Subterranean Waters of Yucatán Peninsula, Mexico Reveal Epigean Species Dominance and Intraspecific Variability in Freshwater Ostracodes (Crustacea: Ostracoda)
Abstract
1. Introduction
2. Materials and Methods
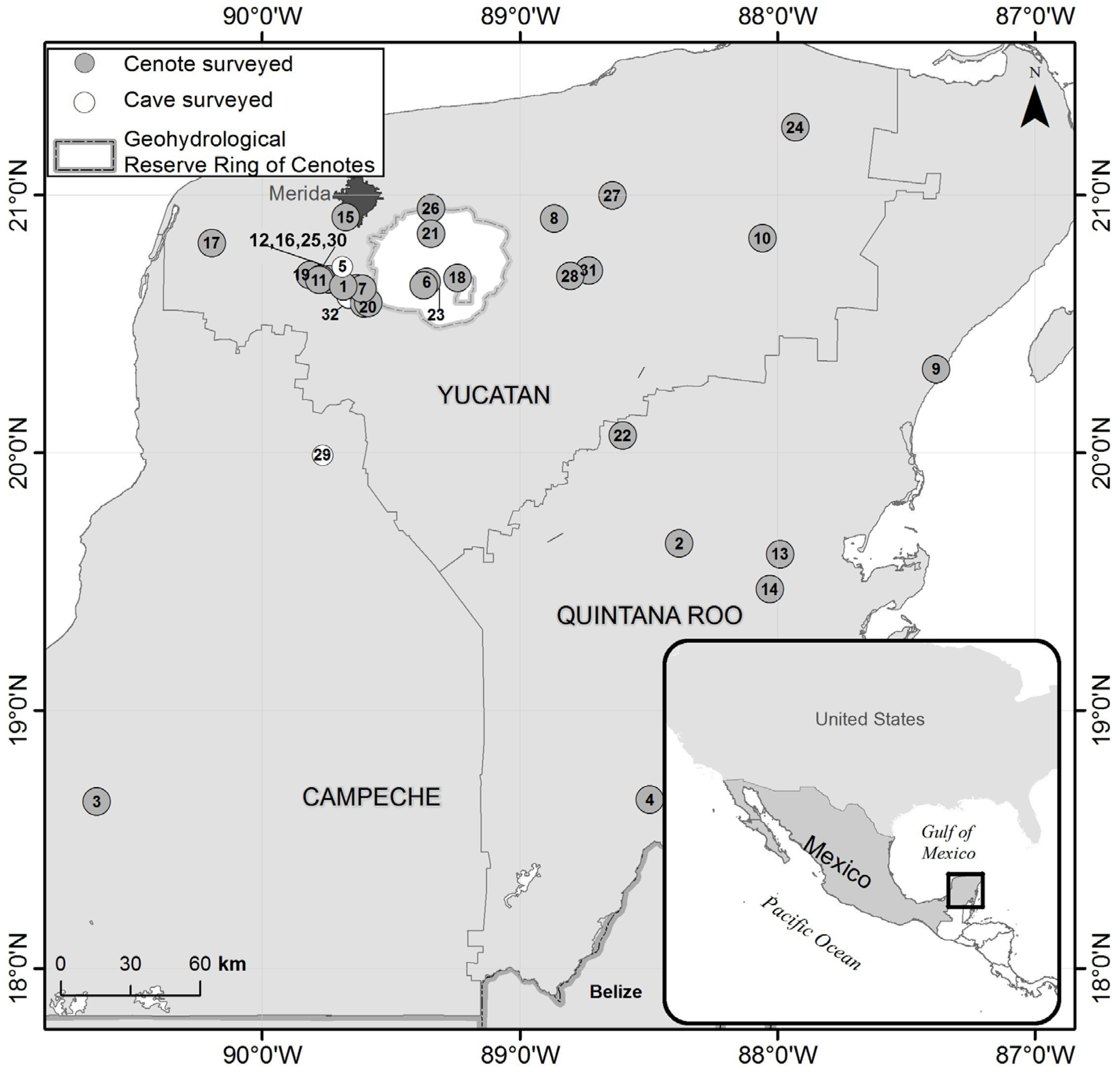
2.1. Sampling, Valve Extraction, Counting and Identification
2.2. Morphometric Analysis of Ostracode Valves
2.3. Molecular Analysis: DNA Extraction, Amplification and Sequencing
2.4. Molecular Analysis: Sequence Alignment and Phylogenetic Analysis
3. Results
3.1. Ostracode Species Composition in Subterranean Waters of the Yucatán Peninsula
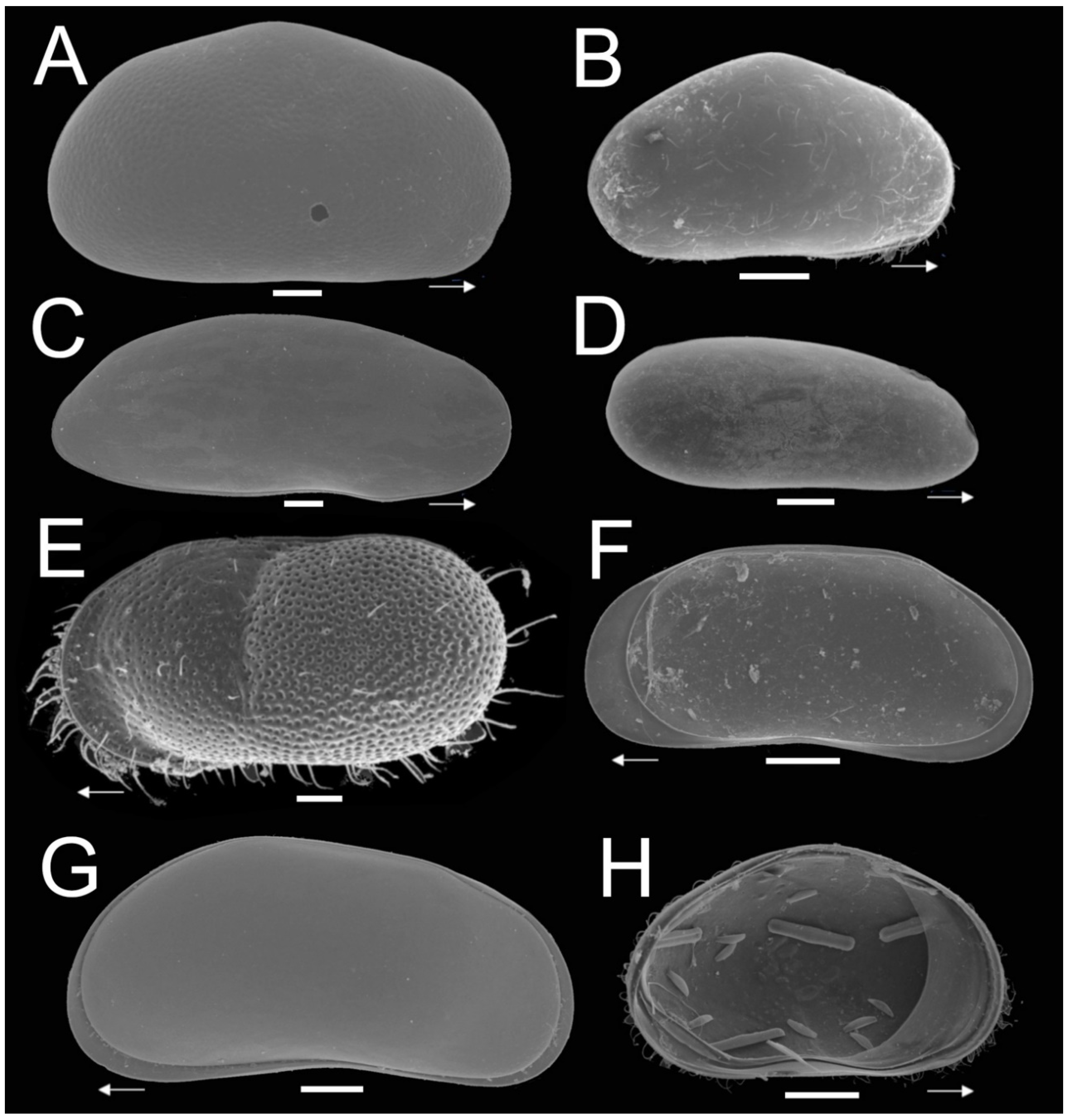
3.2. Shape and Size Morphometric Analysis of Selected Ostracode Species from Caves and Cenotes
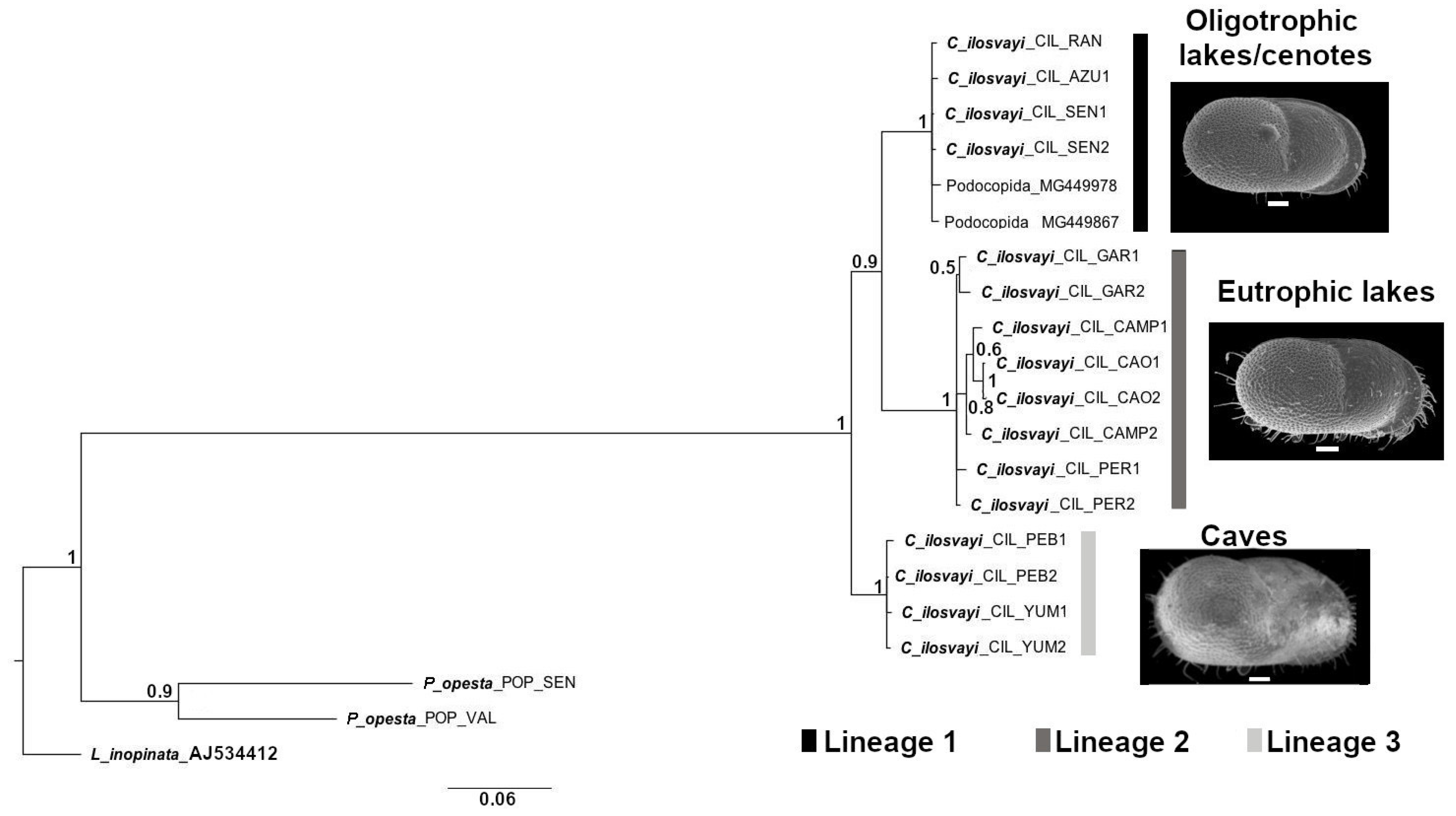
3.3. Molecular Analysis of Cytheridella ilosvayi Epigean and Hypogean Populations
4. Discussion
4.1. Low Diversity and Epigean Species Dominance on Ostracode Assemblages of Subterranean Environments of the Yucatán Peninsula
4.2. Morphological Variability of Freshwater Ostracodes in Subterranean Waters of Yucatán Peninsula
4.3. Genetic Differentiation of Cytheridella ilosvayi in Subterranean Waters of the Yucatán Peninsula
4.4. Late Pleistocene and Early Holocene Climate as the Driver of Subterranean Biodiversity in the Yucatán Peninsula
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, K.S.; Benfield, E.F. Leaf and wood breakdown in cave streams. J. N. Am. Benthol. Soc. 2001, 20, 550–563. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats; Oxford University Press: Oxford, UK, 2009; p. 275. [Google Scholar] [CrossRef]
