Digging Deeper into the Ecology of Subterranean Ants: Diversity and Niche Partitioning across Two Continents
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site
2.2. Sampling Design
2.2.1. Above-Ground Baits
2.2.2. Subterranean Baits
2.2.3. Pitfalls and Winkler Extractions
2.2.4. Species Identification
2.3. Statistical Analysis
2.3.1. Sampling and Baiting Complementarity
2.3.2. Diversity Measures above and below Ground
2.3.3. Trophic and Strata Specialisation
3. Results
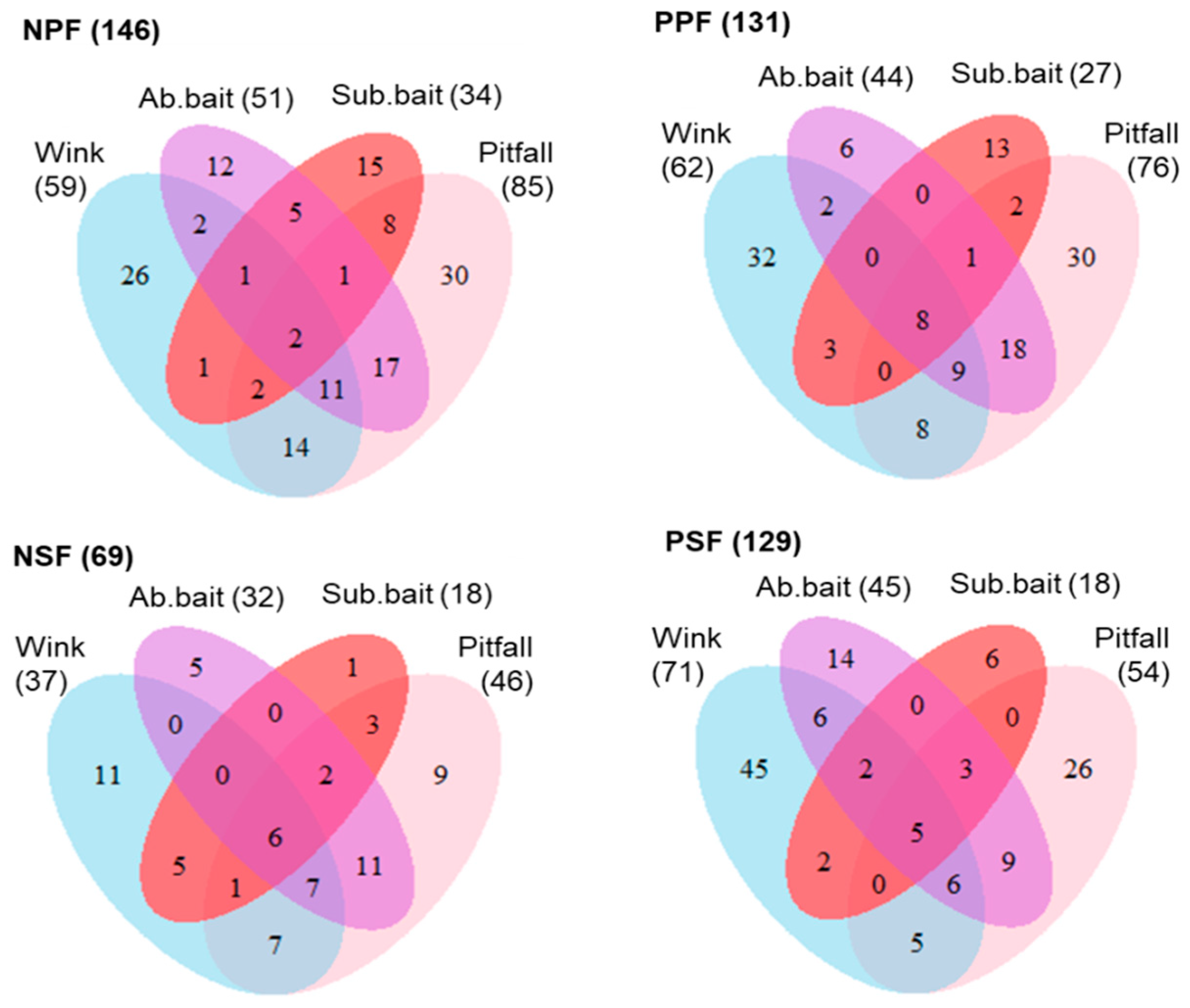
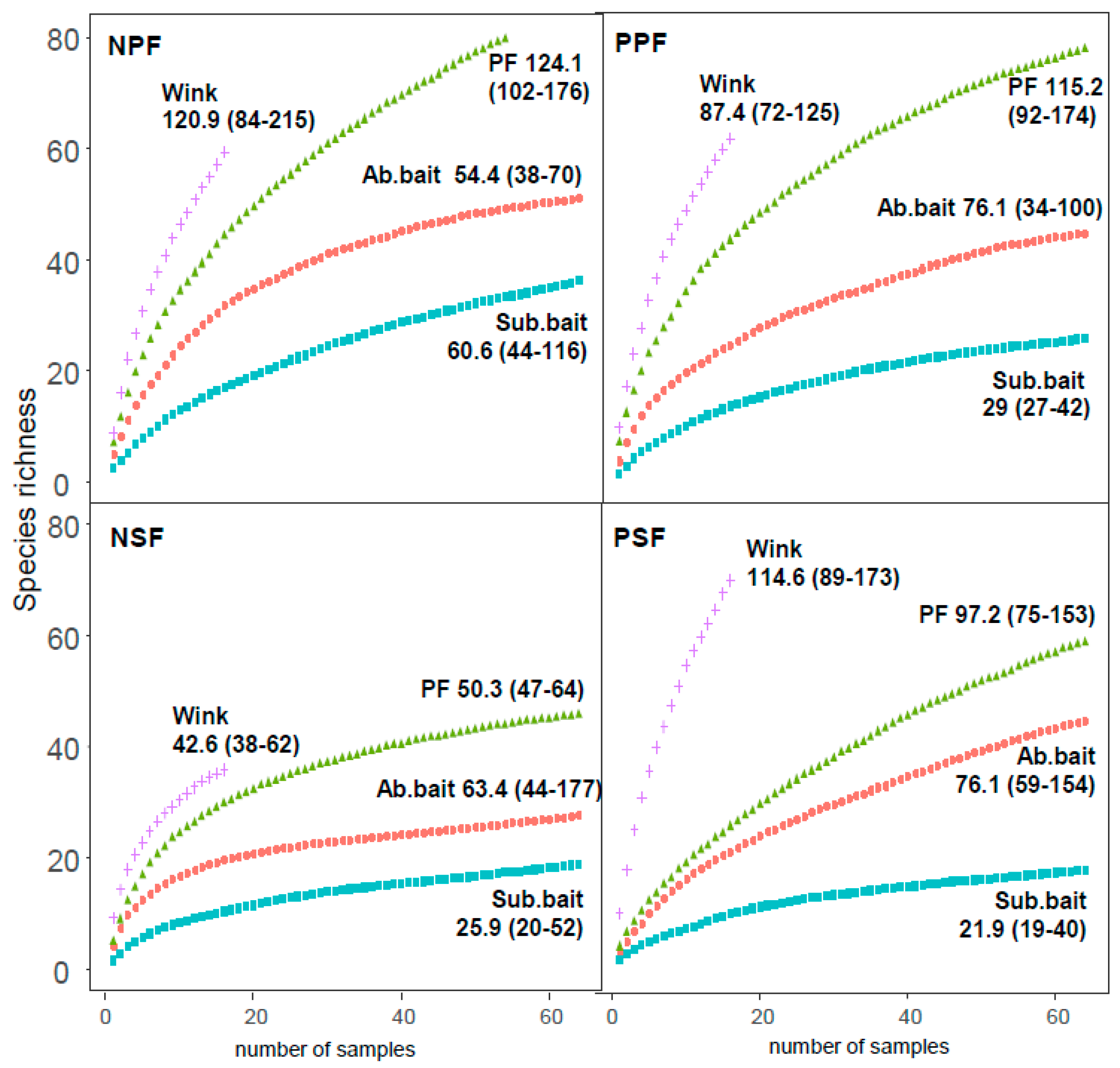
3.1. Species Richness for Different Sampling Techniques and Strata
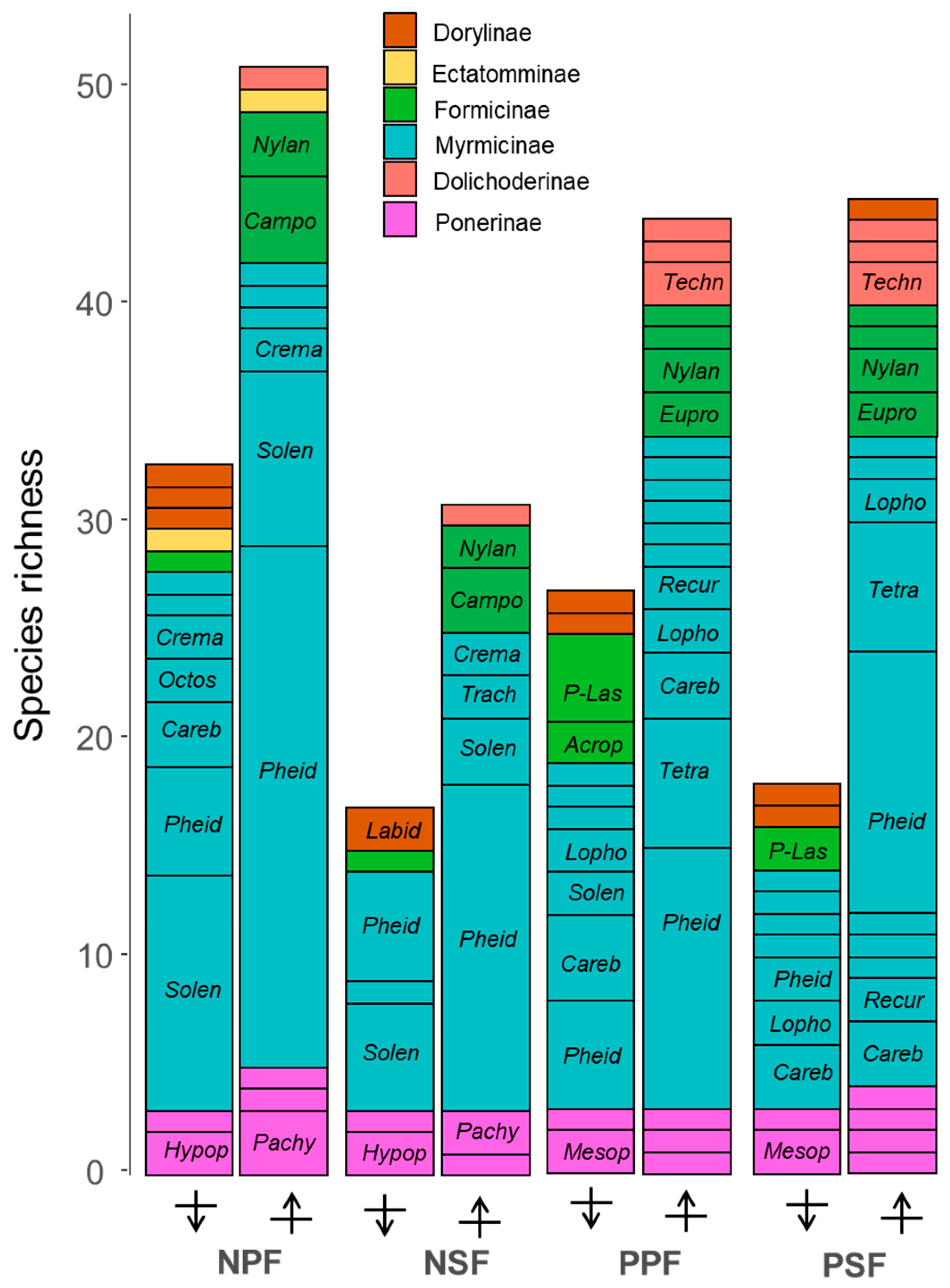
3.2. Taxonomic Composition and Stratum Specialisation at Different Baits
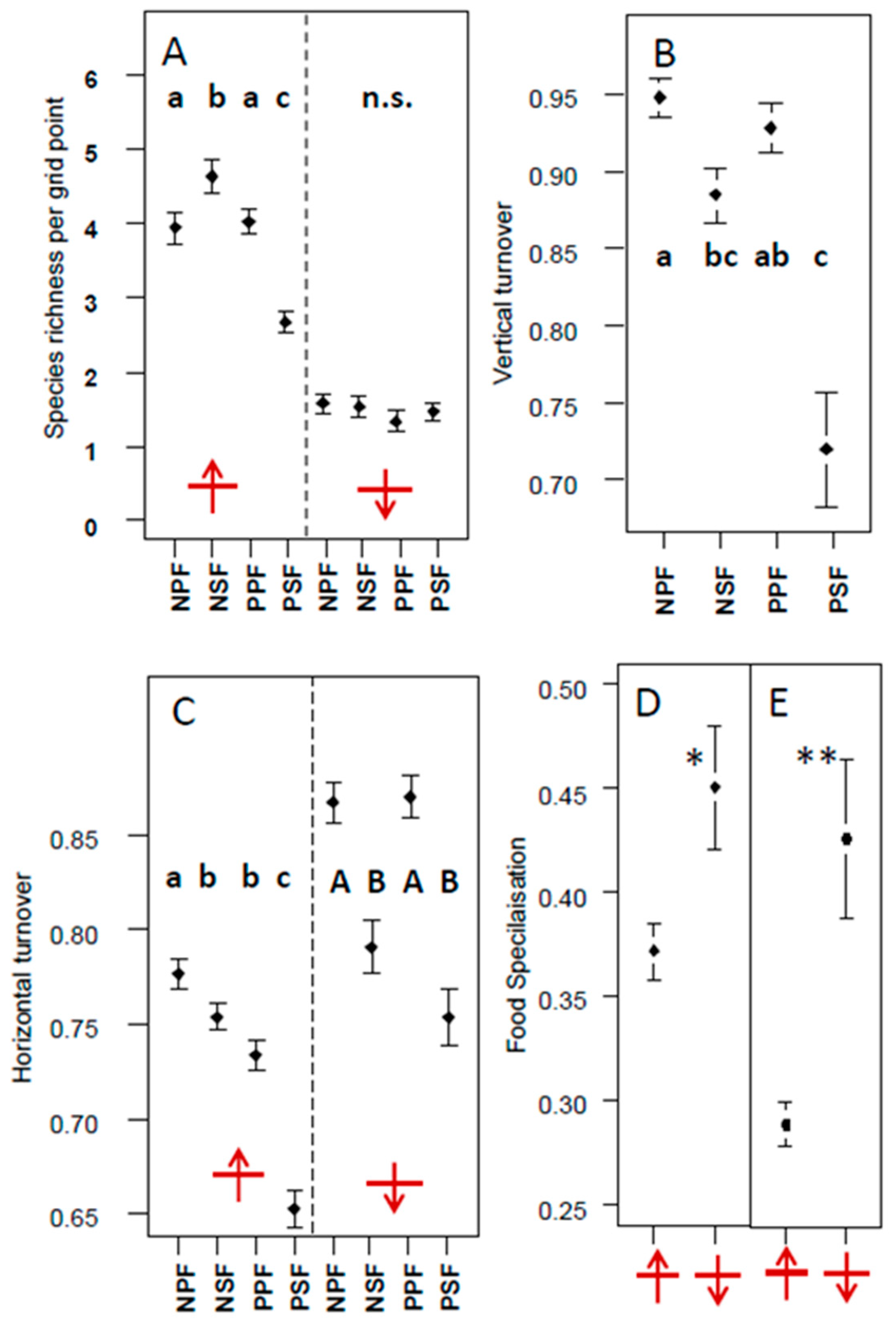
3.3. Vertical and Horizontal Turnover
3.4. Dietary Characteristics for Species and Whole Communities
4. Discussion
4.1. Species Richness in Above-Ground and Subterranean Baits
4.2. Taxonomic Composition above and below Ground
4.3. Vertical and Horizontal Turnover
4.4. Dietary Preferences and Food Specialisation
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berghoff, S.; Weissflog, A.; Linsenmair, K.E.; Hashim, R.; Maschwitz, U. Foraging of a hypogaeic army ant: A long neglected majority. Insectes Sociaux 2002, 49, 133–141. [Google Scholar] [CrossRef]
- Houadria, M.; Menzel, F. Temporal and dietary niche is context-dependent in tropical ants. Ecol. Entomol. 2020, 45. [Google Scholar] [CrossRef]
- Tanaka, H.O.; Yamane, S.; Itioka, T. Within-tree distribution of nest sites and foraging areas of ants on canopy trees in a tropical rainforest in Borneo. Popul. Ecol. 2010, 52, 147–157. [Google Scholar] [CrossRef]
