Abstract
Ongoing destruction of tropical forests makes isolated pasture trees potentially important for the persistence of original forest dwellers such as many vascular epiphytes. We studied epiphyte assemblages on 100 isolated trees at ten pasture sites in southwest Panama along an elevational gradient ranging from 140 to 1240 m a.s.l. We analysed epiphyte species composition (richness, similarity) and registered climate and host trait variables of potential influence on their occurrence. We found a total of 5876 epiphyte individuals belonging to 148 species. Epiphyte abundance, species richness and diversity all varied about 4-fold among the 10 sites, with a high similarity of epiphyte assemblages among sites. Two sites at 870 and 1050 m a.s.l. did not fit into the overall elevational trend of increased abundance, species richness and diversity. However, all three measures were significantly correlated with humidity as the independent variable. This highlights that a gradient in humidity, and not elevation as such, is responsible for the typical elevational changes in epiphyte assemblages, so that special local conditions may lead to deviations from expected patterns. Our documentation of current elevational diversity patterns also provides a baseline for the study of long-term changes in epiphyte assemblages in anthropogenically modified landscapes.
1. Introduction
Despite their important role as the habitat of at least half of the global terrestrial biodiversity, tropical forests keep decreasing in cover due to human activities [1,2]. As human populations grow, there is an increasing need for land to provide food, living space and other resources, which currently represents the principal threat to tropical biodiversity [3,4,5]. Resolving the conflict between environmental conservation and economic globalisation represents a big challenge [6]. In the tropics, land use change rather than climate change or invasive species is the most important cause of decreasing biodiversity [7]. Knowledge of the current status of tropical biodiversity in modified landscapes will allow us to establish management plans for conservation and sustainable development beyond primary forests [8].
In many pristine tropical forests, vascular epiphytes are one of the most species-rich plant groups, with major impacts on nutrient and hydrological cycles in the ecosystem [9,10,11]. Epiphyte diversity can be impressive, e.g., a single tree may harbour almost 200 vascular epiphyte species [12]. However, epiphyte richness in human-modified landscapes is usually substantially reduced (see [13,14]). In the case of pastures, isolated trees kept to offer shade for livestock can provide connectivity and allow gene flow among epiphyte populations in pastures and surrounding forest patches [15,16,17].
Knowledge of the structure and dynamics of vascular epiphyte assemblages on isolated trees is still relatively limited. Traditionally, studies with epiphytes were mainly undertaken in relatively pristine forests and, to a lesser degree, in secondary forests (e.g., [18,19,20,21,22]). More recently, isolated trees in pastures have been included in a number of studies that compared vascular epiphyte assemblages in primary forests, fragmented forests and isolated pasture trees [13,23,24,25,26,27].
Epiphyte communities and their relationship with large-scale environmental gradients has been studied for decades mainly in forests [28] and, lately, in isolated pasture trees in the lowlands (e.g., [11,13,17]). The elevational gradients and the “mid-elevation bulge” of vascular epiphytes are well known. However, diversity changes in vascular epiphyte assemblages in pastures along an elevational gradient in a single region have not been documented. Epiphytes do not show an exceptional elevational pattern: many groups of organisms show an increase in richness from the lowlands until a maximum is reached at intermediate elevations [29]. Maxima of vascular epiphyte diversity are typically reached at c. 1400–1600 m, e.g., Küper, Kreft, Nieder, Köster and Barthlott ([20], at 1500 m a.s.l., in a range from 0 to 3200 m a.s.l. in Ecuador), Krömer, Gradstein and Acebey [19], Krömer, Kessler, Gradstein and Acebey ([10], at 1500 m a.s.l., from 350 to 4000 m a.s.l. in northern Bolivia) and Hietz and Hietz-Seifert ([30], at 1400 m a.s.l., from 720 to 2370 m a.s.l. in Mexico).
The current study focused on epiphyte assemblages on isolated pasture trees along such an elevational gradient in western Panama to test whether elevational trends were comparable to those documented in previous studies in forest settings (see [19,20,21,28,30]). Since the studied elevational gradient ended at c. 1200 m, we expected a steep and continuous increase in species numbers with elevation. We also describe the variation of β-diversity in the elevational gradient and the effects of some important biotic and abiotic variables that typically have a direct effect on epiphyte assemblages [14,23]. Since epiphyte communities on isolated pasture trees tend to have low β-diversity [24], we expected the same, simple pattern along the gradient. Further, we expected clear relationships between changes in local climate and changes in epiphyte assemblages.
2. Materials and Methods
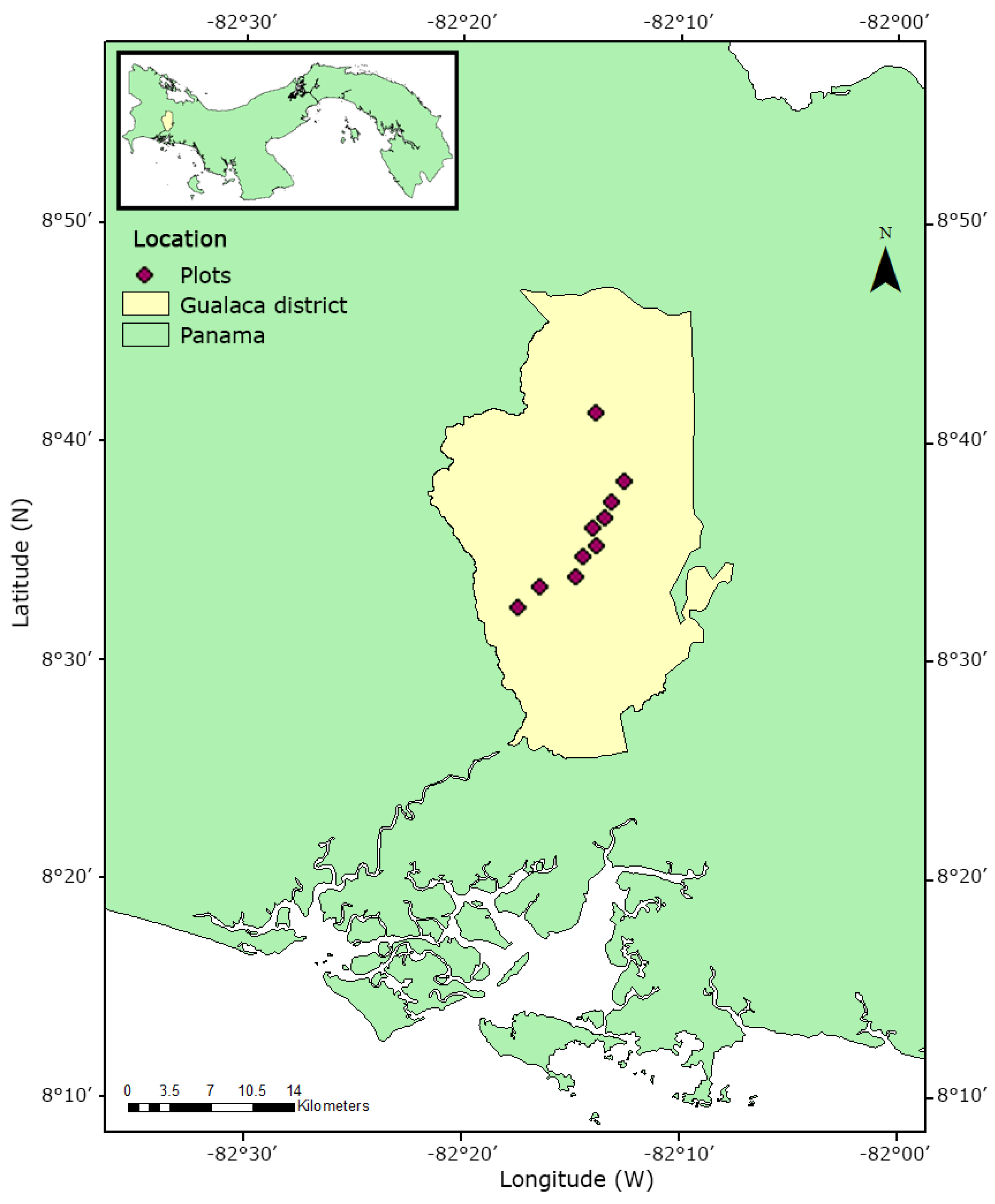
This study was conducted in southwest Panama, Gualaca district (between 8°32′27.36″ N, 82°32′27.36″ W from the lowlands at 140 m a.s.l. and 8°41′27.42″ N, 82°13′47.27″ W at 1240 m a.s.l, near the Cordillera Central). We studied 100 trees distributed in 10 1-ha pasture plots at elevation intervals from 70 to 190 m (Figure 1). According to Tosi Jr [31], the surrounding vegetation type represented patches of tropical lowland forest in the lower regions and pristine montane forest in the highlands. There is a dry season between January and April that governs lowland and highland pastures [17,32]. The pasture trees at 1240 m a.s.l. were mostly remnants of the cleared forest in close proximity to the Fortuna Forest Reserve, whereas most other trees along the gradient were planted, common pasture trees or had established spontaneously after clearance. Most of the pastures were established more than 60 years ago (personal communication of local farmers). Typical tree species were Tabebuia rosea (Bertol.) DC. (Bignoniaceae), Byrsonima crassifolia (L.) Kunth (Malpighiaceae) and Guazuma ulmifolia Lam. (Malvaceae). Within each pasture plot, we randomly selected ten trees with a diameter at breast height (DBH) ≥ 10 cm [following 17] and their geographical coordinates were recorded with a GPS. We recorded the DBH of each host tree. We monitored air temperature and relative humidity at each elevation with Hobo U23 Pro V2 data loggers (Onset Computer Corporation, MA, USA). The devices were installed in one haphazardly chosen tree per site at its first inner branches at approximately 3 m height. Data were logged every 30 min for 186 days (August 2017 to February 2018) and loggers were checked for consistent readings before and after the measurement campaign. We quantified tree density in each 1-ha pasture plot and noted the surrounding forest cover (expressed as area percentage) in a radius of 100 m of the plots using the measuring tools of Google Earth Pro 7.1.7.2606 software and satellite images from 2018. Surrounding forest cover area was studied because it represents a potential source pool of epiphytes for neighbouring pastures.