- Hüppop, K. Adaptation to Low Food. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Academic Press: Washington, DC, USA, 2012; pp. 1–9. [Google Scholar] [CrossRef]
- Liu, W.; Golovatch, S.; Wesener, T.; Tian, M. Convergent Evolution of Unique Morphological Adaptations to a Subterranean Environment in Cave Millipedes (Diplopoda). PLoS ONE 2017, 12, e0170717. [Google Scholar] [CrossRef] [PubMed]
- Pricop, E.; Negrea, B.M. On the adaptations to cave life of some different animal groups (first note). ELBA Bioflux 2009, 1, 41–47. [Google Scholar]
- Soares, D.; Niemiller, M.L. Sensory adaptations of fishes to subterranean environments. Bioscience 2013, 63, 274–283. [Google Scholar] [CrossRef]
- Huntsman, B.M.; Venarsky, M.P.; Benstead, J.P.; Huryn, A.D. Effects of organic matter availability on the life history and production of a top vertebrate predator (Plethodontidae: Gyrinophilus palleucus) in two cave streams. Freshw. Biol. 2011, 56, 1746–1760. [Google Scholar] [CrossRef]
- Pipan, T.; Culver, D.C.; Papi, F.; Kozel, P. Partitioning diversity in subterranean invertebrates: The epikarst fauna of Slovenia. PLoS ONE 2018, 13, e0195991. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Angyal, D.; Balázs, G.; Blick, T.; Brustel, H.; Carter, J.; Ćurčić, S.; Danflous, S.; Dányi, L.; et al. Local- versus broad-scale environmental drivers of continental β-diversity patterns in subterranean spider communities across Europe. Proc. Biol. Sci. 2019, 286, 20191579. [Google Scholar] [CrossRef]