- Floren, A.; Biun, A.; Linsenmair, K.E. Arboreal ants as key predators in tropical lowland rainforest trees. Oecologia 2002, 131, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bruhl, C.A.; Gunsalam, G.; Linsenmair, K.E. Stratification of ants (Hymenoptera, Formicidae) in a primary rain forest in Sabah, Borneo. J. Trop. Ecol. 1998, 14, 285–297. [Google Scholar] [CrossRef]
- Wong, M.K.L.; Guénard, B. Subterranean ants: Summary and perspectives on field sampling methods, with notes on diversity and ecology (Hymenoptera: Formicidae). Myrmecol. News 2017, 25, 1–16. [Google Scholar]
- Ryder Wilkie, K.T.; Mertl, A.L.; Traniello, J.F.A. Biodiversity below ground: Probing the subterranean ant fauna of Amazonia. Naturwissenschaften 2007, 94, 725–731. [Google Scholar] [CrossRef]
- Longino, J.T.; Colwell, R.K. Biodiversity assessment using structured inventory: Capturing the ant fauna of a tropical rain forest. Ecol. Appl. 1997, 7, 1263–1277. [Google Scholar] [CrossRef]
- Agosti, D.; Alonso, L. The ALL Protocol. In Ants: Standard Methods for Measuring and Monitoring Biodiversity; Agosti, D., Majer, J., Alonso, E., Schultz, T., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2000; p. 280. ISBN 1-56098-858-4. [Google Scholar]
- Andersen, A.N.; Brault, A. Exploring a new biodiversity frontier: Subterranean ants in northern Australia. Biodivers. Conserv. 2010, 19, 2741–2750. [Google Scholar] [CrossRef]
- Agosti, D.; Majer, J.D.; Alonso, L.E.; Schultz, T.R. Standard Methods for Measuring and Monitoring Biodiversity, 2000th ed.; Duke, J., Ed.; Smithsonian Institution Press: Washington, DC, USA, 2000; Volume 233, ISBN 1560988584. [Google Scholar]
