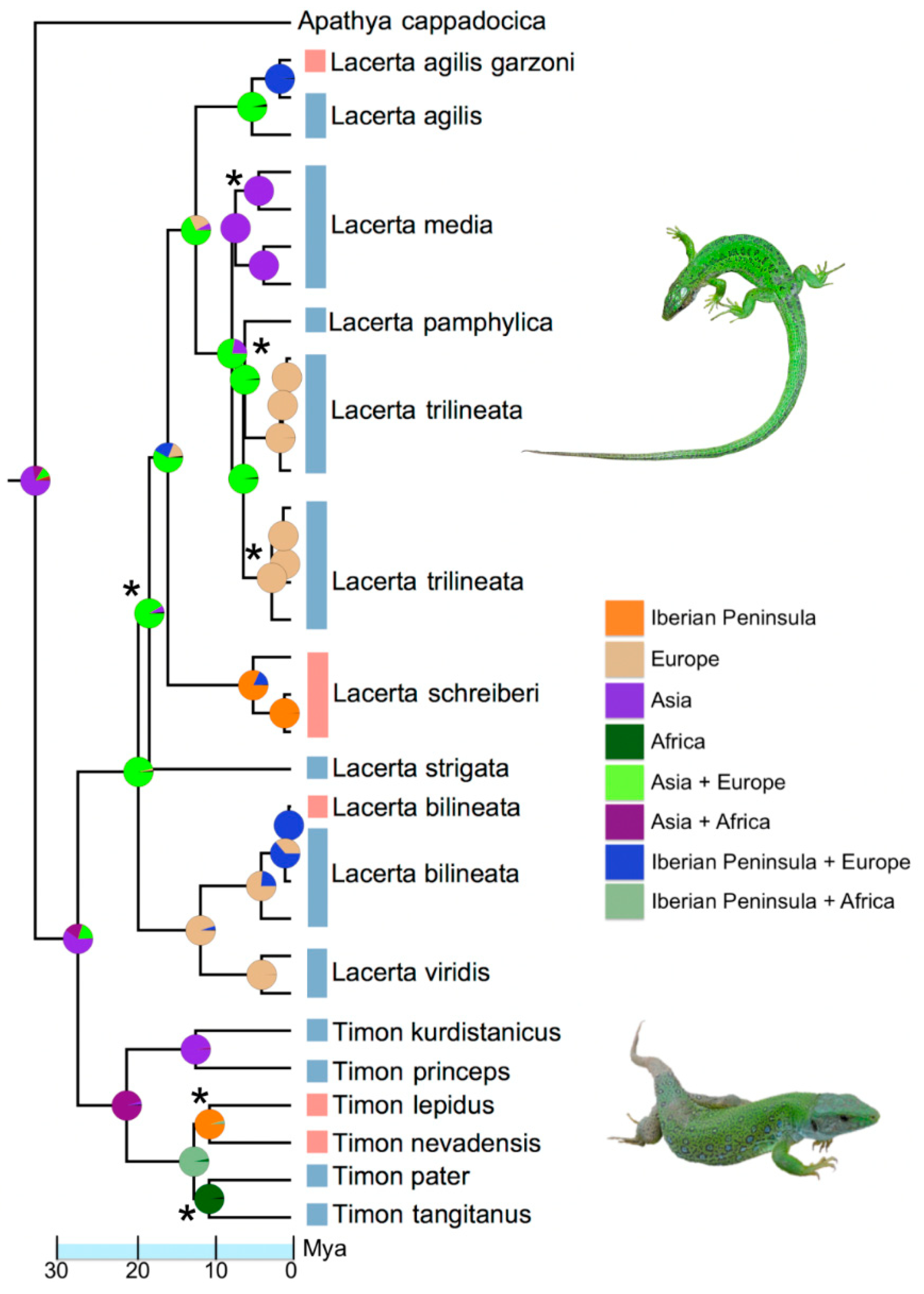
2.4. Molecular Phylogenetics and Biogeography
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jezkova, T.A.; Wiens, J.J. Testing the role of climate in speciation: New methods and applications to squamate reptiles (lizard and snakes). Mol. Ecol. 2018, 27, 2754–2769. [Google Scholar] [CrossRef]
- Velasco, J.A.; Martínez-Meyer, E.; Flores-Villela, O.; García, A.; Algar, A.C.; Köhler, G.; Daza, J.M. Climatic niche attributes and diversification in Anolis lizards. J. Biogeogr. 2016, 43, 134–144. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; Farigoule, P.; Agostini, S.; Badiane, A.; Meylan, S.; Le Galliard, J.F. Short-term change in water availability influences thermoregulation behaviours in a dry-skinned ectotherm. J. Anim. Ecol. 2020, 89, 2099–2110. [Google Scholar] [CrossRef]
- Grigg, J.W.; Buckley, L.B. Conservatism of lizard thermal tolerances and body temperatures across evolutionary history and geography. Biol. Lett. 2013, 29, 20121056. [Google Scholar] [CrossRef]
- Rodríguez, M.Á.; Belmontes, J.A.; Hawkins, B.A. Energy, water and large-scale patterns of reptile and amphibian species richness in Europe. Acta Oecologica 2005, 28, 65–70. [Google Scholar] [CrossRef]
- Law, B.S.; Bradley, R.A. Habitat use and basking site selection in the water skink, Eulamprus quoyii. J. Herpetol. 1990, 24, 235–240. [Google Scholar] [CrossRef]
- Capula, M.; Luiselli, L.; Rugiero, L. Comparative ecology in sympatric Podarcis muralis and P. sicula (Reptilia: Lacertidae) from the historical centre of Rome: What about competition and niche segregation in an urban habitat? Ital. J. Zool. 1993, 60, 287–291. [Google Scholar]
- Escoriza, D. Patterns of alpha diversity among Tunisian lizards (Lacertidae). J. Arid Environ. 2018, 151, 23–30. [Google Scholar] [CrossRef]
- Speybroeck, J.; Beukema, W.; Bok, B.; Van Der Voort, J. Field Guide to the Amphibians and Reptiles of Britain and Europe; Bloomsbury: London, UK, 2016. [Google Scholar]
- Arnold, E.N. Resource partition among lacertid lizards in southern Europe. J. Zool. Lond. B 1987, 1, 739–782. [Google Scholar] [CrossRef]
- Pollo, C.J.; Pérez-Mellado, V. An analysis of a Mediterranean assemblage of three small lacertid lizards in central Spain. Acta Oecologica 1991, 12, 655–671. [Google Scholar]
- Escoriza, D.; Pascual, G.; Sánchez-Vialas, A. Habitat use in south-west European skinks (genus Chalcides). PeerJ 2018, 6, e4274. [Google Scholar] [CrossRef] [PubMed]