- Christman, M.C.; Culver, D.C.; Madden, M.K.; White, D. Patterns of endemism of the eastern North American cave fauna. J. Biogeogr. 2005, 32, 1441–1452. [Google Scholar] [CrossRef]
- Porter, M.L.; Meland, K.; Price, W. Global diversity of mysids (Crustacea-Mysida) in freshwater. Hydrobiologia 2007, 595, 213–218. [Google Scholar] [CrossRef]
- Juberthie, C. Underground Habitats and Their Protection; Council of Europe: Strasbourg, France, 1995; p. 158. [Google Scholar]
- Sket, B. The nature of biodiversity in hypogean waters and how it is endangered. Biodivers. Conserv. 1999, 8, 1319–1338. [Google Scholar] [CrossRef]
- Culver, D.C.; Deharveng, L.; Bedos, A.; Lewis, J.J.; Madden, M.; Reddell, J.R.; Sket, B.; Trontelj, P.; White, D. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 2006, 29, 120–128. [Google Scholar] [CrossRef]
- Stoch, F.; Galassi, D.M.P. Stygobiotic crustacean species richness: A question of numbers, a matter of scale. Hydrobiologia 2010, 653, 217–234. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. Diversity patterns in the tropics. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Amsterdam, The Netherlands, 2012; pp. 38–50. [Google Scholar] [CrossRef]
- Dumnicka, E.; Pipan, T.; Culver, D.C. Habitats and diversity of subterranean macroscopic freshwater invertebrates: Main gaps and future trends. Water 2020, 12, 2170. [Google Scholar] [CrossRef]
- QRSS. Quintana Roo Speleological Survey. Available online: http://caves.org/project/qrss/qrss.htm (accessed on 25 October 2020).