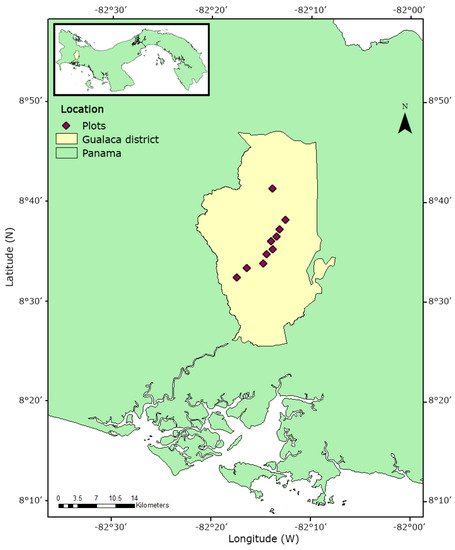
Figure 1.
Study area in Gualaca district in southwest Panama. Ten plots were established along the elevational gradient from 100 to 1240 m a.sl. In each plot, ten trees were selected, and their epiphyte assemblages were studied.
We recorded all vascular epiphytes in the selected trees. Following Poltz and Zotz [17], small seedlings were excluded because of (1) difficulties in species determination and because of (2) their high mortality rates. When growing close together, individual epiphytes cannot always be distinguished from neighbours. In these cases, we defined individuals following Sanford [33] as a “stand”, i.e., a cluster of individuals or stems belonging to the same species separated from other clusters. In our analyses, we only considered true epiphytes as defined by Zotz [34], but hemi-epiphytes, mistletoes and vines were noted to contribute to a general distributional database. Tall trees were accessed with single-rope climbing techniques [35].
Species identification of vascular epiphytes was mostly conducted in the field. When this was not possible, fertile and sterile individuals were sampled and processed in the Herbario de la Universidad Autónoma de Chiriquí (UCH). To identify species, we used several keys of vascular epiphytes from Panama, Costa Rica and Mesoamerica [36,37,38,39]. Some problematic specimens were sent to specialists for identification. In many cases, reproductive structures were lacking and we had to name morphospecies. Scientific names were standardised according to The Plant List [40]. Vouchers are deposited in the Chiriquí Herbarium, UCH.
A profile of the local climate was produced from temperature and relative humidity data. We calculated site averages of both variables. Differences among sites in regard to climate and surrounding forest cover were assessed with regression analyses and Pearson correlation tests.
To compare epiphyte richness per elevation and evaluate sampling effort, we used epiphyte richness (α-diversity) per tree at each site and estimated the richness per elevation with the Chao 1 richness estimator [41]. Further, we produced species accumulation curves of the trees sampled in every pasture by randomising the trees with 100 permutations. We also calculated the abundance, richness and diversity per tree (here as the Shannon–Wiener index) as the average per elevation (-diversity). With these data, changes in diversity components along the elevational gradient were assessed with regression analyses and Pearson correlation tests. Additionally, we tested if local climate variables that may influence epiphyte assemblages were correlated with the elevational gradient (relative air humidity and temperature). Furthermore, to test whether differences among host trees could influence epiphyte assemblages along the elevational gradient, we performed Kruskal–Wallis tests (KW) on tree size (DBH) and also tree density between the pasture plots. Finally, the relationship between tree size and epiphyte richness was analysed with a Pearson correlation test with the aim to find a positive correlation on the trees hosting epiphytes. Diversity types (α, β, γ) are also graphically defined in Figure S1.
To analyse variation in vascular epiphyte assemblage composition along the elevational gradient (β-diversity), we used a general multiple assemblage abundance-based overlap measure (CqN) [42,43], in which we produced similarity profiles at the tree scale for every pasture [43,44]. This analysis allows comparisons of communities by transforming the occurrence and abundance data to Hill numbers, which facilitates the use of numerous diversity parameters. The resulting graph relates similarity as a function of the sensitivity parameter “q” (x-axis). Values on the y-axis range from 0 to 1, indicating how similar the studied assemblages are, with 0 being completely distinct and 1 identical. When q = 0, similarity is calculated as the Sorensen index, which takes into account all species from the database and abundance is irrelevant; when q = 1, similarity is calculated as the Horn overlap index that weighs species proportionally to their frequency; and when q = 2, the measure is based on the Morisita–Horn index that weighs abundant species more than rare species [45]. For clarity, only data of five plots, but spanning the entire elevational range, were considered.
The composition of epiphyte assemblages on every studied tree along the gradient was analysed with non-metric multidimensional scaling (NMDS) using Euclidean distance. The stress value for NMDS was 0.16. To test differences within epiphyte assemblages at each elevation and according to local relative humidity, a permutational analysis of variance (PERMANOVA) was conducted. All tests were run with R v.3.3.2 software [46], where the package “vegan” [47] was used to calculate species accumulative curves, similarity profiles, NMDS and PERMANOVA and the package “vegetarian” was used to elaborate similarity profiles [48].
