Photoprotective Strategies in Mediterranean High-Mountain Grasslands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Material
2.2. Xanthophyll Cycle Pigments Analysis
2.3. Chlorophyll Quantification
2.4. Chlorophyll Fluorescence Parameters
2.5. Statistical Analysis
3. Results
3.1. Chlorophyll Content, Chl a/b, and Cab/xc Ratio
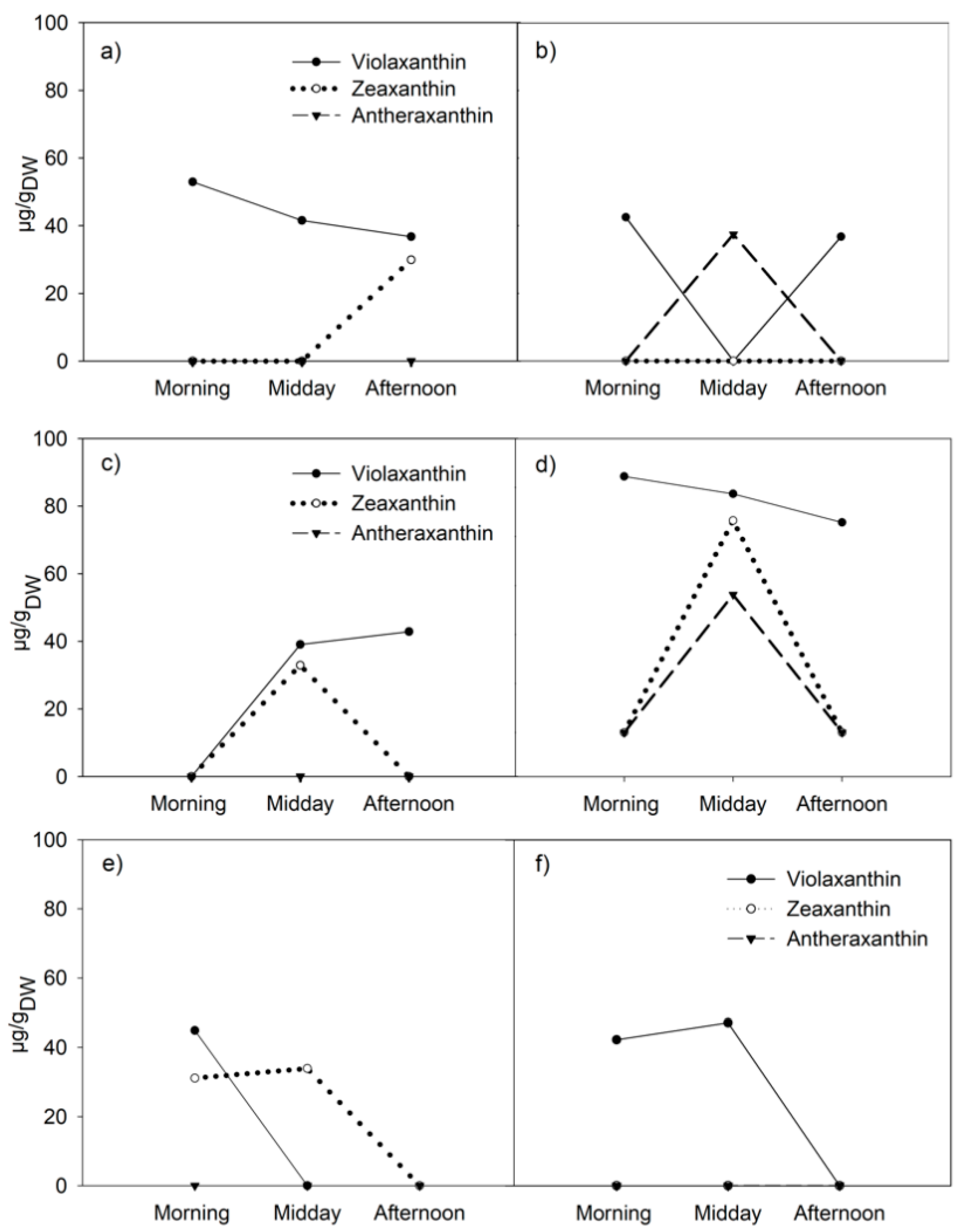
3.2. Diurnal Variations in Xanthophyll-Cycle Pigments
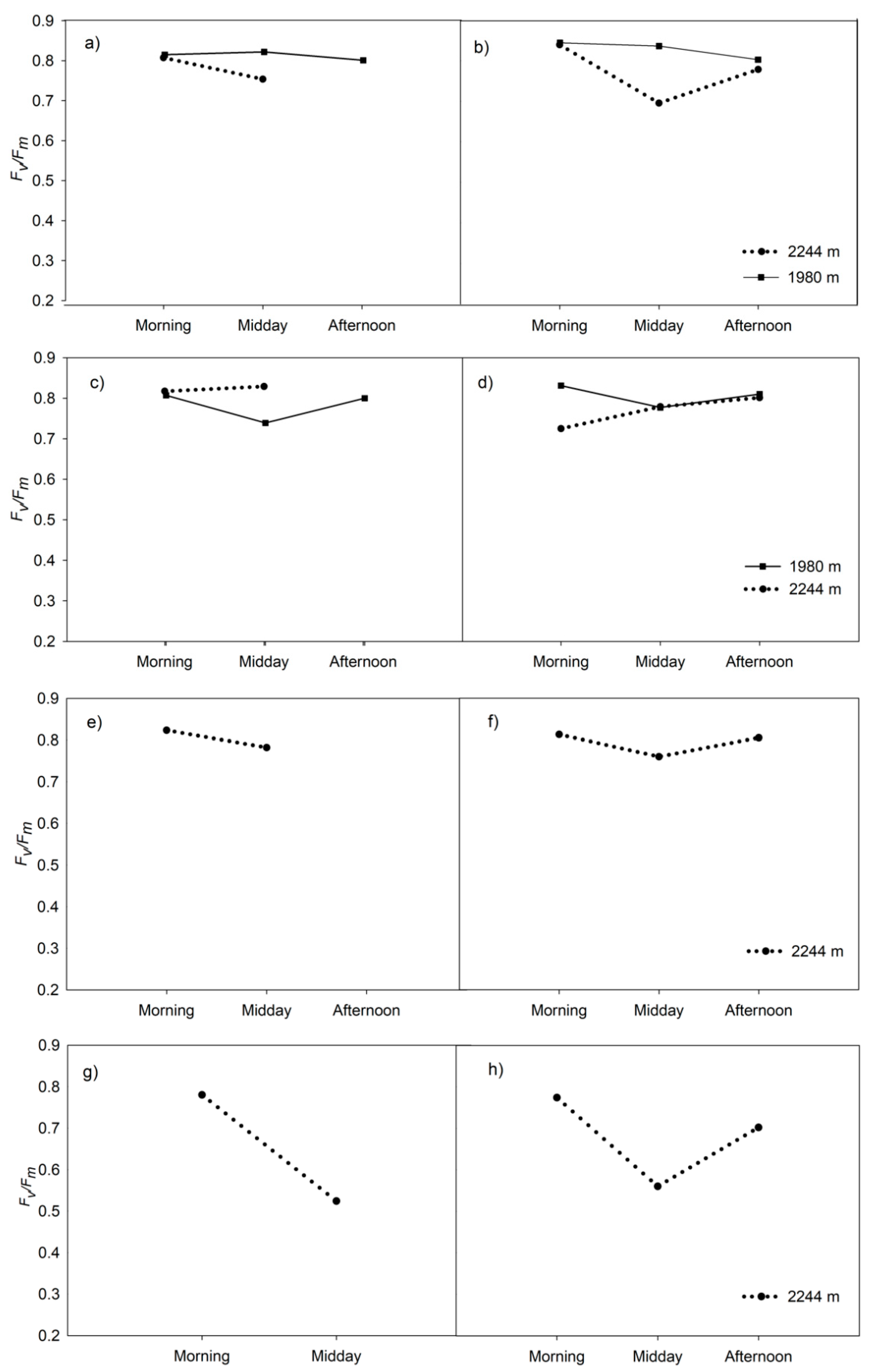
3.3. Chlorophyll Fluorescence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 2nd ed.; Springer: Cham, Switzerland, 2003. [Google Scholar]
- Gutiérrez-Girón, A.; Gavilán, R.G. Plant functional strategies and environmental constraints in Mediterranean high mountain grasslands in central Spain. Plant Ecol. Divers. 2013, 6, 435–446. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A. Molecular design of the photosystem II light-harvesting antenna: Photosynthesis and photo protection. J. Exp. Bot. 2005, 56, 365–373. [Google Scholar] [CrossRef]
- Peñuelas, J.; Munné-Bosch, S. Isoprenoids: An evolutionary pool for photoprotection. Trends Plant Sci. 2005, 10, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Scarpeci, T.E.; Zanor, M.I.; Valle, E.M. Investigating the role of plant heat shock proteins during oxidative stress. Plant Signal Behav. 2008, 3, 856–857. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Ensminger, I.; Busch, F.; Huner, N.P.A. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; García-Plazaola, J.I.; Bravo, L.A.; Corcuera, L.J. Leaf functional and micro-morphological photo protective attributes in two ecotypes of Colobanthus quitensis from the Andes and Maritime Antarctic. Polar. Biol. 2010, 33, 885–896. [Google Scholar] [CrossRef]
- Huner, N.; Öquist, G.; Hurry, V.M.; Krol, M.; Falk, S.; Griffith, M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth. Res. 1993, 17, 19–39. [Google Scholar] [CrossRef]
- Berry, J.; Björkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plan. Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Li, X.; Björkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000, 403, 391–395. [Google Scholar] [CrossRef]
- Buchner, O.; Roach, T.; Gertzen, J.; Schenk, S.; Karadar, M.; Stöggl, W.; Miller, R.; Bertel, C.; Neuner, G.; Kranner, I. Drought affects the heat-hardening capacity of alpine plants as indicated by changes in xanthophyll cycle pigments, singlet oxygen scavenging. Environ. Exp. Bot. 2017, 133, 159–175. [Google Scholar] [CrossRef]