- Rabeling, C.; Brown, J.M.; Verhaagh, M. Newly discovered sister lineage sheds light on early ant evolution. Proc. Natl. Acad. Sci. USA 2008, 105, 14913–14917. [Google Scholar] [CrossRef]
- Brown, B.; Feener, D.H.J. Parasitic phorid flies (diptera: Phoridae) associated with army ants (hymenoptera: Formicidae: Ecitoninae, dorylinae) and their conservation biology. Biotropica 1998, 30, 482–487. [Google Scholar] [CrossRef]
- Berghoff, S.; Maschwitz, U.; Linsenmair, K.E. Influence of the hypogaeic army ant Dorylus (Dichthadia) laevigatus on tropical arthropod Communities. Int. Assoc. Ecol. 2003, 135, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Houadria, M.; Salas-Lopez, A.; Orivel, J.; Blüthgen, N.; Menzel, F. Dietary and temporal niche differentiation in tropical ants-Can they explain local ant coexistence? Biotropica 2015, 47, 208–217. [Google Scholar] [CrossRef]
- Fowler, H.G.; Delabie, J.H.C. Resource partitioning among epigaeic and hypogaeic ants (Hymenoptera: Formicidae) of a Brazilian cocoa plantation. Ecol. Austral. 1995, 5, 117–124. [Google Scholar]
- Díaz, J.A.; Cabezas-Díaz, S. Seasonal variation in the contribution of different behavioural mechanisms to lizard thermoregulation. Funct. Ecol. 2004, 18, 867–875. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hasegawa, M. An experiment on ant predation in soil using a new bait trap method. Ecol. Res. 1996, 11, 11–16. [Google Scholar] [CrossRef]