3. Results
3.1. Climatic Variables and Vegetation along the Elevational Gradient
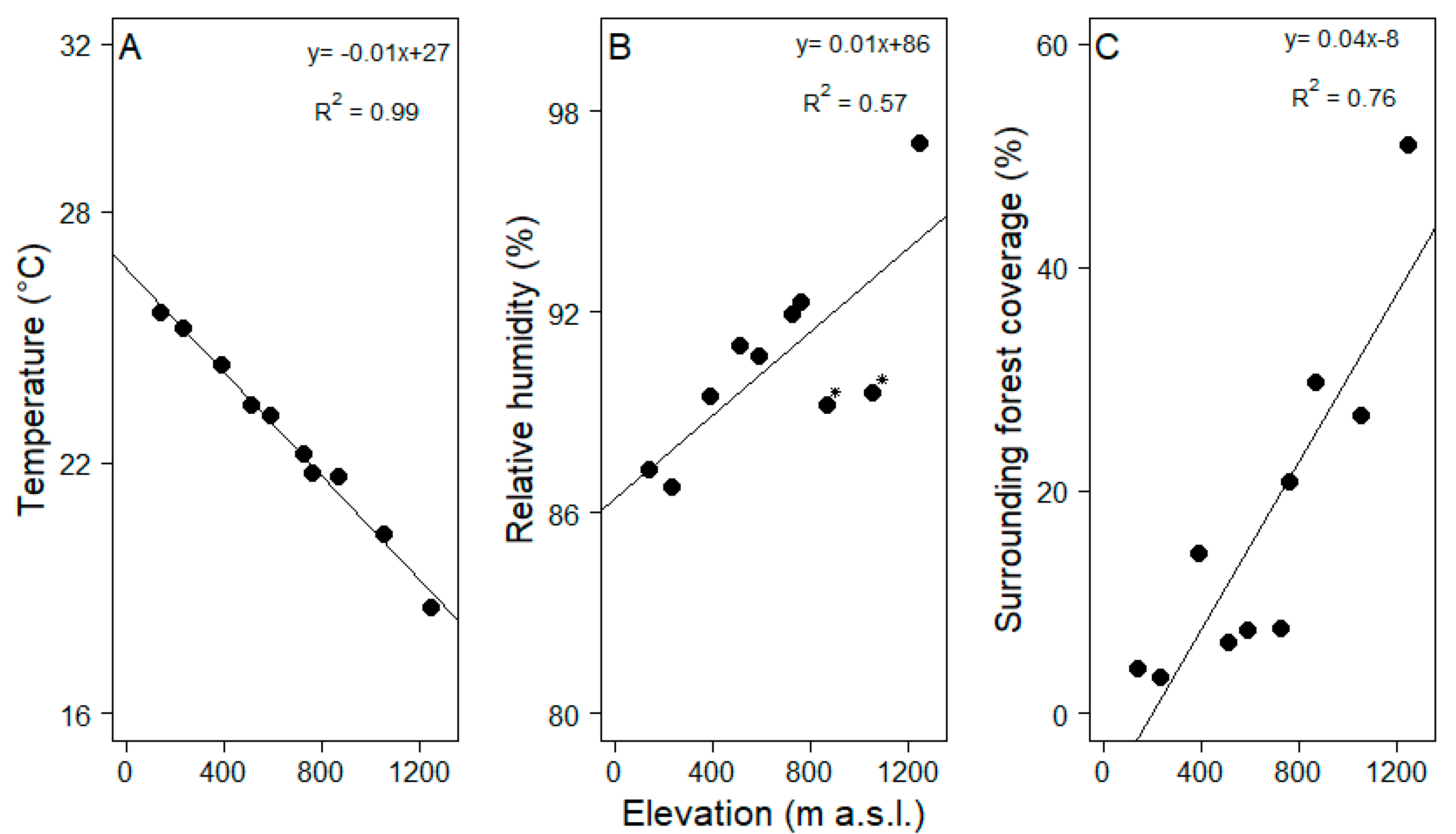
The average temperature decreased by 0.6 °C with each increase of 100 m in elevation (Figure 2A, R2 = 0.99, Pearson correlation test, t = −25.58, df = 8, p = 0.01). Increases in relative humidity were not as tightly coupled with elevation but still significantly related (Figure 2B, R2 = 0.57, Pearson correlation test, t = 3.25, df = 8, p = 0.01). Surrounding forest cover increased more than 5-fold within the studied elevational range (R2= 0.76, Pearson correlation test, t = 4.98, df = 8, p = 0.01), i.e., the proportion of modified vegetation strongly decreased towards the mountain range (Figure 2C). Tree density varied from 12 to 37 tree ha−1 in the pastures but did not vary with elevation (KW = 9, df = 9, p > 0.05, Table 1). Tree size did not vary with elevation either (KW = 15.89, df = 9, p > 0.05).
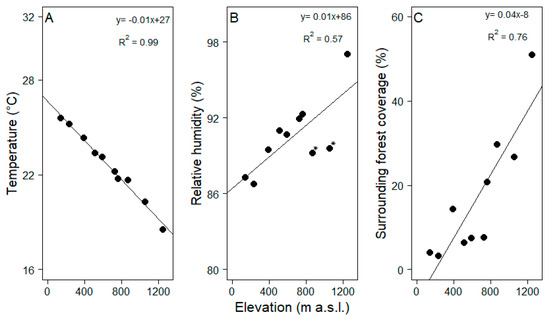
Figure 2.
Changes in abiotic and biotic features that might influence epiphyte assemblages ((A) temperature, (B) relative humidity and (C) surrounding forest coverage) along the elevational gradient (100–1200 m a.s.l.) in Gualaca district, Panama. In graph B, humidity is shown to be low in two pastures, highlighted with asterisks (at 800 and 890 m a.s.l.). Surrounding forest coverage is a measure for the size of the potential epiphyte species pool that acts as a seed source for the epiphyte assemblages on the pasture trees. Equations and coefficient of determination are given for each regression.

Table 1.
Characteristics of study sites along an elevational gradient in southwest Panama. Columns show the abiotic characteristics such as temperature (T) and relative humidity (RH), the biotic characteristics such as surrounding forest cover, diameter at breast height (DBH) and tree density and characteristics of epiphyte assemblages at the landscape scale (total richness, Chao richness and total abundance) and at the tree scale (abundance per host, richness per host, Shannon index per host). Temperature and relative humidity values are the average of 186 days of recording. Relative humidity, DBH, Chao richness, abundance per host, richness per host and Shannon index per host are expressed as the average ± standard deviation ().
3.2. Taxonomic Composition of Vascular Epiphyte Assemblages
We registered 6027 structurally dependent plants, representing 167 species. Approximately 97% of all individuals and 89% of the species were true epiphytes, representing 148 species and 5876 individuals, distributed in 49 genera and 13 families (Table S1). Hemi-epiphytes, mistletoes and vines made up 2% of the plants and 11% of the species.
Orchidaceae was the most important family accounting for more than 50% (75 taxa) of the epiphyte species and 49% (2905) of the individuals, Polypodiaceae represented 17% of the species (841/14% individuals) and Bromeliaceae represented 16% of the species (1809/31% individuals). These three families were abundant over the entire gradient. Tillandsia fasciculata was the single most abundant species, with 569 individuals at eight sites. Other common species were Vriesea sanguinolenta (563 individuals), Polypodium polypodioides (387 individuals), Catopsis nutans (360 individuals) and Epidendrum difforme sensu lato (348 individuals), which were all found at nine sites.