- Zidorn, C. Altitudinal variation of secondary metabolites in flowering heads of the Asteraceae: Trends and causes. Phytochem. Rev. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Buchner, O.; Stoll, M.; Karadar, M.; Kranner, I.; Neuner, G. Application of heat stress in situ demonstrates a protective role of irradiation on photosynthetic performance in alpine plants. Plant Cell Environ. 2015, 38, 812–826. [Google Scholar] [CrossRef]
- Hay, R.; Porter, J. The Physiology of Crop Yield, 2nd ed.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Nicola, S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016, 106, 141–148. [Google Scholar] [CrossRef]
- Baker, N.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Magaña Ugarte, R.; Escudero, A.; Gavilán, R.G. Metabolic and physiological responses of Mediterranean high-mountain and Alpine plants to combined abiotic stresses. Physiol. Plant. 2019, 165, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Streb, P.; Aubert, S.; Gout, E.; Bligny, R. Reversibility of cold- and light-stress tolerance and accompanying changes of metabolite and antioxidant levels in the two high mountain plant species Soldanella alpina and Ranunculus Glacialis. J. Exp. Biol. 2003, 54, 405–418. [Google Scholar] [CrossRef]
- Streb, P.; Shang, W.; Feierabend, J.; Bligny, R. Divergent strategies of photo protection in high-mountain plants. Planta 1998, 207, 313–324. [Google Scholar] [CrossRef]
- Albert, A.; Sareedenchai, V.; Heller, W.; Seidlitz, H.K.; Zidorn, C. Temperature is the key to altitudinal variation of phenolics in Arnica montana L. cv. ARBO. Oecologia 2009, 160, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Laureau, C.; Bligny, R.; Streb, P. The significance of glutathione for photoprotection at contrasting temperatures in the alpine plant species Soldanella alpina and Ranunculus glacialis. Physiol. Plant. 2011, 143, 246–260. [Google Scholar] [CrossRef]
- Spitaler, R.; Winkler, A.; Lins, I.; Yanar, S.; Stuppner, H.; Zidorn, C. Altitudinal variation of phenolic contents in flowering heads of Arnica montana cv. ARBO: A 3-year comparison. J. Chem. Ecol. 2008, 34, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Protective effects of lichen metabolites evernic and usnic acids against redox impairment-mediated cytotoxicity in centra nervous system-like cells. Food Chem. Tox. 2017, 105, 262–277. [Google Scholar] [CrossRef]
- Wildi, B.; Lütz, C. Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ. 1996, 19, 138–146. [Google Scholar] [CrossRef]
- Larcher, W. Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst. 2000, 134, 279–295. [Google Scholar] [CrossRef]