- Vera, I.; Mariño-Tapia, I.; Enriquez, C. Effects of drought and subtidal sea-level variability on salt intrusion in a coastal karst aquifer. Mar. Freshw. Res. 2012, 63, 485–493. [Google Scholar] [CrossRef]
- Parra, S.M.; Valle-Levinson, A.; Marino-Tapia, I.; Enriquez, C. Salt intrusion at a submarine spring in a fringing reef lagoon. J. Geophys. Res. Ocean. 2015, 120, 2736–2750. [Google Scholar] [CrossRef]
- Benítez, S.; Iliffe, T.M.; Quiroz-Martínez, B.; Alvarez, F. How is the anchialine fauna distributed within a cave? A study of the Ox Bel Ha System, Yucatan Peninsula, Mexico. Subterr. Biol. 2019, 31, 15–28. [Google Scholar] [CrossRef]
- Schmitter-Soto, J.; Comín, F.; Escobar-Briones, E.; Herrera, J.; Alcocer, J.; Suarez-Morales, E.; Elías-Gutiérrez, M.; Díaz, V.; Marin, L.; Steinich, B. Hydrogeochemical and biological characteristics of cenotes in the Yucatan Peninsula (SE Mexico). Hydrobiologia 2002, 467, 215–228. [Google Scholar] [CrossRef]
- Cervantes-Martínez, A.M.; Mezeta-Barrera, M.; Gutiérrez-Aguirre, M.A. Limnología básica del lago cárstico turístico Cenote Azul en Quintana Roo, México. Hidrobiológica 2009, 19, 177–180. [Google Scholar]
- Camargo-Guerra, T.; Escalera-Vázquez, L.H.; Zambrano, L. Fish community structure dynamics in cenotes of the Biosphere Reserve of Sian Ka’an, Yucatán Peninsula, Mexico. Rev. Mex. Biodivers. 2013, 84, 901–911. [Google Scholar] [CrossRef]
- Cervantes-Martínez, A.M.; Elías-Gutiérrez, M.; Arce-Ibarra, A.M.; Gutiérrez-Aguirre, M.A. Aquatic biodiversity in cenotes from the Yucatan Peninsula (Quintana Roo, Mexico). Teor. Prax. 2018, 25, 49–68. [Google Scholar]
- Angyal, D.; Chávez-Solís, E.M.; Liévano-Beltrán, L.A.; Magaña, B.; Simões, N.; Mascaró, M. New distribution records of subterranean crustaceans from cenotes in Yucatan (Mexico). Zookeys 2020, 911, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Salas, N.F.; Morales-Vela, B.; Suárez-Morales, E.; Iliffe, T. Conservation status of the inland aquatic crustaceans in the Yucatan Peninsula, Mexico: Shortcomings of a protection strategy. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 939–951. [Google Scholar] [CrossRef]
- Bauer-Gottwein, P.; Gondwe, B.R.N.; Charvet, G.; Marín, L.E.; Rebolledo-Vieyra, M.; Merediz-Alonso, G. Review: The Yucatán Peninsula karst aquifer, Mexico. Hydrogeol. J. 2011, 19, 507–524. [Google Scholar] [CrossRef]
- Sars, G.O. On Megalocypris princeps, a gigantic fresh-water ostracod from South Africa. Arch. Math. Naturv. 1898, 20, 1–18. [Google Scholar]
- Meisch, C. Freshwater Ostracoda of Western and Central Europe; Süsswasserfauna von Mitteleuropa 8/3; Spektrum Akademischer Verlag: Heidelberg/Berlin, Germany, 2000; pp. 1–522. [Google Scholar]
- Karanovic, I. Recent Freshwater Ostracods of the World. Crustacea, Ostracoda, Podocopida; Springer: Berlin/Heidelberg, Germany, 2012; 610p. [Google Scholar]
- Danielopol, D.L.; Marmonier, P.; Boulton, A.J.; Bonaduce, G. World subterranean ostracod biogeography: Dispersal or vicariance. In Culver DC, Holsinger JR Biogeography of Subterranean Crustaceans: The effects of different scales. Hydrobiologia 1994, 287, 119–129. [Google Scholar] [CrossRef]
- Karanovic, I.; Perina, G.; Eberhard, S. Austromesocypris bluffensis sp. n. (Crustacea, Ostracoda, Cypridoidea, Scottiinae) from subterranean aquatic habitats in Tasmania, with a key to world species of the subfamily. Zookeys 2012, 215, 1–31. [Google Scholar] [CrossRef][Green Version]
- Simões, L.; Ferreira, T.; Bichuette, M. Aquatic biota of different karst habitats in epigean and subterranean systems of Central Brazil—visibility versus relevance of taxa. Subterr. Biol. 2013, 11, 55–74. [Google Scholar] [CrossRef][Green Version]
- Karanovic, I.; Sidorov, D.; Marmonier, P. Zoogeography of the ostracod genus Nannocandona (Podocopa) with description of two new species from Europe and East Asia. Ann. Limnol. Int. J. Lim. 2015, 51, 297–313. [Google Scholar] [CrossRef]
- Artheau, M. Geographical review of the ostracod genus Vestalenula (Darwinulidae) and a new subterranean species from southern France. Invertebr. Syst. 2007, 21, 471–486. [Google Scholar] [CrossRef]
- Mazzini, I.; Marrone, F.; Arculeo, M.; Rossetti, G. Revision of Recent and fossil Mixtacandona Klie 1938 (Ostracoda, Candonidae) from Italy, with description of a new species. Zootaxa 2017, 4221, 323–340. [Google Scholar] [CrossRef]