- Tonini, J.F.R.; Beard, K.H.; Ferreira, R.B.; Jetz, W.; Pyron, R.A. Fully-sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biol. Conserv. 2016, 204, 23–31. [Google Scholar] [CrossRef]
- Labra, A.; Pienaar, J.; Hansen, T.F. Evolution of thermal physiology in Liolaemus lizards: Adaptation, phylogenetic inertia, and niche tracking. Am. Nat. 2009, 174, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Amat, F.A.; Llorente, G.A.; Carretero, M.A. A preliminary study on thermal ecology, activity times and microhabitat use of Lacerta agilis (Squamata: Lacertidae) in the Pyrenees. J. Zool. 2003, 52, 413–422. [Google Scholar]
- Salvador, A. Reptiles, 2a Edición Revisada y Aumentada; Fauna Ibérica; MNCN-CSIC: Madrid, Spain, 2014; Volume 10. [Google Scholar]
- Wiens, J.J.; Graham, C.H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Cuttelod, A.; García, N.; Abdul Malak, D.; Temple, H.; Katariya, V. The Mediterranean: A Biodiversity Hotspot under Threat. In The 2008 Review of The IUCN Red List of Threatened Species; Vié, J.-C., Hilton-Taylor, C., Stuart, S.N., Eds.; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- Losos, J.B.; Marks, J.C.; Schoener, T.W. Habitat use and ecological interactions of an introduced and a native species of Anolis lizard on Grand Cayman, with a review of the outcomes of anole introductions. Oecologia 1993, 95, 525–532. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Waring, R.H.; Coops, N.C.; Fan, W.; Nightingale, J.M. MODIS enhanced vegetation index predicts tree species richness across forested ecoregions in the contiguous USA. Remote Sens. Environ. 2006, 103, 218–226. [Google Scholar] [CrossRef]
- Didan, K. MOD13Q1 Modis/Terra Vegetation Indices 16-Day L3 Global 250m SIN Grid V006. Nasa Eosdis Land Processes DAAC. Available online: https://modis.ornl.gov (accessed on 10 October 2020).
- de Oliveira, B.S.; Ferreira, M.E.; Coutinho, A.C.; Esquerdo, J.C. Phenological-metric algorithm for mapping soybean in savanna biome in Brazil. Aust. J. Crop Sci. 2019, 13, 1456–1466. [Google Scholar] [CrossRef]
- Templeton, A.R.; Brazeal, H.; Neuwald, J.L. The transition from isolated patches to a metapopulation in the eastern collared lizard in response to prescribed fires. Ecology 2011, 92, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Dolédec, S.; Chessel, D.; Gimaret-Carpentier, C. Niche separation in community analysis: A new method. Ecology 2000, 81, 2914–2927. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. Available online: https://www.R-project.org (accessed on 10 September 2020).