- Magaña Ugarte, R.; Escudero, A.; Sánchez-Mata, D.; Gavilán, R.G. Changes in foliar functional traits of S. pirenaicus subsp. carpetanus under the ongoing climate change: A retrospective survey. Plants 2020, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Labourdette, D.; Génova, M.; Schmitz, M.F.; Urrutia, R.; Pineda, F.D. Summer rainfall variability in European Mediterranean mountains from the sixteenth to the twentieth century reconstructed from tree rings. Int. J. Biometeorol. 2014, 58, 1627–1639. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; García-Camacho, R.; García-Fernández, A.; Iriondo, J.M.; Lara-Romero, C.; Morente-López, J. How does climate change affect regeneration of Mediterranean high-mountain plants? An integration and synthesis of current knowledge. Plant Biol. 2018, 20, 50–62. [Google Scholar] [PubMed]
- Gutiérrez-Girón, A.; Gavilán, R.G. Monitoring Mediterranean high-mountain vegetation in the Sistema Central (Spain): GLORIA project and collateral ecological studies. Lazaroa 2013, 34, 77–87. [Google Scholar] [CrossRef]
- Sierra-Almeida, A.; Cavieres, L.A.; Bravo, L.A. Freezing resistance varies within the growing season and with elevation in high-Andean species of central Chile. New Phytol. 2009, 182, 461–469. [Google Scholar] [CrossRef]
- Abeli, T.; Orsenigo, S.; Guzzon, F.; Faè, M.; Balestrazzi, A.; Carlsson-Graner, U.; Müller, J.V.; Mondoni, A. Geographical pattern in the response of the arctic-alpine Silene suecica (Cariophyllaceae) to the interaction between water availability and photoperiod. Ecol. Res. 2015, 30, 327–335. [Google Scholar] [CrossRef]
- Pescador, D.; Sierra-Almeida, Á.; Torres, P.J.; Escudero, A. Summer Freezing Resistance: A Critical Factor for Plant Community Assemblies in Mediterranean High Mountains. Front. Plant Sci. 2016, 7, 194. [Google Scholar]
- Singh, S.; Agrawal, M.; Agrawal, S. Differential sensitivity of spinach and Amaranthus to enhanced UV-B at varying soil nutrient levels: Association with gas exchange, UV-Babsorbing compounds and membrane damage. Photosynt. Res. 2013, 115, 123–128. [Google Scholar] [CrossRef] [PubMed]
- García-Plazaola, J.; Artetxe, U.; Becerril, J. Diurnal changes in antioxidant and carotenoid composition in the Mediterranean schlerophyll tree Quercus ilex (L) during winter. Plant Sci. 1999, 143, 125–133. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.B.; Lasanta, T.; Moreno, J.I.L. Climate Change in Mediterranean Mountains during the 21st Century. AMBIO 2008, 37, 280–285. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Gavilán, R.G.; Escudero, A.; Iriondo, J.M.; Fernández-González, F. Decline of dry grassland specialists in Mediterranean high-mountain communities influenced by recent climate warming. J. Veg. Sci. 2014, 25, 1394–1404. [Google Scholar] [CrossRef]