- Danielopol, D.L. An essay to assess the age of the freshwater interstitial ostracods of Europe. Bijdr. Dierkd. 1980, 50, 243–291. [Google Scholar] [CrossRef]
- Karanovic, I. Candoninae ostracodes from the Pilbara region in Western Australia. Crustac. Monogr. 2007, 7. [Google Scholar] [CrossRef]
- De Deckker, P. Terrestrial ostracods in Australia. In Papers from the Conference on the Biology and Evolution of Crustacea; Australian Museum Memoir; Lowry, J.K., Ed.; The Australian Museum: Sydney, Australia, 1983; Volume 18, pp. 87–100. [Google Scholar]
- Karanovic, I. Towards a revision of Candoninae (Crustacea, Ostracoda): Description of two new genera from Australian groundwater. Species Divers. 2003, 8, 353–383. [Google Scholar] [CrossRef][Green Version]
- Iliffe, T.M. Fauna troglobia acuatica de la Peninsula de Yucatan. In Biodiversidad Marina y Costera de México; Salazar-Vallejo, S.I., Gonzalez, N.E., Eds.; Com. Nal. Biodiversidad y CIQRO: México, 1993; p. 865. [Google Scholar]
- Fiers, F.; Reid, J.W.; Iliffe, T.M.; Suárez-Morales, E. New hypogean cyclopoid copepods (Crustacea) from the Yucatan Peninsula, Mexico. Contrib. Zool. 1996, 66, 65–102. [Google Scholar] [CrossRef]
- Angyal, D.; Chávez-Solís, E.; Magana, B.; Balázs, G.; Simoes, N. Mayaweckelia troglomorpha (Amphipoda, Hadziidae), a new subterranean amphipod species from Yucatán state (Yucatán Peninsula, Mexico). Zookeys 2018, 735, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Furtos, N.C. On the Ostracoda from the Cenotes of Yucatan and vicinity. Carnegie Inst. Wash. Publ. 1936, 457, 89–115. [Google Scholar]
- Kornicker, L.S.; Iliffe, T.M. New Ostracoda (Halocyprida: Thaumatocyprididae and Halocyprididae) from Anchialine Caves in the Bahamas, Palau, and Mexico. Smithson. Contrib. Zool. 1989, 470, 1–47. [Google Scholar] [CrossRef]
- Kornicker, L.S.; Iliffe, T.M. Myodocopid Ostracoda (Halocypridina, Cladocopina) from Anchialine Caves in the Bahamas, Canary Islands, and Mexico. Smithson. Contrib. Zool. 1998, 599, 1–93. [Google Scholar] [CrossRef]
- Cohuo, S.; Macario-González, L.; Pérez, L. Schwalb, A. Overview of Neotropical-Caribbean freshwater ostracode fauna (Crustacea, Ostracoda): Identifying areas of endemism and assessing biogeographical affinities. Hydrobiologia 2017, 786, 5–21. [Google Scholar] [CrossRef]
- Wrozyna, C.; Neubauer, T.A.; Meyer, J.; Piller, W.E. Shape variation in Neotropical Cytheridella (Ostracoda) using semiland marks based geometric morphometrics: A methodological approach and possible biogeographical implications. PLoS ONE 2016, 11, e0168438. [Google Scholar] [CrossRef]
- Wrozyna, C.; Meyer, J.; Gross, M.; Ramos, M.I.; Piller, W. Definition of regional ostracod (Cytheridella) morphotypes by use of landmark-based morphometrics. Freshw. Sci. 2018, 37, 573–592. [Google Scholar] [CrossRef]
- Rohlf, F.J. The tps series of software. Hystrix. It. J. Mamm. 2015, 26, 1–4. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Bookstein, F. Linear discrimination, ordination, and the visualization of selection gradients in modern morphometrics. Evol. Biol. 2011, 38, 100–114. [Google Scholar] [CrossRef]
- Hammer, Q.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Macario-González, L.; Cohuo, S.; Elías-Gutiérez, M.; Vences, M.; Pérez, L.; Schwalb, A. Integrative taxonomy of freshwater ostracodes (Crustacea: Ostracoda) of the Yucatán Peninsula, implications for paleoenvironmental reconstructions in the northern Neotropical region. Zool. Anz. 2018, 275, 20–36. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Prosser, S.; Martínez-Arce, A.; Elías-Gutiérrez, M. A new set of primers for COI amplification from freshwater microcrustaceans. Mol. Ecol. Resour. 2013, 13, 1151–1155. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Endo, K. Molecular phylogeny of Ostracoda (Crustacea) inferred from 18S ribosomal DNA sequences: Implication for its origin and diversification. Mar. Biol. 2003, 143, 23–38. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gate- way for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop, GCE 2010, New Orleans, LA, USA, 14 November 2010; pp. 1–8. Available online: http://ieeexplore.ieee.org/document/5676129/ (accessed on 10 October 2020).
