Abstract
Palaeoannelida Weigert and Bleidorn, 2016 is an old clade branching off at the base of the Annelida radiation. It includes two morphologically and ecological divergent groups of sedentary burrowers and tube-dwellers: Magelonidae Cunningham and Ramage, 1888, and Oweniidae Rioja, 1917. Magelonids are characterised by a flattened, shovel-shaped prostomium and a pair of ventral papillated palps. Oweniids have simplified bodies lacking parapodia or appendages and are easily distinguished by the presence of oval patches of packed uncini, each with two distal curved teeth. The present review aims to summarise available information about the diversity of forms and life strategies displayed in the group, providing some guidelines for species identification and the techniques commonly used for their study. In addition, the assumed geographic distributions of some taxa are critically discussed. A brief introduction about the evolutionary relationships, systematics, and taxonomic history is given for both Magelonidae and Oweniidae. The motivation of this review is to highlight the main knowledge gaps from a taxonomic, methodological, and geographic perspective, aiming at stimulating further research into members of this clade.
1. Introduction
The term Palaeoannelida was proposed by Weigert and Bleidorn [1] for the clade formed by Oweniidae Rioja, 1917 and Magelonidae Cunningham and Ramage, 1888, branching of at the base of the annelid tree and sister to all the rest of annelids (see also [2,3]). The basal position of these two groups was assessed after analyses of phylogenomic data [4,5,6]. Previous analyses of just a few molecular markers had unveiled Oweniidae diverging early in the annelid radiation but only weakly supported (e.g., [7,8,9,10,11,12,13,14,15]). The position of these sedentary organisms at the base of the annelid tree generated some controversy with the fossil record, as most Cambrian annelid fossils showed morphologies corresponding to an epibenthic lifestyle. However, a recent finding of a tubicolous annelid dated in the early Cambrian [16], which was proposed within the Magelonidae, has suggested that a diversity of life modes, including sedentary and errant forms, may have inhabited the oceans at that time. The relationship between Magelonidae and Oweniidae, whilst supported by some morphological features such as the presence of a monociliated epidermis and lack of nuchal organs [17], still needs further work.
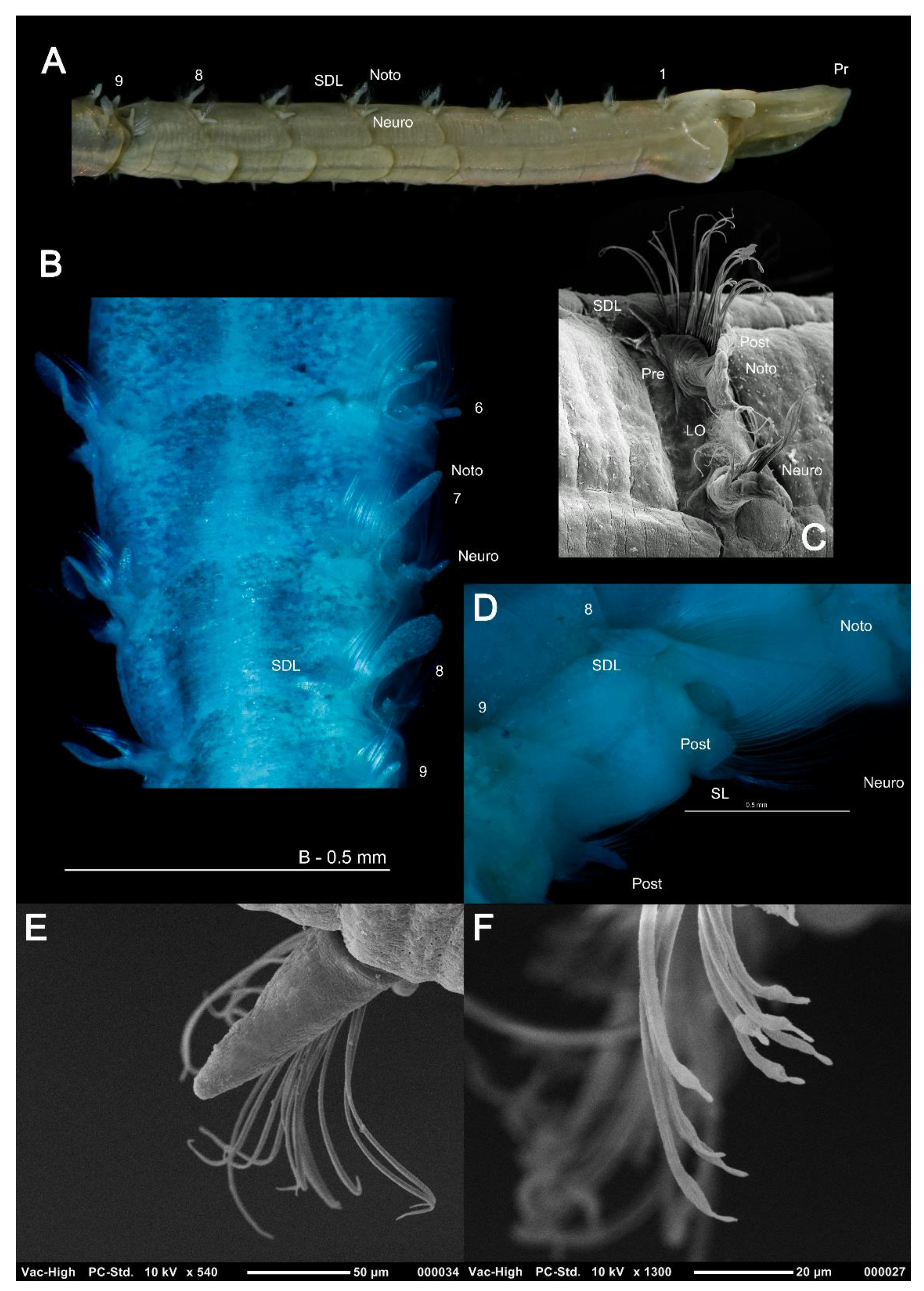