3.3. α-Diversity along the Elevational Gradient
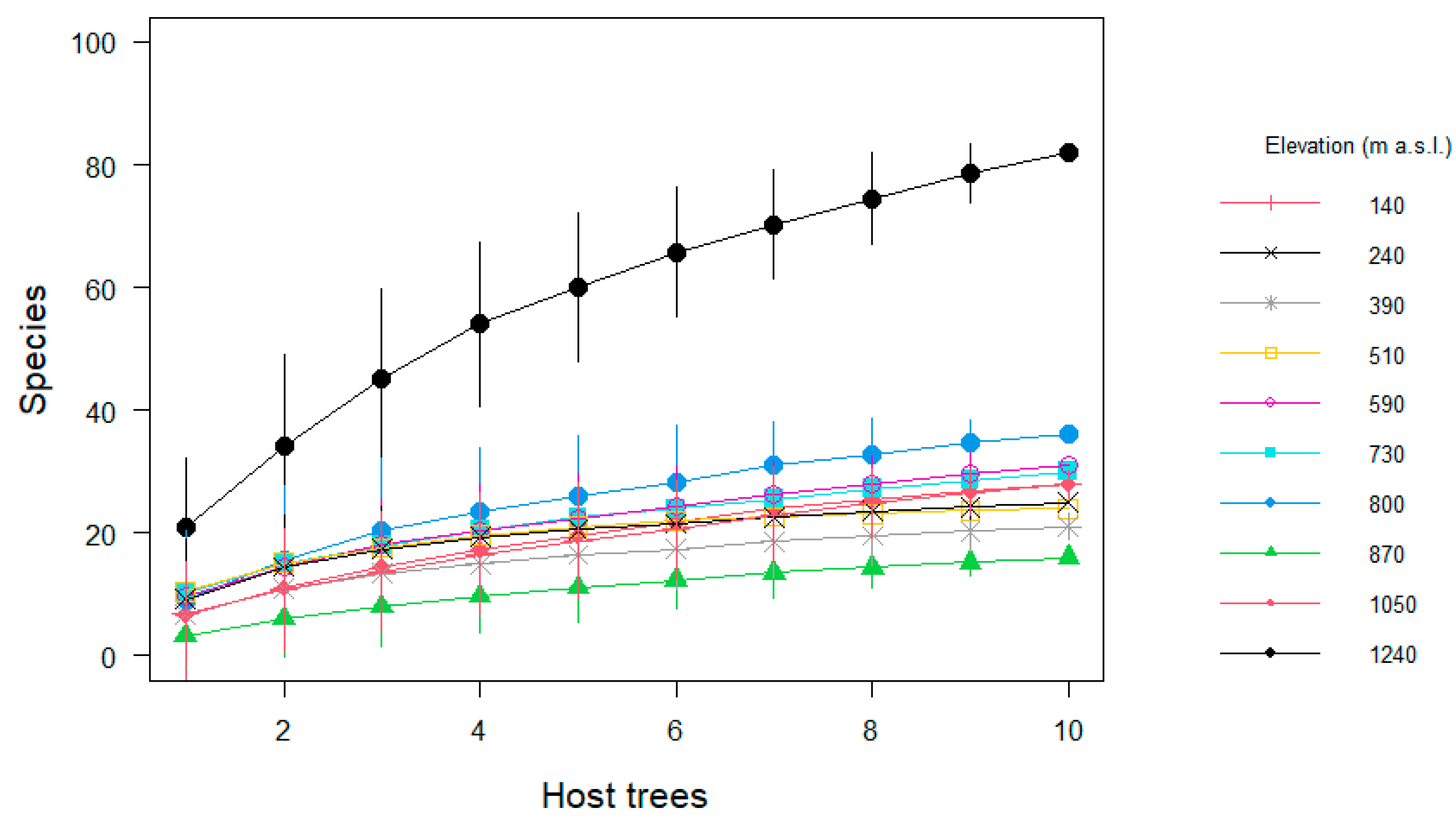
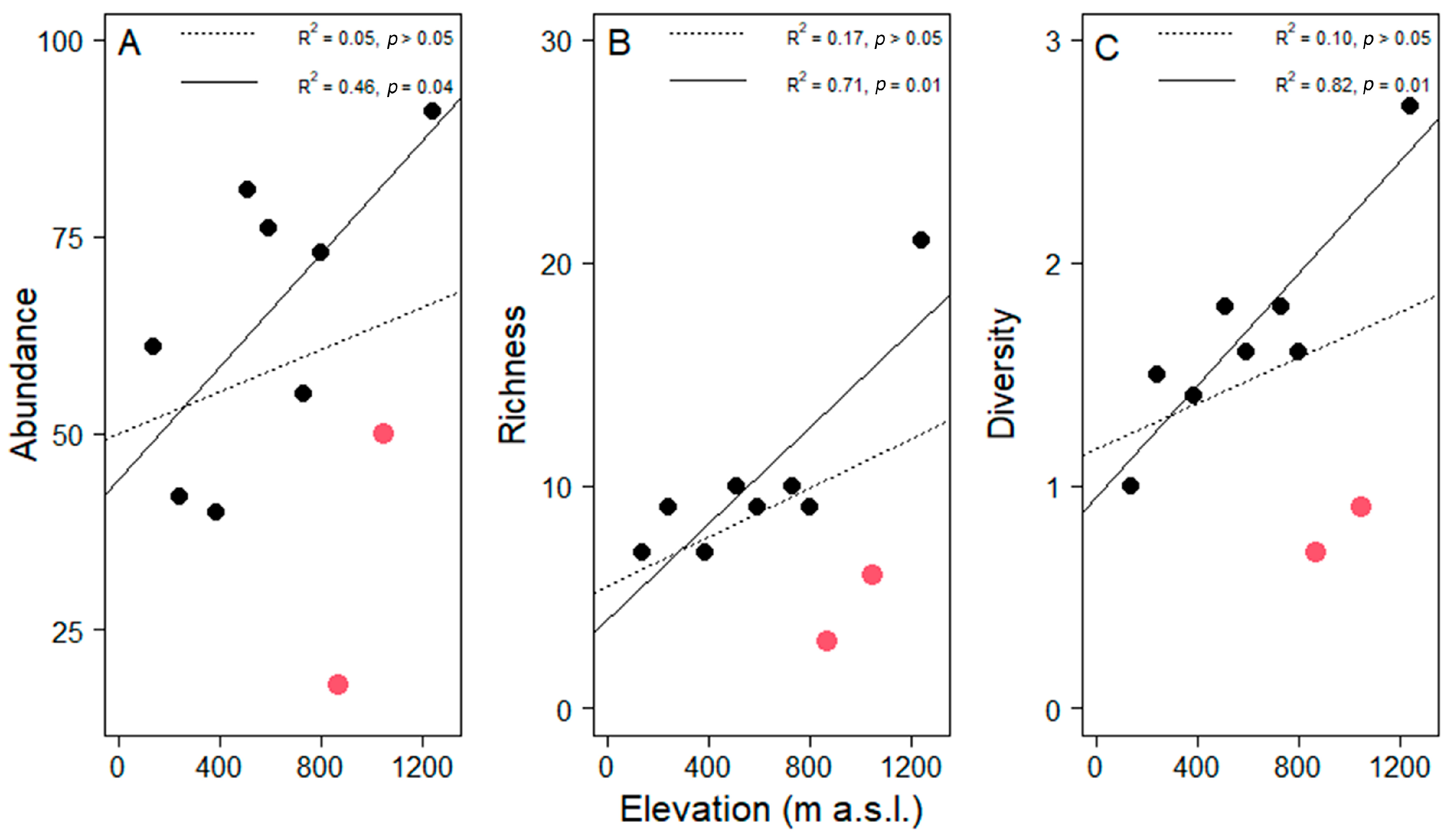
Regarding the sampling effort, the observed species represented between 45 and 95% of the estimated richness per site (Table 1). Species richness at 1240 m a.s.l. was substantially higher than at all other elevations (Figure 3). Not surprisingly, we found richness per tree was correlated with tree size (R2 = 0.11, t = 3.59, df = 98, p = 0.01). However, neither epiphyte abundance (Pearson correlation R2 = 0.05, t = 0.60, df = 8, p > 0.05,), richness (R2= 0.17, t = 1.42, df = 8, p > 0.05) nor diversity per host (R2 = 0.10, t = 0.95, df = 8, p > 0.05) correlated significantly with elevation along the entire gradient (Figure 4).
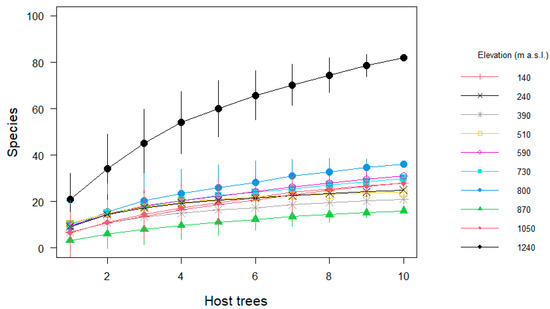
Figure 3.
Species accumulation curves of epiphyte assemblages on host trees from pasture sites distributed along an elevational gradient in Gualaca district, Panama. Data show the mean ± the standard deviation of species in every pasture.
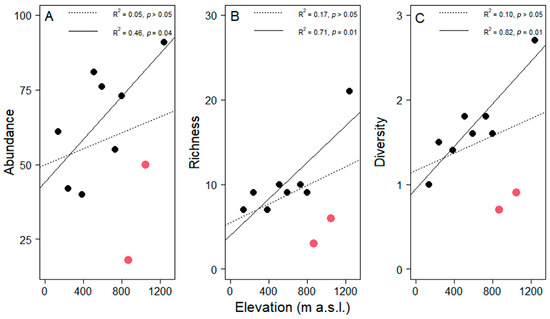
Figure 4.