- Broodbakker, N.W. The distribution and zoogeography of freshwater Ostracoda (Crustacea, Ostracoda) in the West Indies, with emphasis on species inhabiting wells. Bijdr. Dierkd. 1984, 54, 25–50. [Google Scholar] [CrossRef]
- Pérez, L.; Lorenschat, J.; Brenner, M.; Scharf, B.; Schwalb, A. Extant freshwater ostracodes (Crustacea: Ostracoda) from Lago Petén Itzá, Guatemala. Rev. Biol. Trop. 2010, 58, 871–895. [Google Scholar] [CrossRef]
- Cohuo-Durán, S. M; Elías-Gutiérrez M; Karanovic, I. On three new species of Cypretta Vivra, 1895 (Crustacea: Ostracoda) from the Yucatan Peninsula, Mexico. Zootaxa 2013, 3636, 501–524. [Google Scholar] [CrossRef]
- Culver, D.; Trontelj, P.; Zagmajster, M.; Pipan, T. Paving the Way for Standardized and Comparable Subterranean Biodiversity Studies. Subterr. Biol. 2013, 10, 43–50. [Google Scholar] [CrossRef]
- Iepure, S.; Feurdean, A.; Bădăluţă, C.; Nagavciuc, V.; Perşoiu, A. Pattern of richness and distribution of groundwater Copepoda (Cyclopoida: Harpacticoida) and Ostracoda in Romania: An evolutionary perspective. Biol. J. Linn. Soc. 2016, 119, 593–608. [Google Scholar] [CrossRef]
- Meisch, C.; Smith, R.J.; Martens, K. A subjective global checklist of the extant non-marine Ostracoda (Crustacea). Eur. J. Taxon. 2019, 492, 1–135. [Google Scholar] [CrossRef]
- Broodbakker, N.W. The genus Heterocypris (Crustacea, Ostracoda) in the West Indies, Part 1: Taxonomic characters. Bijdr. Dierkd. 1982, 52, 207–227. [Google Scholar] [CrossRef]
- Broodbakker, N.W. The genus Strandesia and other Cypricercini (Crustacea, Ostracoda) in the West Indies, Part 1: Taxonomy. Bijdr. Dierkd. 1983, 53, 327–368. [Google Scholar] [CrossRef]
- Broodbakker, N.W. The genus Hemicypris (Crustacea, Ostracoda) in the West Indies. Bijdr. Dierkd. 1983, 53, 135–157. [Google Scholar] [CrossRef]
- Broodbakker, N.W. The subfamily Candoninae (Crustacea, Ostracoda) in the West Indies. Bijdr. Dierkd. 1983, 53, 287–326. [Google Scholar] [CrossRef]
- Alvarez, F.; Iliffe, T.M.; Benitez, S.; Brankovits, D.; Villalobos, J.L. New records of anchialine fauna from the Yucatan Peninsula, Mexico. Check List 2015, 11, 1505. [Google Scholar] [CrossRef]
- Elewa, A.M.T. Application of geometric morphometrics to the study of shape polymorphism in Eocene ostracodes from Egypt and Spain. In Morphometrics; Elewa, A.M.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 7–28. [Google Scholar]
- Karanovic, I.; Lavtižar, V.; Djurakic, M. A complete survey of normal pores on a smooth shell ostracod (Crustacea): Landmark-based versus outline geometric morphometrics. J. Morphol. 2017, 278, 1091–1104. [Google Scholar] [CrossRef]
- Aiello, G.; Barattolo, F.; Barra, D.; Fiorito, G.; Mazzarella, A.; Raia, P.; Viola, R. Fractal analysis of ostracod shell variability: A comparison with geometric and classic morphometrics. Acta Palaeontol. Pol. 2007, 52, 563–573. [Google Scholar] [CrossRef]
- Karanovic, I.; Huyen, P.T.M.; Yoo, H.; Nakao, Y.; Tsukagoshi, A. Shell and appendages variability in two allopatric ostracod species seen through the light of molecular data. Contrib. Zool. 2020, 89, 247–269. [Google Scholar] [CrossRef]
- Pérez, A.P.; Coviaga, C.A.; Ramos, L.Y.; Lancelotti, J.; Alperin, M.S.; Cusminsky, G.C. Taxonomic revision of Cypridopsis silvestrii comb. nov. (Ostracoda, Crustacea) from Patagonia, Argentina with morphometric analysis of their intraspecific shape variability and sexual dimorphism. Zootaxa 2019, 4563. [Google Scholar] [CrossRef]
- Ramos, L.; Cusminsky, G.; Schwalb, A.; Alperin, M. Morphotypes of the lacustrine ostracod Limnocythere rionegroensis Cusminsky & Whatley from Patagonia, Argentina, shaped by aquatic environments. Hydrobiologia 2017, 786, 137–148. [Google Scholar] [CrossRef]
- Ramos, L.Y.; Alperin, M.; Pérez, A.P.; Coviaga, C.A.; Schwalb, A.; Cusminsky, G.C. Eucypris fontana (Graf, 1931) (Crustacea, Ostracoda) in permanent environments of Patagonia Argentina: A geometric morphometric approach. Ann. Limnol. 2015, 51, 125–138. [Google Scholar] [CrossRef]
- Wrozyna, C.; Neubauer, T.A.; Meyer, J.; Ramos, M.I.F.; Piller, W.E. Significance of climate and hydrochemistry on shape variation—A case study on Neotropical cytheroidean Ostracoda. Biogeosciences 2018, 15, 5489–5502. [Google Scholar] [CrossRef]
- Ruiz-Jiménez, J.A.; Beltrán-Gutiérrez, M.; Criales-Hernández, M.I. Primer registro de Cytheridella ilosvayi Daday, 1905 (Ostracoda: Limnocytheridae) en la ciénaga de San Silvestre, Colombia. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2020, 44, 554–559. [Google Scholar] [CrossRef]
- Elewa, A.M.T. Morphometric studies on three ostracod species of the genus Digmocythere Mandelstam from the middle Eocene of Egypt. Palaeontol. Electron. 2003, 6, 1–11. [Google Scholar]
- Rossetti, G.; Martens, K. Redescription and morphological variability of Darwinula stevensoni (Brady & Robertson, 1870) (Crustacea, Ostracoda). Bull. Inst. R. Sci. Nat. Belg. 1996, 66, 73–92. [Google Scholar]