Magelonidae are known colloquially as the ‘shovel head worms’ due to their uniquely flattened and spade shaped prostomia (Figure 1) utilised in burrowing. The family is relatively small, containing 72 species worldwide [18]. Based on the diversity of species reported from relatively small geographic areas, the number of species is likely to be drastically underestimated [19]. The first species to be described was Magelona papillicornis F. Müller, 1858 from Brazil, with the group being raised to the rank of family by Cunningham and Ramage [20]. Their unusual morphology has often led to difficulties in relating them to other annelid groups. However, in spite of this they are easily recognised by their characteristic prostomia, and two uniquely papillated and ventrally inserted palps (Figure 1A and Figure 2A,B), characters which support the monophyly of the group [21] (and Mortimer et al., in preparation A). Magelonid species are relatively uniform in appearance and this has posed issues with species identification and the understanding of generic delineations within the family.
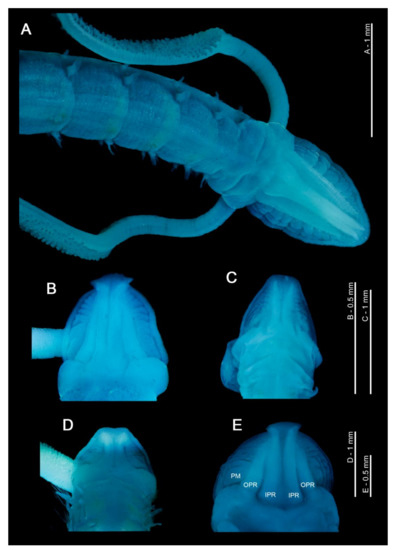
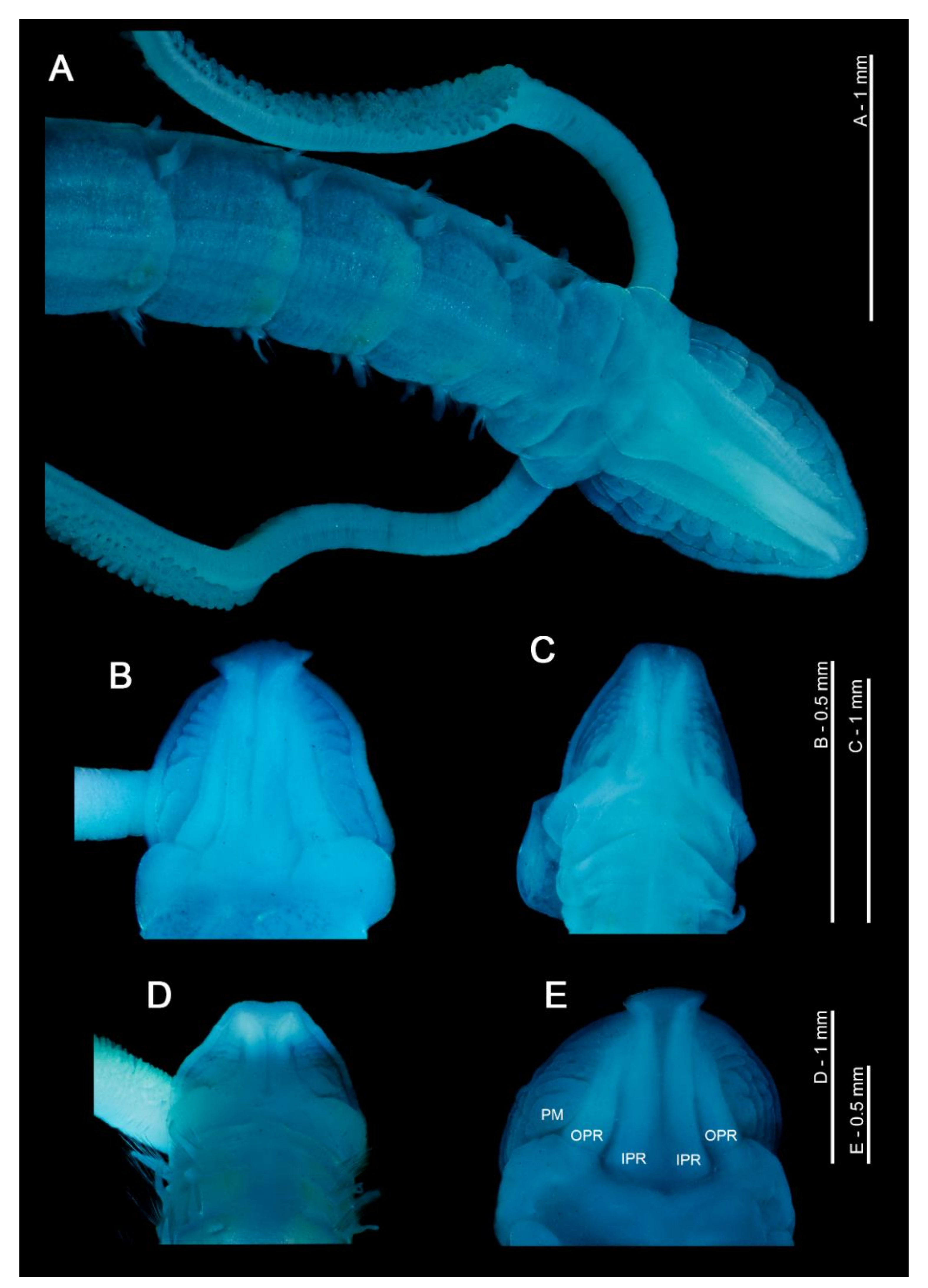
Figure 1.
(A) Anterior end of Magelona mirabilis, showing prostomium, palps, achaetous first segment and first five chaetigers (dorsal view); (B–E) magelonid prostomia (dorsal views) of M. crenulifrons, M. mahensis, M. symmetrica and M. wilsoni respectively. All stained with methyl green. Abbreviations: IPR—inner prostomial ridge, OPR—outer prostomial ridge, PM—prostomial markings.