- Andersen, A.N.; Penman, T.D.; Debas, N.; Houadria, M. Ant community responses to experimental fire and logging in a eucalypt forest of south-eastern Australia. For. Ecol. Manag. 2009, 258, 188–197. [Google Scholar] [CrossRef]
- Houadria, M.; Menzel, F. What determines the importance of a species for ecosystem processes? Insights from tropical ant assemblages. Oecologia 2017, 184. [Google Scholar] [CrossRef]
- Houadria, M.; Blüthgen, N.; Salas-Lopez, A.; Schmitt, M.I.; Arndt, J.; Schneider, E.; Orivel, J.; Menzel, F. The relation between circadian asynchrony, functional redundancy, and trophic performance in tropical ant communities. Ecology 2016, 97, 225–235. [Google Scholar] [CrossRef]
- Grevé, M.E.; Houadria, M.; Andersen, A.N.; Menzel, F. Niche differentiation in rainforest ant communities across three continents. Ecol. Evol. 2019, 1–15. [Google Scholar] [CrossRef]
- Eguchi, K.; Bui, T.V. Exploration of subterranean ant fauna by underground bait-trapping—A case study in Binh Chau-Phuoc Buu Nature Reserve, Vietnam (Insecta: Hymenoptera: Formicidae). ARI 2009, 32, 20–24. [Google Scholar]
- Bolton, B. Identification Guide to the Ant Genera of the World; Harvard University Press: Cambridge, MA, USA, 1997; ISBN 0-674-44280-6. [Google Scholar]
- Schmidt, C.; Shattuck, S. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 2014, 3817, 1–242. [Google Scholar] [CrossRef] [PubMed]
- Fayle, T.M. Key to the Workers of the 100 Ant Genera and 12 Ant Subfamilies of Borneo in English and Malay. Available online: http://www.tomfayle.com/Key%20to%20the%20ant%20genera%20of%20Borneo%20v1%20(English-Malay).pdf (accessed on 21 October 2020).