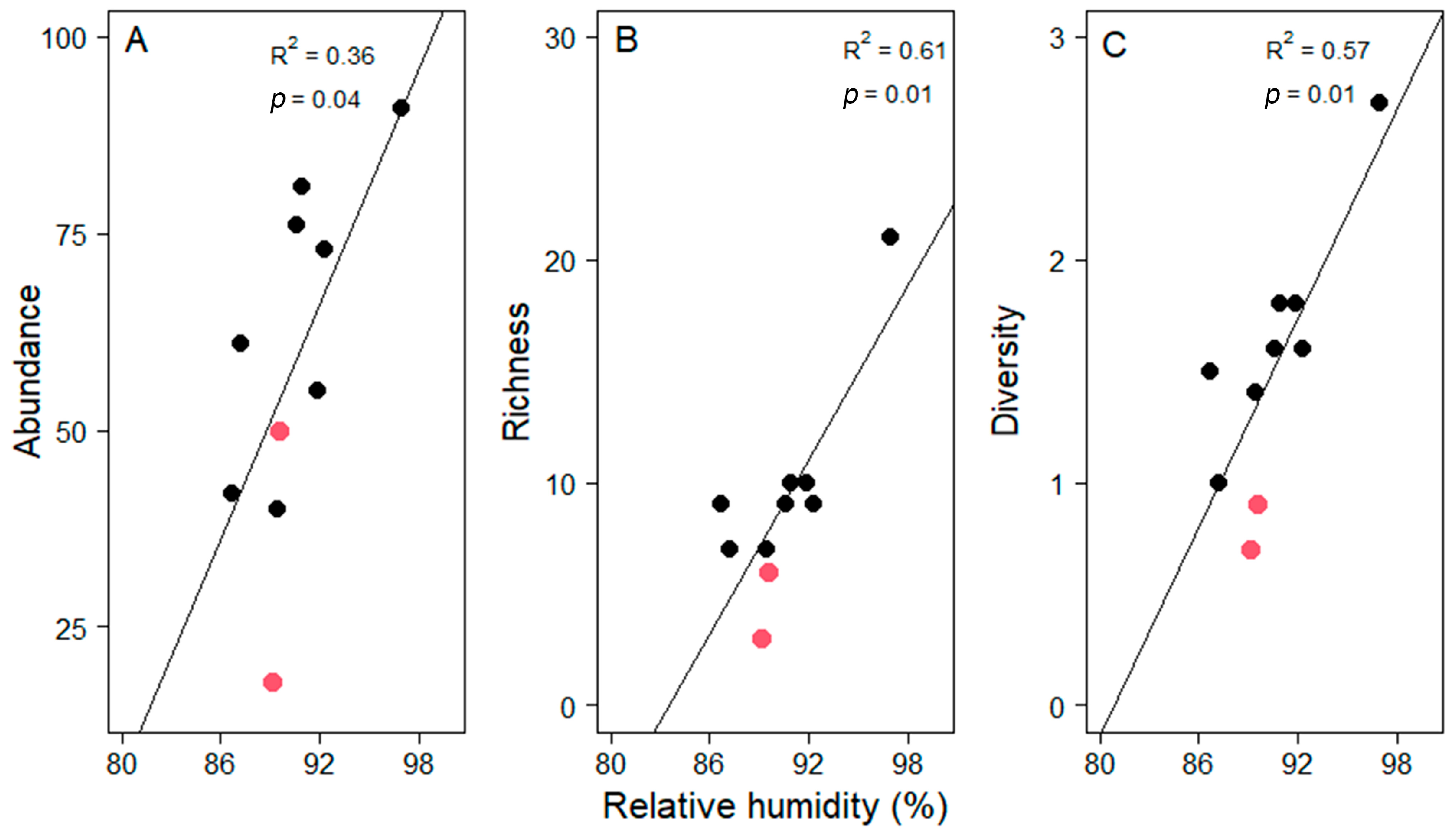
Variation in the average of (A) abundance, (B) richness and (C) diversity (as the Shannon index) of epiphyte assemblages in pasture trees (n = 10 per plot) along the elevational gradient in Gualaca district. Dotted lines represent linear regressions including all plots. Solid lines represent the correlation without the plots at 870 and 1050 m a.s.l. (highlighted with red symbols). Coefficients of determination and probability values are given in each plot.
This unexpected finding was driven by the low values of the two study sites at 870 and 1050 m a.s.l. Noteworthy is that relative humidity was very low at these two sites given the elevation (Figure 2B). Excluding these two plots from the analysis yielded significant increases with elevation in all three assemblage attributes (Figure 4). These significant relationships were strongly influenced, but not entirely driven by, the site with the highest values in all three measures at 1240 m. Including only the seven lowest plots in a third analysis still yielded a significant relationship for diversity (R2 = 0.57, p < 0.05) and a trend for abundance (R2 = 0.39, p = 0.13). Alternatively, when relating abundance, richness and diversity to relative humidity, all three relationships were highly significant (Pearson correlation, abundance, t = 2.45, df = 8, p = 0.04; richness, t = 3.85, df = 8, p = 0.01; diversity, t = 3.78, df = 8, p = 0.01, Figure 5).
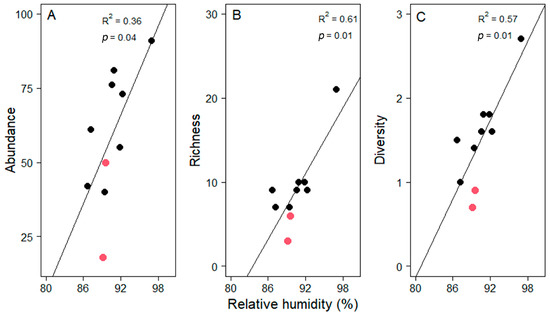
Figure 5.
Variation in the average of (A) abundance, (B) richness and (C) diversity (as the Shannon index) of epiphyte assemblages in pasture trees (n = 10 per plot) as a function of relative humidity along an elevational gradient in Gualaca district. Pastures at 870 and 1050 m a.s.l. are highlighted with red symbols. Coefficients of determination and p-values of regression analyses are given in each plot.
3.4. β-Diversity along the Elevational Gradient
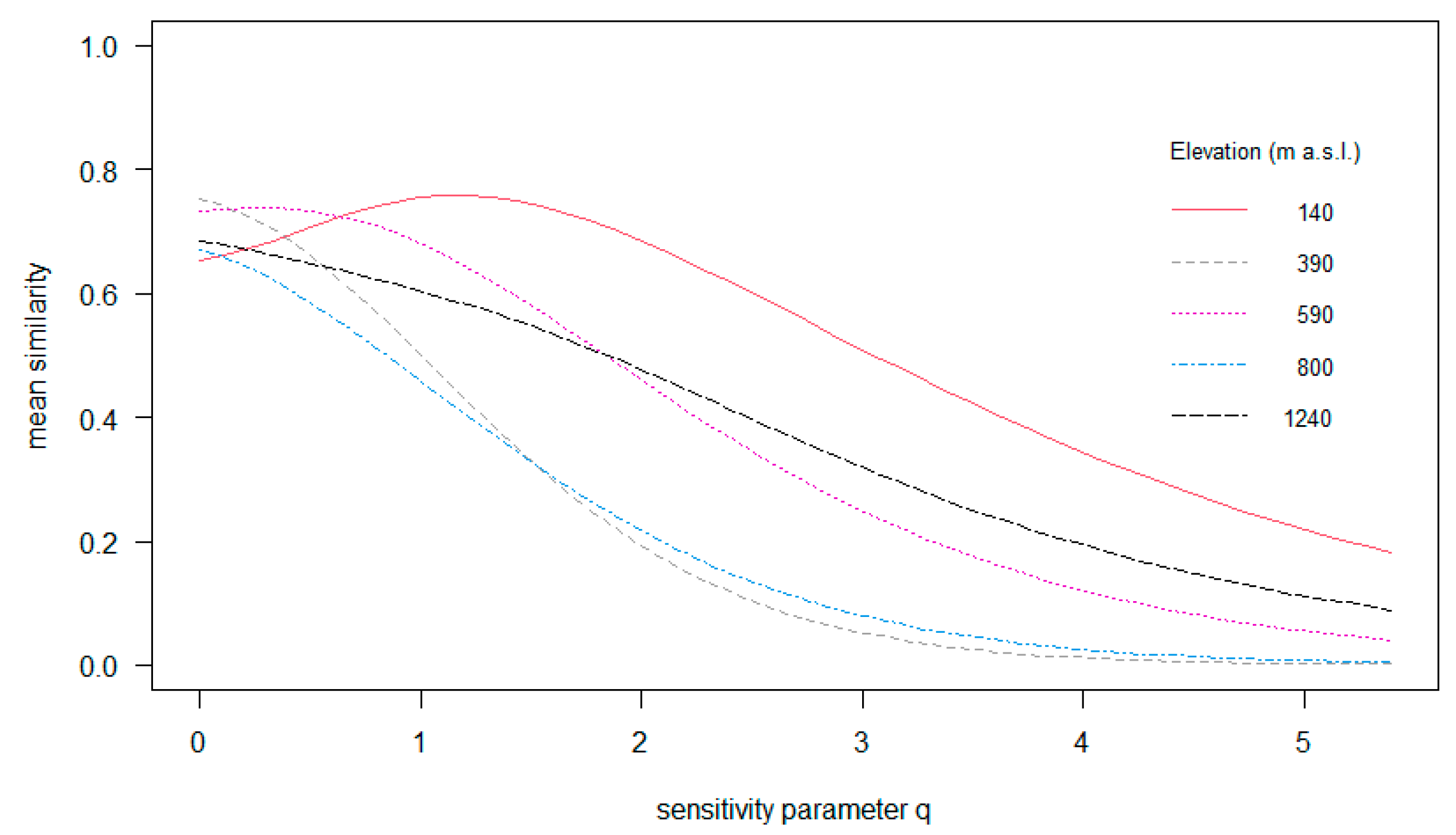
The among-tree similarity of the epiphyte assemblages ranged between 65 and 85% (q = 0; Figure 6). Among-tree similarity in regard to the most common species (q = 1) ranged between 45 and 75% along the gradient, while variation regarding very abundant species was largest with 15–70% (q = 2). In brief, among-tree similarity of epiphyte assemblages varied little between elevations considering species richness (q = 0), but considering common and also abundant species (q = 1 and q = 2, Figure 6), the structure of epiphyte assemblages varied strongly between the different elevations.