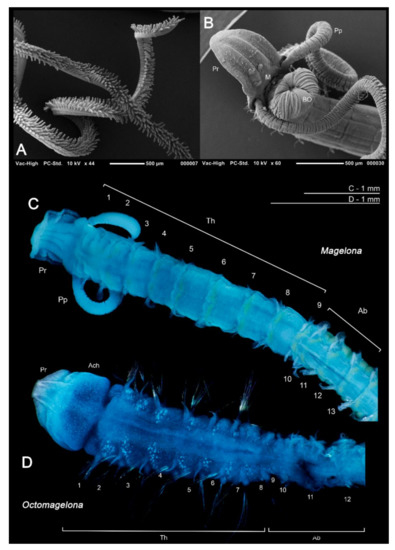
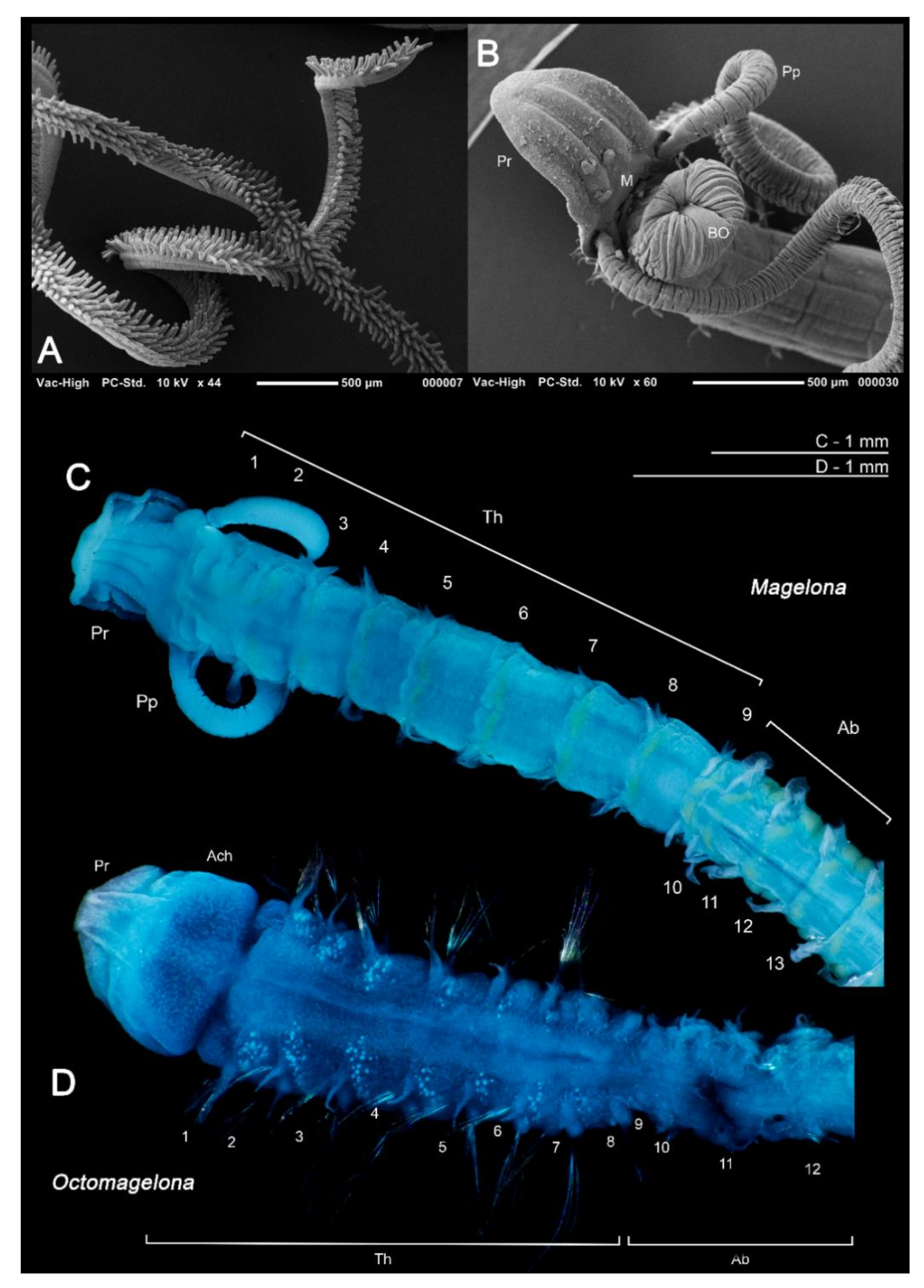
Figure 2.