- Winkler, M.; Lamprecht, A.; Steinbauer, K.; Hülber, K.; Theurillat, J.P.; Breiner, F.; Choler, P.; Ertl, S.; Girón, A.G.; Rossi, G.; et al. The rich sides of mountain summits- a pan European view on aspect preferences of alpine plants. J. Biogeogr. 2016, 43, 2261–2273. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress:Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Hernández-Fuentes, C.; Bravo, L.; Cavieres, L.A. Photosynthetic responses and photoprotection strategies of Phacelia secunda plants exposed to experimental warming at different elevations in the central Chilean Andes. Alp. Bot. 2015, 125, 87–99. [Google Scholar] [CrossRef]
- Hernández-Fuentes, C.; Coopman, R.E.; Cavieres, L.A.; Bravo, L.A. Photoprotective strategies against drought are depending on the elevation provenance in Phacelia Secunda. Alp. Bot. 2019, 129, 123–135. [Google Scholar] [CrossRef]
- Pescador, D.S.; Chacón-Labella, J.; de la Cruz, M.; Escudero, A. Maintaining distances with the engineer: Patterns of coexistence in plant communities beyond the patch-bare dichotomy. New Phytol. 2014, 204, 140–148. [Google Scholar] [CrossRef] [PubMed]
- García-Plazaola, J.; Becerril, J. A Rapid High-performance Liquid Chromatography Method to Measure Lipophilic Antioxidants in Stressed Plants: Simultaneous Determination of Carotenoids and Tocopherols. Phytochem. Anal. 1999, 10, 307–313. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation; R Package Version 0.7.6; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Patterson, H.; Thompson, R. Recovery of inter-black information when block sizes are unequal. Biometrika 1971, 58, 545–554. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. The Nmle: Linear and Nonlinear Mixed Effects Models; R Version 3; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- García-Plazaola, J.; Hernández, A.; Olano, J.M.; Becerril, J.M. The operation of the lutein epoxide cycle correlates with energy dissipation. Funct. Plant Biol. 2003, 30, 319–324. [Google Scholar] [CrossRef]
- Costa, A.C.; Rezende-Silva, S.L.; Megguer, C.A.; Moura LM, F.; Rosa, M.; Silva, A.A. The effect of irradiance and water restriction on photosynthesis in young jatobá-do-cerrado (Hymenaea stigonocarpa) plants. Photosynthetica 2015, 53, 118–127. [Google Scholar] [CrossRef]