- Swanson, H.K.; Lysy, M.; Power, M.; Stasko, A.D.; Johnson, J.D.; Reist, J.D. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 2015, 96, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Lysy, M.; Stasko, A.D.; Swanson, H.K. nicheROVER: (Niche) (R)Egion and Niche (Over)Lap Metrics for Multidimensional Ecological Niches. R Package Version 1.0. Available online: https://CRAN.R-project.org/package=nicheROVER (accessed on 4 December 2020).
- Legendre, P. Spatial autocorrelation: Trouble or new paradigm? Ecology 1993, 7, 1659–1673. [Google Scholar] [CrossRef]
- Waller, L.A. Spatial Models for Categorical Data. In Wiley StatsRef: Statistics Reference Online; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. A practical information-theoretic approach. In Model Selection and Multimodel Inference, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bivand, R.S.; Wong, D.W. Comparing implementations of global and local indicators of spatial association. Test 2018, 27, 716–748. [Google Scholar] [CrossRef]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package Version 2.3-1. Available online: https://cran.r-project.org/package=AICcmodavg (accessed on 6 January 2021).
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Carranza, S.; Arnold, E.N.; Amat, F. DNA phylogeny of Lacerta (Iberolacerta) and other lacertine lizards (Reptilia: Lacertidae): Did competition cause long-term mountain restriction? Syst. Biod. 2004, 2, 57–77. [Google Scholar] [CrossRef]
- Cernansky, A. Earliest world record of green lizards (Lacertilia, Lacertidae) from the Lower Miocene of Central Europe. Biologia 2010, 65, 737–741. [Google Scholar] [CrossRef]
- Venczel, M. Lizards from the late Miocene of Polgárdi (W-Hungary). Nymphaea 2006, 33, 25–38. [Google Scholar]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comp. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J. Tracer, Version 1.4. 2007. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 10 September 2015).
- Yu, Y.; Harris, A.J.; Blair, C.; He, X. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phyl. Evol. 2015, 87, 46–49. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Tran. Autom. Cont. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Saint Girons, M.C. Relations interspécifiques et cycle d’activité chez Lacerta viridis et Lacerta agilis (Sauria, Lacertidae). Vie et Milieu 1976, 26, 115–132. [Google Scholar]
- Geniez, P.; Cheylan, M. Les Amphibiens et Les Reptiles du Languedoc-Roussillon et Régions Limitrophes: Atlas Biogéographique; Biotope: Mèze, France, 2012. [Google Scholar]
- Arribas, O.J. Distribución y estatus de Lacerta agilis y Zootoca vivipara en Cataluña. Bull. Soc. Catalana Herpetol. 1999, 14, 10–21. [Google Scholar]
- House, S.M.; Taylor, P.J.; Spellerberg, I.F. Patterns of daily behaviour in two lizard species Lacerta agilis L. and Lacerta vivipara Jacquin. Oecologia 1979, 44, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Sercu, B.K.; Baeten, L.; van Coillie, F.; Martel, A.; Lens, L.; Verheyen, K.; Bonte, D. How tree species identity and diversity affect light transmittance to the understory in mature temperate forests. Ecol. Evol. 2017, 7, 10861–10870. [Google Scholar] [CrossRef] [PubMed]
- García, J.D.D.; Arévalo, J.R.; Fernández-Palacios, J.M. Road edge effect on the abundance of the lizard Gallotia galloti (Sauria: Lacertidae) in two Canary Islands forests. Biodivers. Conserv. 2007, 16, 2949–2963. [Google Scholar] [CrossRef]
- Amo, L.; López, P.; Martín, J. Natural oak forest vs. ancient pine plantations: Lizard microhabitat use may explain the effects of ancient reforestations on distribution and conservation of Iberian lizards. Biodivers. Conserv. 2007, 16, 3409–3422. [Google Scholar] [CrossRef]