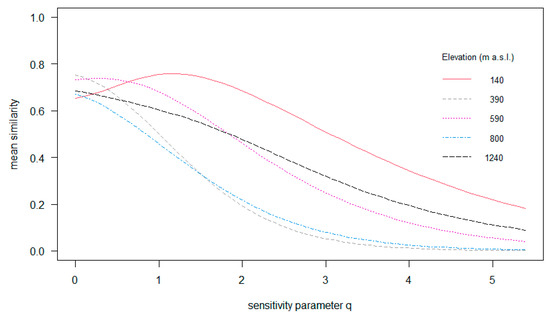
Figure 6.
Multiple-assemblage similarity profile of epiphyte assemblages growing along the elevational gradient in Gualaca district. The graph illustrates the compositional differences in the epiphyte assemblages from different elevations using similarity indices that differ in their sensitivity to relative species abundance. The x-axis shows the orders of q (sensitivity parameter) and the y-axis shows the values of mean similarity, from 0 = assemblages being completely distinct to 1.0 = assemblages being identical.
3.5. Ordination Analyses
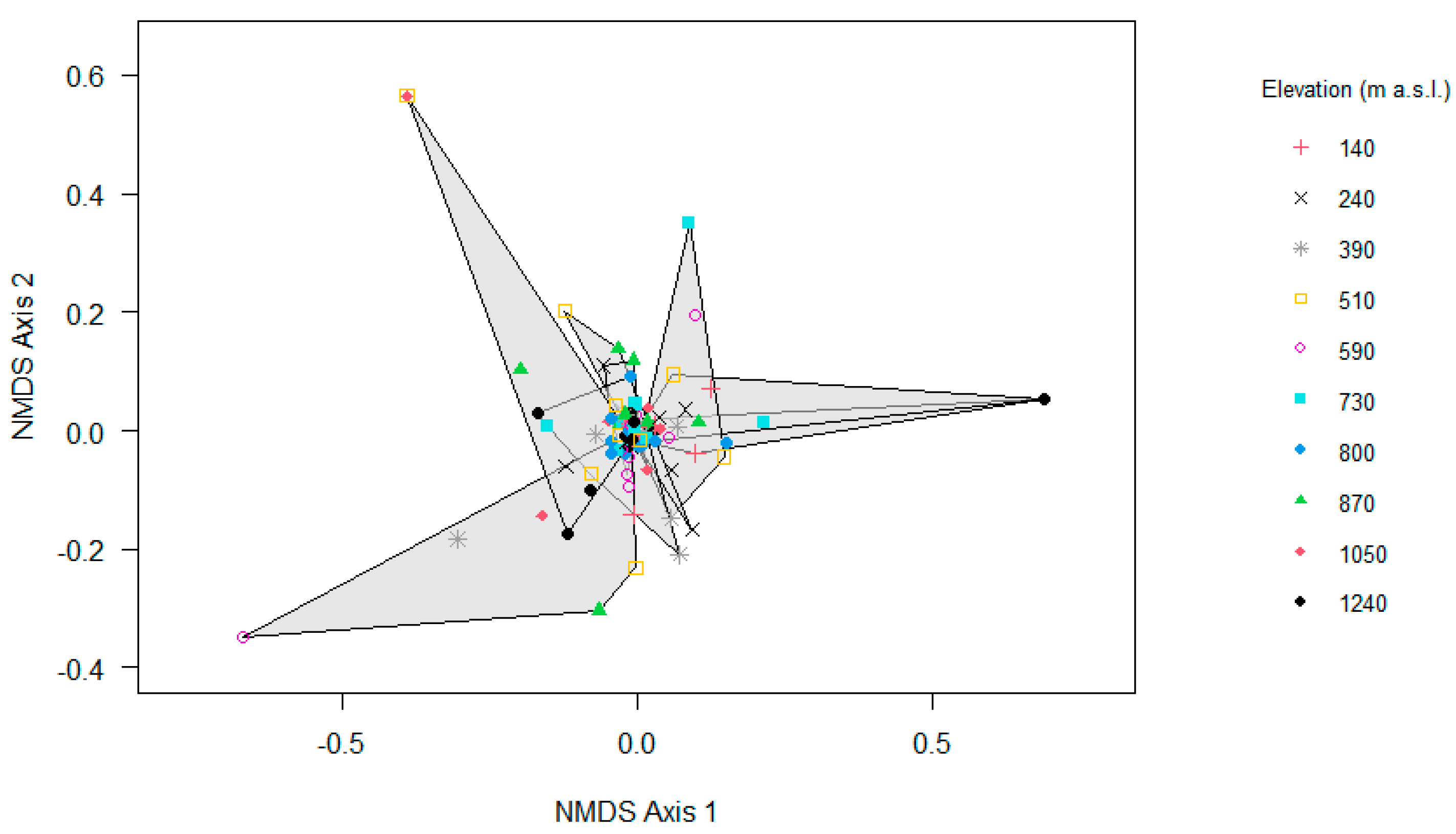
Consistent with the relatively high similarity of epiphyte assemblages among elevations, the NMDS indicated a substantial overlap of epiphyte assemblages along the gradient (Figure 7), which was further supported by the results of the PERMANOVA regarding the elevation (F = 6.69, R2 = 0.06, p = 0.01) and the local relative humidity (F = 4.20, R2 = 0.04, p = 0.01).
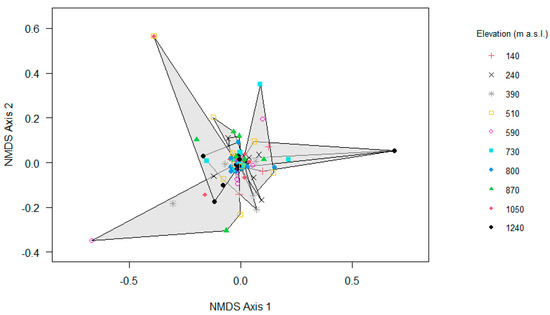
Figure 7.
Non-metric multidimensional scaling (NMDS) ordination analyses of epiphyte assemblages on trees along the elevational gradient in Gualaca district, Panama (n = 100). Stress value for NMDS is 0.16.