(A) Papillated palps of Magelona johnstoni; (B) anterior thorax of M. johnstoni showing partially everted burrowing organ, mouth at prostomium base, and basal portion of the palps (ventral view); (C) M. gemmata (dorsal view); (D) undescribed species of Octomagelona from Western Africa (dorsal view). Abbreviations: Ab—abdomen, Ach—achaetous first segment, BO—burrowing organ, M—mouth, Pp—palp, Pr—prostomium, Th—thorax. Numbers indicate chaetiger. (C,D) stained with methyl green.
At present, the family contains two genera: the type genus Magelona F. Müller, 1858 for species possessing thoracic regions of nine chaetigers (Figure 2C), and the monotypic Octomagelona Aguirrezabalaga, Ceberio and Fiege, 2001 for those possessing a thoracic region of only eight (Figure 2D). Whilst the latter genus contains only one described species, Octomagelona bizkaiensis Aguirrezabalaga, Ceberio and Fiege, 2001 from the Bay of Biscay (North East Atlantic), several other undescribed species (from Mexico, Australia and West Africa) are known but have not yet been formally described [22]. All previously introduced generic names (Maea Johnston, 1865; Rhynophylla Carrington, 1865 and Meredithia Hernández-Alcántara and Solís-Weiss, 2000) have been synonymised with Magelona.
Several key researchers have worked on the family: McIntosh [23,24,25,26,27,28] provided much early knowledge on the morphology and anatomy of the group, and later Jones [29,30,31,32,33] published a series of taxonomic papers and a paper on the morphology, feeding and behaviour of an undescribed species from Woods Hole, Massachusetts. Jones [29] introduced unique terminology for the group, particularly relating to parapodial structures (e.g., lateral lamellae, ventral neuropodial lobes, dorsal medial and ventral medial