- Meyer, J.; Wrozyna, C.; Gross, M.; Leis, A.; Piller, W.E. Morphological and geochemical variations of Cyprideis (Ostracoda) from modern waters of the northern Neotropics. Limnology 2017, 18, 251–273. [Google Scholar] [CrossRef] [PubMed]
- Keyser, D.; Aladin, N. Noding in Cyprideis torosa and its causes. Stud. Quat. 2004, 21, 19–24. [Google Scholar]
- Minati, K.; Cabral, M.C.; Pipík, R.; Danielopol, D.L.; Linhart, J.; Neubauer, W. Morphological variability among European populations ofVestalenula cylindrica (STRAUB) (Crustacea, Ostracoda). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 264, 296–305. [Google Scholar] [CrossRef]
- Karan-Žnidaršič, T.; Vujić, V.; Baltanás, Á. Analysing morphological variation of appendages and labrum in 10 species of Heterocypris Claus, 1893 (Podocopida : Cyprididae) with additional description of Heterocypris exigua. Invertebr. Syst. 2018, 32, 1448–1464. [Google Scholar] [CrossRef]
- Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 2015, 85, 407–415. [Google Scholar] [CrossRef]
- Costa, F.; deWaard, J.R.; Boutillier, J.; Ratnasingham, S.; Dooh, R.T.; Hajibabaei, M.; Hebert, P.D.N. Biological identifications through DNA barcodes: The case of the Crustacea. Can. J. Fish. Aquat. Sci. 2007, 64, 272–295. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, P.A.; Carter, L.; Brinker, A.M.; da Silva, A.J.; Chu, D.M.; Lampel, K.A.; Monday, S.R. Targeting single-nucleotide polymorphisms in the 18S rRNA gene to differentiate Cyclospora species from Eimeria species by multiplex PCR. Appl. Environ Microbiol. 2003, 69, 4806–4813. [Google Scholar] [CrossRef] [PubMed]
- Salim, B.; Bakheit, M.A.; Kamau, J.; Nakamura, I.; Sugimoto, C. Nucleotide sequence heterogeneity in the small subunit ribosomal RNA gene within Theileria equi from horses in Sudan. Parasitol. Res. 2010, 106, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xiong, J.; Yu, Y. Taxonomic resolutions based on 18S rRNA genes: A case study of subclass Copepoda. PLoS ONE 2015, 10, e0131498. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Lambeck, K.; Johnston, P.; De Deckker, P.; Fifield, L.K. Timing of the last glacial maximum from observed sea-level minima. Nature 2000, 406, 713–716. [Google Scholar] [CrossRef]
- Grant, K.M.; Rohling, E.J.; Bar-Matthews, M.; Ayalon, A.; Medina-Elizalde, M.; Ramsey, C.B.; Satow, C.; Roberts, A.P. Rapid coupling between ice volume and polar temperature over the past 150,000 years. Nature 2012, 491, 744–747. [Google Scholar] [CrossRef]
- Mueller, A.D.; Anselmetti, F.S.; Ariztegui, D.; Brenner, M.; Hodell, D.A.; Curtis, J.H.; Escobar, J.; Gilli, A.; Grzesik, D.A.; Guilderson, T.P.; et al. Late Quaternary palaeoenvironment of northern Guatemala: Evidence from deep drill cores and seismic stratigraphy of Lake Petén Itzá. Sedimentology 2010, 57, 1220–1245. [Google Scholar] [CrossRef]
- Cohuo, S.; Macario-González, L.; Pérez, L.; Sylvestre, F.; Paillès, C.; Curtis, J.; Kutterolf, S.; Wojewódka, M.; Zawisza, E.; Szeroczynska, K.; et al. Ultrastructure and aquatic community response to Heinrich Stadial (HS5a-HS1) in the continental northern Neotropics. Quat. Sci. Rev. 2018, 19, 75–91. [Google Scholar] [CrossRef]
- Hillesheim, M.B.; Hodell, D.A.; Leyden, B.W.; Brenner, M.; Curtis, J.H.; Anselmetti, F.S.; Ariztegui, D.; Buck, D.G.; Guilderson, T.P.; Rosenmeier, M.F.; et al. Lowland neotropical climate change during the late deglacial and early Holocene. J. Quat. Sci. 2005, 20, 363–376. [Google Scholar] [CrossRef]
- Anselmetti, F.S.; Ariztegui, D.; Hodell, D.A.; Hillesheim, M.B.; Brenner, M.; McKenzie, J.A.; Mueller, A.D. Late Quaternary climate-induced variations in Lake Petén Itzá, Guatemala, inferred from seismic stratigraphic analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 230, 52–69. [Google Scholar] [CrossRef]
- Cohuo, S.; Macario-González, L.; Wagner, S.; Naumann, K.; Echeverría-Galindo, P.; Pérez, L.; Curtis, J.; Brenner, M.; Schwalb, A. Influence of late Quaternary climate on the biogeography of Neotropical aquatic species as reflected by non-marine ostracodes. Biogeosciences 2020, 17, 145–161. [Google Scholar] [CrossRef]
- Pérez, L.; Frenzel, P.; Brenner, M.; Escobar, J.; Hoelzmann, P.; Scharf, B.; Schwalb, A. Late Quaternary (24~10 ka BP) environmental history of the Neotropical lowlands inferred from ostracodes in sediments of Lago Petén Itzá, Guatemala. J. Paleolimnol. 2011, 46, 59–74. [Google Scholar] [CrossRef]
- Sugden, A.M. Drivers of Diversity. Science 2012, 337, 15. [Google Scholar] [CrossRef]
- Fine, P.V.A. Ecological and Evolutionary Drivers of Geographic Variation in Species Diversity. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 369–392. [Google Scholar] [CrossRef]