- Miyake, C.; Horiguchi, S.; Makino, A.; Shinzaki, Y.; Yamamoto, H.; Tomizawa, K.I. Effects of light intensity on cyclic electron flow around PSI and its relationship to non-photochemical quenching of Chi fluorescence in tobacco leaves. Plant Cell Physiol. 2005, 46, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Gilmore, A.; Osmond, C. Diurnal and acclamatory responses of violaxanthin and lutein epoxide in the Australian mistletoe Amyema miquelii. Aust J. Plant Physiol. 2001, 28, 793–800. [Google Scholar]
- Shi, C.; Sun, G.; Zhang, H.; Xiao, B.; Ze, B.; Zhang, N.; Wu, N. Effects of Warming on Chlorophyll Degradation and Carbohydrate Accumulation of Alpine Herbaceous Species during Plant Senescence on the Tibetan Plateau. PLoS ONE 2014, 9, e107874. [Google Scholar] [CrossRef]
- Di Cecco, V.; Catoni, R.; Puglielli, G.; Di Martino, L.; Frattaroli, A.R.; Gratani, L. Ecophysiology of Adonis distorta, a high-mountain species endemic to the Central Apenines. Lazaroa 2016, 37, 125–134. [Google Scholar] [CrossRef]
- Pruzinská, A.; Tanner, G.; Aubry, S.; Anders, I.; Moser, S.; Müller, T.; Ongania, K.-H.; Kräutler, B.; Youn, J.-Y.; Liljegren, S.J.; et al. Chlorophyll breakdown in senescent Arabidopsis leaves. Characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreasing reaction. Plant Physiol 2005, 139, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Abadía, J.; Morales, F.; Abadía, A. Photosystem II efficiency in low chlorophyll, iron-deficient leaves. Plant Soil 1999, 215, 183–192. [Google Scholar] [CrossRef]


| Temperature (°C) | 1980 m.a.s.l. | |||
| Year | Morning | Midday | Afternoon | |
| 2018 | 19.1 | 21.03 | 22.6 | |
| 2019 | 19.0 | 24.4 | 21.6 | |
| 2244 m.a.s.l. | ||||
| 2018 | 19.86 | 20.02 | 19.86 | |
| 2019 | 18.9 | 22.3 | 19.8 | |
| SWC (%, by vol.) | 1980 m.a.s.l. | |||
| 2018 | 5.10 | |||
| 2019 | 1.4 | |||
| 2244 m.a.s.l. | ||||
| 2018 | 2.37 | |||
| 2019 | 1.68 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaña Ugarte, R.; Gómez-Serranillos, M.P.; Escudero, A.; Gavilán, R.G. Photoprotective Strategies in Mediterranean High-Mountain Grasslands. Diversity 2021, 13, 137. https://doi.org/10.3390/d13030137
Magaña Ugarte R, Gómez-Serranillos MP, Escudero A, Gavilán RG. Photoprotective Strategies in Mediterranean High-Mountain Grasslands. Diversity. 2021; 13(3):137. https://doi.org/10.3390/d13030137
Chicago/Turabian StyleMagaña Ugarte, Rosina, María Pilar Gómez-Serranillos, Adrián Escudero, and Rosario G. Gavilán. 2021. "Photoprotective Strategies in Mediterranean High-Mountain Grasslands" Diversity 13, no. 3: 137. https://doi.org/10.3390/d13030137
APA StyleMagaña Ugarte, R., Gómez-Serranillos, M. P., Escudero, A., & Gavilán, R. G. (2021). Photoprotective Strategies in Mediterranean High-Mountain Grasslands. Diversity, 13(3), 137. https://doi.org/10.3390/d13030137