- Rigato, F. Revision of the myrmicine ant genus Lophomyrmex, with a review of its taxonomic position (Hymenoptera: Formicidae). Syst. Entomol. 1994, 19, 47–60. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Version 9; User’s Guide and application; Department of Ecology & Evolutionary Biology, University of Connecticut: Storrs, CT, USA, 2013; Available online: http://purl.oclc.org/estimates (accessed on 1 April 2020).
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 67, 9. [Google Scholar]
- Ryder Wilkie, K.T.; Mertl, A.L.; Traniello, J.F.A. Species diversity and distribution patterns of the ants of Amazonian ecuador. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Schmidt, F.A.; Diehl, E. What is the effect of soil use on ant communities? Neotrop. Entomol. 2008, 37, 381–388. [Google Scholar] [CrossRef]
- De Souza, D.R.; Stingel, E.; De Almeida, L.C.; Lazarini, M.A.; Munhae, C.D.B.; Bueno, O.C.; Archangelo, C.R.; Morini, M.S.D.C. Field methods for the study of ants in sugarcane plantations in Southeastern Brazil. Sci. Agric. 2010, 67, 651–657. [Google Scholar] [CrossRef][Green Version]
- Schmidt, F.A.; Solar, R.R.C. Hypogaeic pitfall traps: Methodological advances and remarks to improve the sampling of a hidden ant fauna. Insectes Sociaux 2010, 57, 261–266. [Google Scholar] [CrossRef]
- Torres, M.T.; Souza, J.L.P.; Baccaro, F.B. Distribution of epigeic and hypogeic ants (Hymenoptera: Formicidae) in ombrophilous forests in the Brazilian Amazon. Sociobiology 2020, 67, 186–200. [Google Scholar] [CrossRef]
- Pacheco, R.; Vasconcelos, H.L. Subterranean pitfall traps: Is it worth including them in your ant sampling protocol? Psyche (London) 2012, 2012, 20–23. [Google Scholar] [CrossRef]