4. Discussion
As epiphytes lack access to soil, they respond more than other life forms such as trees or terrestrial herbs to variation in humidity [28]. Thus, the typically observed increase in epiphyte abundance and species richness with elevation up to a maximum at intermediate elevations [19,20,21,28,30] is usually explained by an increase in water availability. Surprisingly, our results did not fulfil the expectation of a steady increase because neither epiphyte richness, abundance nor diversity showed significant trends (Figure 4). However, when these parameters were correlated directly with the hypothesised driver, i.e., with local relative humidity, a significant relationship emerged (Figure 5). This suggests that the two sites at 870 and 1050 m are drier than expected for their elevation and that this deviation causes the unexpected result. Since any elevational trend in diversity is not due to elevation as such, but rather due to spatial constraints (e.g., [49]) or co-varying abiotic factors such as temperature or moisture availability (Figure 2, [50,51]), our results actually lend support to the general mechanistic explanation for differences in epiphyte community structure.
Together with local climate, variables such as tree density, surrounding forest coverage, time since original disturbance and presence of nearby human settlements all represent local factors that can modify general patterns. For example, we found that isolated trees at 1240 m a.s.l. were remnants of the primary forest with much forest in the vicinity (Figure 3), which provides a straightforward explanation for the high α-diversity, while most trees at lower elevations established after clearance and the remaining forest as source areas are scarce. A high degree of local variability in epiphyte communities related to a varying number of remnant trees and newly established ones was also reported for pastures in lowland Panama [11].
Tree density did not differ much among sites, similar to observations in pastures in the lowlands of Panama [17]. Previous studies emphasised the role of the spacing of isolated trees for the connectivity and genetic restoration of isolated plant populations [15]. Maintaining or even increasing tree density may be an important factor for the long-term persistence of vascular epiphyte assemblages in pastures. Further, the positive correlation of epiphyte abundance and host DBH found in this study supports the general notion that large and old trees are of disproportionate importance for epiphytes [15,23].
As expected, assemblages varied little in species composition (Figure 6 and Figure 7), although there was variation in assemblage structure (i.e., a change in the most abundant species) along the elevational gradient. Moreover, the overlap of epiphyte assemblages in the NMDS and PERMANOVA analyses supports the notion of small differences among elevations or by comparing them with their local relative humidity. Despite that, similarity profiles suggest that heterogeneity in the structure of epiphyte assemblages was mainly caused by changes in the most abundant species at the highest elevation where humidity is highest (Figure 6). Werner, Homeier and Gradstein [27] also reported a high species richness on isolated remnant trees in Ecuador, which was still substantially reduced compared to a forest habitat.
Noteworthy is that all long-term studies (e.g., [17,27,52]) suggest that epiphyte assemblages are currently not saturated (for a theoretical treatise, see [53]). Even on isolated trees in pastures, epiphytes tend to increase in abundance and species numbers over time: Einzmann and Zotz [54] reported a 3-fold increase in abundance over just eight years. Our study provides the basis to test whether such increases are really a universal phenomenon by repeating the census on the same pasture trees in a few years.
5. Conclusions
We documented the occurrence of vascular epiphytes in pasture trees along an elevational gradient in western Panama. Surprisingly, diversity was not significantly correlated with elevation, but variations in abundance, species richness and diversity were significantly related to differences in relative humidity. The proximity of surrounding forest and land use history influenced the number of potential species that can reach isolated trees. Both introduce a high level of local idiosyncrasy. We emphasise that the value of this study is not restricted to the analysis of the status quo but also lends itself as a starting point for the investigation of long-term changes in epiphyte assemblages in this human-modified landscape.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/13/2/49/s1: Figure S1: Analytical design. Alpha (α-) diversity represents the number of species in each individual tree. At each elevation, beta (β-) diversity represents the turnover between trees and gamma (γ-) represents the total diversity of a plot. We calculated the average diversity at the tree scale (-diversity) to perform regression analyses (n = 10) and we compared the β-diversity of each plot. Table S1: Epiphyte abundance and composition in isolated pasture trees along an elevational gradient in southwest Panama.
Author Contributions
Conceptualisation G.Z.; investigation, C.R.Q. and G.Z.; methodology, C.R.Q. and G.Z.; data collection, C.R.Q.; resources, C.R.Q. and G.Z.; funding acquisition, C.R.Q. and G.Z.; data curation and formal analysis, C.R.Q.; data interpretation, C.R.Q. and G.Z.; writing—review and editing, C.R.Q. and G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation was funded by the project APY-NI-2017-11 of Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT) of the Republic of Panama Government and the Programa de Maestría de Biología Vegetal from SENACYT and Universidad Autónoma de Chiriquí.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the supplementary material.
Acknowledgments
We are grateful to Helena Einzmann, Katrin Wagner (both Oldenburg) and Iris Fossatti (Panama) for their help in field work, to the pasture owners of the study areas, specialists in taxonomic determination, Zabdy Samudio, Zuleika Serracín, Rodolfo Flores (UCH) and other collaborators in this investigation. We also acknowledge the support of governmental institutions, especially the Ministerio de Ambiente (Scientific Permit SE/P-23-18), SENACYT and the Herbario UCH of the Universidad Autónoma de Chiriquí.
Conflicts of Interest
The authors declare no conflict of interest.
References
- MacDicken, J.; Jonsson, Ö.; Adikari, Y.; Garzuglia, M.; Lindquist, E.; Reams, G.; D’Annunzio, R. Global Forest Resources Assesment 2015: How are the World’s Forests Changing? Taylor, D., Miller, D., Eds.; FAO: Rome, Italy, 2016. [Google Scholar]
- Pimm, S.L.; Raven, P. Extinction by numbers. Nature 2000, 403, 843–845. [Google Scholar] [CrossRef] [PubMed]
- FAO. El Estado de los Bosques del Mundo 2016. Los Bosques y la Agricultura: Desafíos y Oportunidades en Relación Con el Uso de la Tierra; FAO: Rome, Italy, 2016. [Google Scholar]
- Gibson, L.; Ming Lee, T.; Pin Koh, L.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Borger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lambin, E.F.; Meyfroidt, P. Global land use change, economic globalization, and the looming land scarcity. Proc Natl Acad Sci USA 2011, 108, 3465–3472. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin III, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global diversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Harvey, C.A.; Komar, O.; Griffith, D.M.; Ferguson, B.G.; Martinez-Ramos, M.; Morales, H.; Nigh, R.; Soto-Pinto, L.; Breugel, M.; et al. Beyond reserves: A research agenda for conserving biodiversity in human-modified tropical landscapes. Biotropica 2009, 41, 142–153. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Horng, F.-W.; Kuo, C.-M. Epiphyte biomass and nutrient capital of a moist subtropical forest in north-eastern Taiwan. J. Trop. Ecol. 2002, 18, 659–670. [Google Scholar] [CrossRef]
- Krömer, T.; Kessler, M.; Gradstein, S.R.; Acebey, A. Diversity patterns of vascular epiphytes along an elevational gradient in the Andes. J. Biogeogr. 2005, 32, 1799–1809. [Google Scholar] [CrossRef]