- Schiavo, R.M.; Ferri, V. Ciclo annuale di Lacerta bilineata (Daudin, 1802) nella pianura padana lombarda. Pianura 1996, 10, 5–12. [Google Scholar]
- Nettmann, H.K.; Rykena, S. Lacerta viridis (Laurenti, 1768)—Smaragdeidechse. In Handbuch der Reptilien und Amphibien Europas; Band 2/I. Echsen II (Lacerta); Bohme, W., Ed.; Aula-Verlag: Wiesbaden, Germany, 1984; pp. 129–189. [Google Scholar]
- Suárez, F.; Manrique, J. Low breeding success in Mediterranean shrubsteppe passerines: Thekla lark Galerida theklae, lesser short-toed lark Calandrella rufescens, and black-eared wheatear Oenanthe hispanica. Ornis Scand. 1992, 23, 24–28. [Google Scholar] [CrossRef]
- Miraldo, A.; Faria, C.; Hewitt, G.M.; Paulo, O.S.; Emerson, B.C. Genetic analysis of a contact zone between two lineages of the ocellated lizard (Lacerta lepida Daudin 1802) in south-eastern I beria reveal a steep and narrow hybrid zone. J. Zool. Syst. Evol. Res. 2013, 51, 45–54. [Google Scholar] [CrossRef][Green Version]
- Barbadillo, L.J. Nuevas citas herpetológicas para la Provincia de Burgos. Rev. Esp. Herpetol. 1986, 1, 57–61. [Google Scholar]
- Delibes, A.; Salvador, A. Censos de lacértidos en la cordillera Cantábrica. Rev. Esp. Herpetol. 1986, 1, 335–361. [Google Scholar]
- Barbault, R.; Stearns, S. Towards an evolutionary ecology linking species interactions, life-history strategies and community dynamics: An introduction. Acta Oecologica 1991, 12, 3–10. [Google Scholar]
- Spawls, S.; Rotich, D. An annotated checklist of the lizards of Kenya. J. East Afr. Nat. Hist. 1997, 86, 61–83. [Google Scholar] [CrossRef]
- Thompson, J.D.; Lavergne, S.; Affre, L.; Gaudeul, M.; Debussche, M. Ecological differentiation of Mediterranean endemic plants. Taxon 2005, 54, 967–976. [Google Scholar] [CrossRef]
- Hampe, A.; Arroyo, J.; Jordano, P.; Petit, R.J. Rangewide phylogeography of a bird-dispersed Eurasian shrub: Contrasting Mediterranean and temperate glacial refugia. Mol. Ecol. 2003, 12, 3415–3426. [Google Scholar] [CrossRef] [PubMed]
| OMI | Tol | Rtol | |
|---|---|---|---|
| L. agilis | 74.4 | 6.4 | 19.2 |
| L. bilineata | 30.3 | 18.3 | 51.5 |
| L. schreiberi | 20.2 | 12.8 | 66.9 |
| T. lepidus | 24.1 | 22.6 | 53.3 |
| T. nevadensis | 66.0 | 7.2 | 26.8 |
| L. agilis | L. bilineata | L. schreiberi | T. lepidus | T. nevadensis | |
|---|---|---|---|---|---|
| L. agilis | – | 53.54 | 14.48 | 14.76 | 0.0003 |
| L. bilineata | 10.43 | – | 65.56 | 86.03 | 4.28 |
| L. schreiberi | 4.54 | 61.59 | – | 71.93 | 5.69 |
| T. lepidus | 1.07 | 51.21 | 30.58 | – | 13.17 |
| T. nevadensis | 0.0 | 22.27 | 18.28 | 83.03 | – |
| AIC | AICWt | R2 | Variables | Estimates | |
|---|---|---|---|---|---|
| LAG-LBI | 35.97 | 0.31 | 0.241 | Tmin | –2.971 |
| 37.33 | 0.16 | 0.257 | Tmin Tmax | –2.288 –0.789 | |
| LAG-TLE | 23.95 | 0.37 | 0.411 | Tmax | –3.060 |
| 25.80 | 0.15 | 0.416 | Tmax Tmin | –2.726 –0.566 | |
| 26.31 | 0.11 | 0.469 | Tmax Tmin EVIm | –2.408 –0.684 –1.534 | |
| LBI-TLE | 63.31 | 0.30 | 0.144 | EVIm | 1.264 |
| 64.64 | 0.16 | 0.154 | EVIm EVIcv | 1.286 0.275 | |
| 64.85 | 0.14 | 0.181 | EVIm Tmax Prec | 1.133 –0.658 0.301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escoriza, D.; Amat, F. Habitat Partitioning and Overlap by Large Lacertid Lizards in Southern Europe. Diversity 2021, 13, 155. https://doi.org/10.3390/d13040155
Escoriza D, Amat F. Habitat Partitioning and Overlap by Large Lacertid Lizards in Southern Europe. Diversity. 2021; 13(4):155. https://doi.org/10.3390/d13040155
Chicago/Turabian StyleEscoriza, Daniel, and Félix Amat. 2021. "Habitat Partitioning and Overlap by Large Lacertid Lizards in Southern Europe" Diversity 13, no. 4: 155. https://doi.org/10.3390/d13040155
APA StyleEscoriza, D., & Amat, F. (2021). Habitat Partitioning and Overlap by Large Lacertid Lizards in Southern Europe. Diversity, 13(4), 155. https://doi.org/10.3390/d13040155