- Sheikh, A.H.; Ganaie, G.A.; Thomas, M.; Bhandari, R.; Rather, Y.A. Ant pitfall trap sampling: An overview. J. Entomol. Res. 2018, 42, 421–436. [Google Scholar] [CrossRef]
- Weissflog, A.; Sternheim, E.; Dorow, W.H.O.; Berghoff, S.; Maschwitz, U. How to study subterranean army ants: A novel method for locating and monitoring field populations of the South East Asian army ant Dorylus (Dichthadia) laevigatus Smith, 1857 (Formicidae, Dorylinae) with observations on their ecology. Insectes Sociaux 2000, 47, 317–324. [Google Scholar] [CrossRef]
- Wong, M.K.L.; Guénard, B. Leptanilla hypodracos sp. n., a new species of the cryptic ant genus Leptanilla (Hymenoptera, Formicidae) from Singapore, with new distribution data and an updated key to Oriental Leptanilla species. Zookeys 2016, 551, 129–144. [Google Scholar] [CrossRef]
- Jacquemin, J.; Drouet, T.; Delsinne, T.; Roisin, Y.; Leponce, M. Soil properties only weakly affect subterranean ant distribution at small spatial scales. Appl. Soil Ecol. 2012, 62, 163–169. [Google Scholar] [CrossRef]
- Davidson, D.W. Resource discovery versus resource domination in ants: A functional mechanism for breaking the trade-off. Ecol. Entomol. 1998, 484–490. [Google Scholar] [CrossRef]
- Wong, M.K.L.; Guénard, B.; Lewis, O.T. Trait-based ecology of terrestrial arthropods. Biol. Rev. 2019, 94, 999–1022. [Google Scholar] [CrossRef]
- Delabie, J.H.C. Trophobiosis between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): An Overview. Neotrop. Entomol. 2001, 30, 501–516. [Google Scholar] [CrossRef]