- Einzmann, H.J.R.; Döcke, L.; Zotz, G. Epiphytes in human settlements in rural Panama. Plant Ecol. Divers. 2016, 9, 277–287. [Google Scholar] [CrossRef]
- Catchpole, D.J.; Kirkpatrick, J.B. The outstandingly speciose epiphytic flora of a single strangler fig (Ficus crassiuscula) in a Peruvian montane cloud forest. In Tropical Montane Cloud Forests: Science for Conservation and Management; Bruijnzeel, L.A., Scatena, F.N., Hamilton, L.S., Eds.; Cambridge University Press New York: New York, NY, USA, 2010; pp. 142–146. [Google Scholar]
- Einzmann, H.J.R.; Zotz, G. How diverse are epiphyte assemblages in plantations and secondary forests in tropical lowlands? Trop. Conserv. Sci. 2016, 9, 629–647. [Google Scholar] [CrossRef]
- Werner, F.A.; Köster, N.; Kessler, M.; Gradstein, S.R. Is the resilience of epiphyte assemblages to human disturbance a function of local climate? Ecotropica 2011, 17, 15–20. [Google Scholar]
- Manning, A.D.; Fischer, J.; Lindenmayer, D.B. Scattered trees are keystone structures – Implications for conservation. Biol. Conserv. 2006, 132, 311–321. [Google Scholar] [CrossRef]
- Ozolins, A.; Brack, C.; Freudenberger, D. Abundance and decline of isolated trees in the agricultural landscapes of central New South Wales, Australia. Pac. Conserv. Biol. 2001, 7, 195–203. [Google Scholar] [CrossRef]
- Poltz, K.; Zotz, G. Vascular epiphytes on isolated pasture trees along a rainfall gradient in the lowlands of Panama. Biotropica 2011, 43, 165–172. [Google Scholar] [CrossRef]
- Johansson, D. Ecology of vascular epiphytes in west African rain forest. Suec. Acta Phytogeogr. 1974, 59, 1–136. [Google Scholar]
- Krömer, T.; Gradstein, S.R.; Acebey, A. Diversidad y ecología de epífitas vasculares en bosques montanos primarios y secundarios de Bolivia. Ecol. Boliv. 2007, 42, 23–33. [Google Scholar]
- Küper, W.; Kreft, H.; Nieder, J.; Köster, N.; Barthlott, W. Large-scale diversity patterns of vascular epiphytes in neotropical montane rain forests. J. Biogeogr. 2004, 31, 1477–1487. [Google Scholar] [CrossRef]
- Wolf, J.H.D.; Flamenco-S, A. Patterns in species richness and distribution of vascular epiphytes in Chiapas, Mexico. J. Biogeogr. 2003, 30, 1689–1707. [Google Scholar] [CrossRef]
- Zimmerman, J.K.; Olmsted, I.C. Host tree utilization by vascular epiphytes in a seasonally inundated forest (Tintal) in Mexico. Biotropica 1992, 24, 402–407. [Google Scholar] [CrossRef]
- Flores-Palacios, A.; García-Franco, J.G. The relationship between tree size and epiphyte species richness: Testing four different hypotheses. J. Biogeogr. 2006, 33, 323–330. [Google Scholar] [CrossRef]
- Flores-Palacios, A.; García-Franco, J.G. Habitat isolation changes the beta diversity of the vascular epiphyte community in lower montane forest, Veracruz, Mexico. Biodivers. Conserv. 2008, 17, 191–207. [Google Scholar] [CrossRef]
- Nöske, N.M.; Hilt, N.; Werner, F.A.; Brehm, G.; Fiedler, K.; Sipman, H.J.M.; Gradstein, S.R. Disturbance effects on diversity of epiphytes and moths in a montane forest in Ecuador. Basic Appl. Ecol. 2008, 9, 4–12. [Google Scholar] [CrossRef]
- Werner, F.A. Reduced growth and survival of vascular epiphytes on isolated remnant trees in a recent tropical montane forest clear-cut. Basic Appl. Ecol. 2011, 12, 172–181. [Google Scholar] [CrossRef]
- Werner, F.A.; Homeier, J.; Gradstein, S.R. Diversity of vascular epiphytes on isolated remnant trees in the montane forest belt of southern Ecuador. Ecotropica 2005, 11, 21–40. [Google Scholar]
- Gentry, A.H.; Dodson, C.H. Diversity and biogeography of neotropical vascular epiphytes. Ann. Mo. Bot. Gard. 1987, 74, 205–233. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Hietz, P.; Hietz-Seifert, U. Composition and ecology of vascular epiphyte communities along an altitudinal gradient in central Veracruz, Mexico. J. Veg. Sci. 1995, 6, 487–498. [Google Scholar] [CrossRef]
- Tosi, J.A., Jr. Inventariación y Demostraciones Forestales, Panamá. Zonas de vida: Una Base Ecológica Para Investigaciones Silvícolas e Inventariación Forestal en la República de Panamá; FAO: Rome, Italy, 1971. [Google Scholar]
- Andersen, K.M.; Turner, B.L.; Dalling, J.W. Soil-based habitat partitioning in understorey palms in lower montane tropical forests. J. Biogeogr. 2010, 37, 278–292. [Google Scholar] [CrossRef]
- Sanford, W.W. Distribution of epiphytic orchids in semi-deciduous tropical forest in southern Nigeria. J. Ecol. 1968, 56, 697–705. [Google Scholar] [CrossRef]
- Zotz, G. Plants on Plants. The Biology of Vascular Epiphytes; Springer Nature: Cham, Switzerland, 2016; p. 282. [Google Scholar] [CrossRef]
- Perry, D.R. A method of access into the crowns of emergent and canopy trees. Biotropica 1978, 10, 155–157. [Google Scholar] [CrossRef]
- Davidse, G.; Sousa, M.; Knapp, S. (Eds.) Flora Mesoamericana Volumen 1. Psilotaceae a Salvinaceae; Universidad Nacional Autónoma de México: Mexico City, Mexico, 1995. [Google Scholar]
- Hammel, B.E.; Grayum, M.; Herrera, C.; Zamora, N.A. Manual de plantas de Costa Rica, Volumen III: Monocotiledóneas (Orchidaceae-Zingiberaceae). In Monographs in Systematic Botany from the Missouri Botanical Garden; Hollowell, V.C., McPherson, A., Gunter, D., Eds.; Missouri Botanical Garden Press: St. Louis, MO, USA, 2003; Volume 93. [Google Scholar]
- Lellinger, D.B. The ferns and fern-allies of Costa Rica, Panama, and the Chocó (Part I: Psilotaceae through Dicksoniaceae); American Fern Society: Washington, DC, USA, 1989. [Google Scholar]
- Woodson, R.E.; Schery, R.W. Flora of Panama. Annals of the Missouri Botanical Garden; Missouri Botanical Garden: St. Louis, MO, USA, 1943. [Google Scholar]
- The Plant List Version 1. Available online: http://www.theplantlist.org/ (accessed on 23 March 2019).
- Chao, A. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L.; Chiang, S.C.; Jiang, Y.H.; Chazdon, R.L. A two-stage probabilistic approach to multiple-community similarity indices. Biometrics 2008, 64, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Jost, L.; Chao, A.; Chazdon, R.L. Compositional similarity and ß (beta) diversity. In Biological Dversity: Frontiers in Measurement and Assesment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 66–84. [Google Scholar]
- Chao, A.; Chiu, C.-H.; Hsie, T.C. Proposing a resolution to debates on diversity partitioning. Ecology 2012, 93, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Mendieta-Leiva, G.; Zotz, G. A conceptual framework for the analysis of vascular epiphyte assemblages. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 510–521. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 12 January 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, R.; et al. Vegan: Community Ecology Package, Version 2.5-6 from CRAN. R Topics Documented. 2019. Available online: https://rdrr.io/cran/vegan/ (accessed on 7 January 2021).
- Charney, N.; Record, S. Jost diversity measure for community data. Package ‘vegetarian’ R Package 2015, 2, 3. [Google Scholar]
- Colwell, R.K.; Lees, D.C. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol. Evol. 2000, 15, 70–76. [Google Scholar] [CrossRef]
- Flenley, J.R. Tropical montane cloud forests. In Cloud Forest, the Massenerhebung Effect, and Ultraviolet Insolation; Hamilton, L.S., Juvik, J.O., Scatena, N.E., Eds.; Springer: New York, NY, USA, 1994; pp. 150–155. [Google Scholar]
- Sanders, N.J.; Lessard, J.P.; Fitzpatrick, M.C.; Dunn, R.R. Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Glob. Ecol. Biogeogr. 2007, 16, 640–649. [Google Scholar] [CrossRef]
- Laube, S.; Zotz, G. A metapopulation approach to the analysis of long-term changes in the epiphyte vegetation on the host tree Annona glabra. J. Veg. Sci. 2007, 18, 613–624. [Google Scholar] [CrossRef]
- Spruch, L.; Hellwig, J.; Zotz, G.; Blasius, B. Modeling community assembly on growing habitat “islands”: A case study on trees and their vascular epiphyte communities. Theor. Ecol. 2019, 12, 513–529. [Google Scholar] [CrossRef]
- Einzmann, H.J.R.; Zotz, G. “No signs of saturation”: Long-term dynamics of vascular epiphyte communities in a human-modified landscape. Biodivers. Conserv. 2017, 26, 1393–1410. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).