- Álvarez, F.; Iliffe, T.M.; Villalobos, J.L. New species of the genus Typhlatya (Decapoda: Atyidae) from anchialine caves in Mexico, the Bahamas and Honduras. J. Crust. Biol. 2005, 25, 81–94. [Google Scholar] [CrossRef]
- Suárez-Morales, E.; Ferrari, F.D.; Iliffe, T.M. A new epacteriscid copepod (Calanoida: Epacteriscidae) from the Yucatan Peninsula, Mexico, with comments on the biogeography of the family. Proc. Biol. Soc. Wash. 2006, 119, 222–238. [Google Scholar] [CrossRef]
- Kahn, N.S.; Ashe, E.; Horton, B.P.; Dutton, A.; Kopp, R.E.; Brocard, G.; Engelhart, S.E.; Hill, D.F.; Peltier, W.R.; Vane, C.H.; et al. Drivers of Holocene sea-level change in the Caribbean. Quat. Sci. Rev. 2017, 155, 13–36. [Google Scholar] [CrossRef]
- Moseley, G.E.; Richards, D.A.; Smart, P.L.; Standish, C.D.; Hoffmann, D.L.; ten Hove, H.; Vinn, O. Early–middle Holocene relative sea-level oscillation events recorded in a submerged speleothem from the Yucatán Peninsula, Mexico. Holocene 2015, 25, 1511–1521. [Google Scholar] [CrossRef]
- Gabriel, J.J.; Reinhardt, E.G.; Peros, M.C.; Davidson, D.E.; van Hengstum, P.J.; Beddows, P.A. Palaeoenvironmental evolution of Cenote Aktun Ha (Carwash) on the Yucatan Peninsula, Mexico and its response to Holocene sea-level rise. J. Paleolimnol. 2009, 42, 199–213. [Google Scholar] [CrossRef]
- Horne, D.J.; Martens, K. Geographical parthenogenesis in European non-marine ostracods: Post-glacial invasion or Holocene stability? Hydrobiologia 1998, 391, 1–7. [Google Scholar] [CrossRef]
- Rouch, R.; Danielopol, D.L. Species richness of microcrustacea in subterranean freshwater habitats. Comparative analysis and approximate evaluation. Int. Rev. Ges. Hydrobiol. Hydrogr. 1997, 82, 121–145. [Google Scholar] [CrossRef]
- Danielopol, D.L.; Rouch, R. Invasion, active versus passive. In Encyclopedia of Caves; Culver, D., White, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 305–310. [Google Scholar] [CrossRef]



| Taxon | Family | Cenote/Cave |
|---|---|---|
| Alicenula sp. | Darwinulidae Brady and Robertson, 1885 | Calcuch, Dos Palmas, Dzonotila, Kankirixché, X’Batún |
| Alicenula yucatanensis Macario-González et al., 2018 | Chihuo-Hol, Dzonbacal, Galeana, Kankirixché, Tanimax, Xtojil, Huul K’in, Oxolá. | |
| Darwinula stevensoni (Brady and Robertson, 1870) | Abala, Dzonotila, Huul K’in, Oxolá, Pol Box, Sabtún-1, X’kokob, Xoch, Yumku | |
| Vestalenula pagliolii (Pinto and Kotzian, 1961) | Azul (in Campeche), Azul (in Quintana Roo), X’Batún | |
| Chlamydotheca unispinosa (Baird, 1862) | Cyprididae Baird, 1845 | Antum |
| Cypridopsis sp. | Cave X’tacumbilxuna’an, Tanimax | |
| Cypridopsis niagranensis Furtos, 1936 | Abala | |
| Cypridopsis vidua (O. F. Müller, 1776) | Abala, Antum, Azul (in Quintana Roo), Colac, Dzonotila, Galeana, Huul K’in, Noh’Chunck, Sabakha, Sabtún-1, Tzitzila, X’Batún, Xoch, Yokdzonot, Yumku | |
| Diaphanocypris meridana (Furtos, 1936) | Colac, Oxolá | |
| Heterocypris sp. | Dzalbay | |
| Strandesia intrepida Furtos, 1936 | Azul (in Campeche), Azul (in Quintana Roo) | |
| Cypria sp. | Candonidae Kaufmann, 1900 | Cave X’tacumbilxuna’an |
| Cypria petenensis Ferguson et al., 1964 | Azul (in Campeche) | |
| Cypria cf. pseudocrenulata Furtos, 1936 | El Padre | |
| Keysercypria sp. | Dzonotila | |
| Keysercypria xanabanica (Furtos, 1936) | Xtojil | |
| Pseudocandona antilliana Broodbakker, 1983 | Xoch | |
| Pseudocandona sp. | Dzonotila, Sacalaca | |
| Cytheridella ilosvayi Daday, 1905 | Limnocytheridae Sars, 1925 | Antum, Azul (in Campeche), Azul (in Quintana Roo), Cave Peba, El Padre, Galeana, Kankirixché, Sabtún-1, Xtojil, Xoch, Yokdzonot, Yax Há, Yumku |
| Cyprideis sp. | Cytherideidae Sars, 1925 | Xoch |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macario-González, L.; Cohuo, S.; Angyal, D.; Pérez, L.; Mascaró, M. Subterranean Waters of Yucatán Peninsula, Mexico Reveal Epigean Species Dominance and Intraspecific Variability in Freshwater Ostracodes (Crustacea: Ostracoda). Diversity 2021, 13, 44. https://doi.org/10.3390/d13020044
Macario-González L, Cohuo S, Angyal D, Pérez L, Mascaró M. Subterranean Waters of Yucatán Peninsula, Mexico Reveal Epigean Species Dominance and Intraspecific Variability in Freshwater Ostracodes (Crustacea: Ostracoda). Diversity. 2021; 13(2):44. https://doi.org/10.3390/d13020044
Chicago/Turabian StyleMacario-González, Laura, Sergio Cohuo, Dorottya Angyal, Liseth Pérez, and Maite Mascaró. 2021. "Subterranean Waters of Yucatán Peninsula, Mexico Reveal Epigean Species Dominance and Intraspecific Variability in Freshwater Ostracodes (Crustacea: Ostracoda)" Diversity 13, no. 2: 44. https://doi.org/10.3390/d13020044
APA StyleMacario-González, L., Cohuo, S., Angyal, D., Pérez, L., & Mascaró, M. (2021). Subterranean Waters of Yucatán Peninsula, Mexico Reveal Epigean Species Dominance and Intraspecific Variability in Freshwater Ostracodes (Crustacea: Ostracoda). Diversity, 13(2), 44. https://doi.org/10.3390/d13020044