- Rogerio, R.S.; Brandao, C.R.F. Morphological patterns and community organization in leaf-litter ant assemblages. Ecol. Monogr. 2010, 80, 107–124. [Google Scholar]
- Gibb, H.; Stoklosa, J.; Warton, D.I.; Brown, A.M.; Andrew, N.R.; Cunningham, S.A. Does morphology predict trophic position and habitat use of ant species and assemblages? Oecologia 2015, 177, 519–531. [Google Scholar] [CrossRef]
- Tuma, J.; Eggleton, P.; Fayle, T.M. Ant-termite interactions: An important but under-explored ecological linkage. Biol. Rev. 2019, 95. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Culver, D.C.; Deharveng, L.; Ferreira, R.L.; Fišer, C.; Galassi, D.M.P.; Griebler, C.; Halse, S.; Humphreys, W.F.; et al. Scientists’ warning on the conservation of subterranean ecosystems. Bioscience 2019, 69, 641–650. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houadria, M.; Menzel, F. Digging Deeper into the Ecology of Subterranean Ants: Diversity and Niche Partitioning across Two Continents. Diversity 2021, 13, 53. https://doi.org/10.3390/d13020053
Houadria M, Menzel F. Digging Deeper into the Ecology of Subterranean Ants: Diversity and Niche Partitioning across Two Continents. Diversity. 2021; 13(2):53. https://doi.org/10.3390/d13020053
Chicago/Turabian StyleHouadria, Mickal, and Florian Menzel. 2021. "Digging Deeper into the Ecology of Subterranean Ants: Diversity and Niche Partitioning across Two Continents" Diversity 13, no. 2: 53. https://doi.org/10.3390/d13020053
APA StyleHouadria, M., & Menzel, F. (2021). Digging Deeper into the Ecology of Subterranean Ants: Diversity and Niche Partitioning across Two Continents. Diversity, 13(2), 53. https://doi.org/10.3390/d13020053