lobes), which he subsequently modified [31,33]. The terms have been inconsistently applied [22] and their continued use may obscure potential homologies with conditions seen in other polychaete groups [19]. During a phylogenetic analysis of the family [21] standardisation of the terminology was attempted, with parapodial structures described in terms of shape, size and position. More recent papers include those from the Indian Ocean [34,35,36,37,38,39], Chinese waters [40,41], Gulf of Mexico [42,43,44]; California [45]; Brazil [46,47] and European waters [18,48,49,50]. Several observational papers looking at the behaviour of British species have also been published [51,52,53].
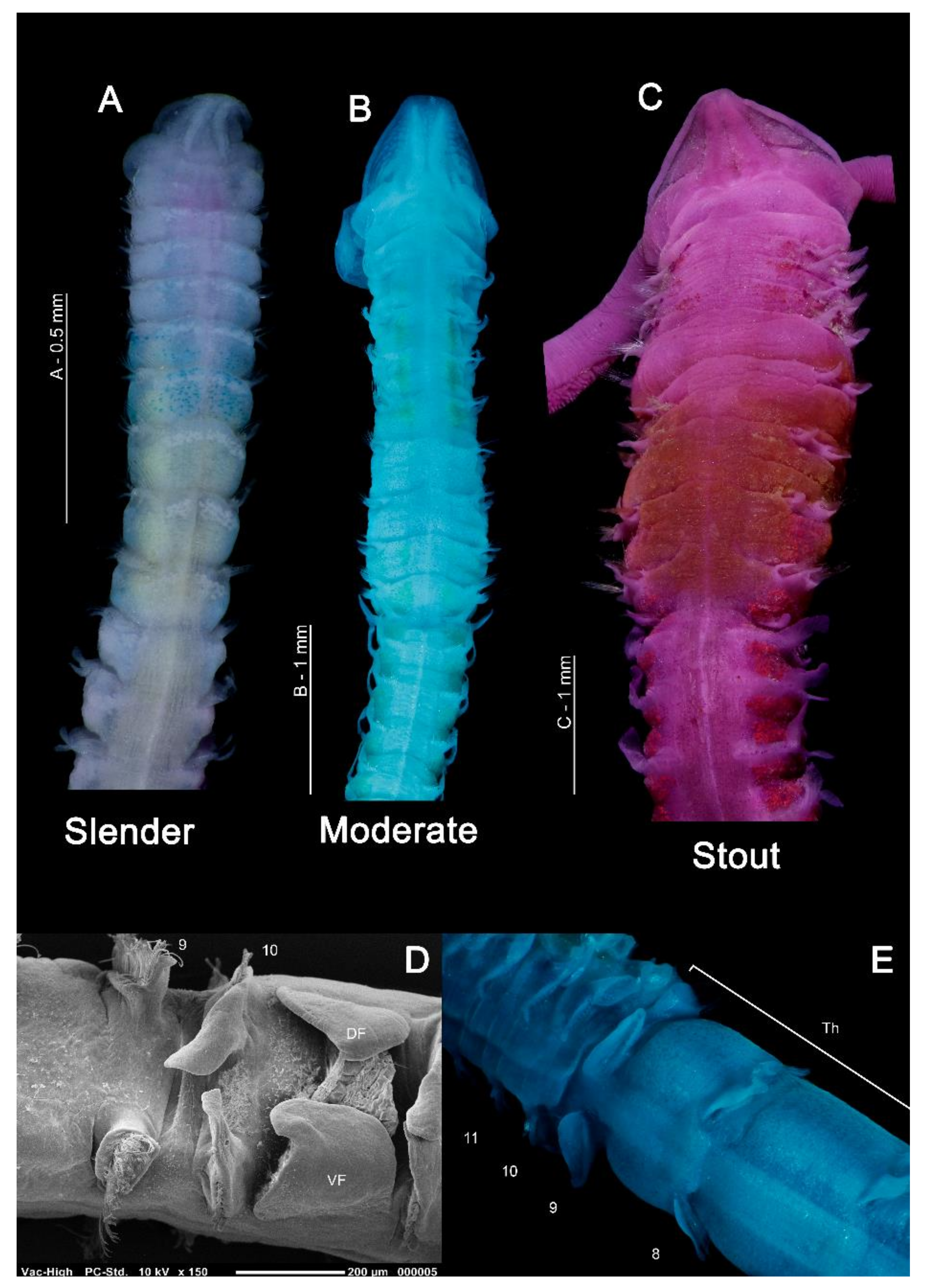
In general, magelonids are less than 1 mm wide (Figure 3A,B), but can reach over 100–150 mm in length [19,48]. Due to their fragility, average lengths of most species are unknown and thus total length is not generally a helpful characteristic for separation of species. However, body proportions are of use for identification. Whilst some species such as Magelona minuta Eliason, 1962, rarely attain widths greater than 0.5 mm, being slender animals (Figure 3A), others such as Magelona alleni Wilson, 1958 (Figure 3C), are more robust, attaining widths up to 1.5 mm (Mortimer et al., in preparation B). The terms slender, moderate and stout are terms often used in descriptions to describe overall body proportions and this is certainly something which warrants defining (Figure 3A–C).
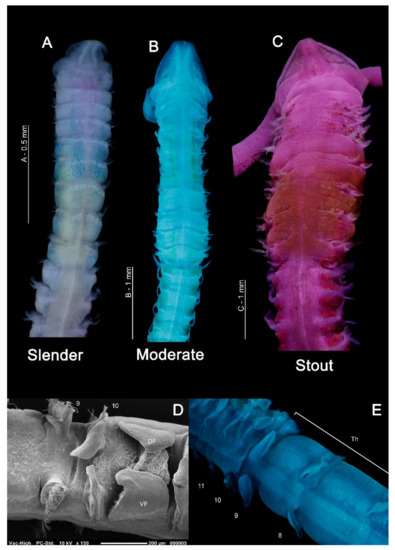
Figure 3.
(A) Anterior of Magelona minuta (dorsal view, specimen stained with rose bengal and methyl green); (B) anterior of M. mahensis (dorsal view, stained with methyl green); (C) anterior of M. alleni, showing distinct pigment band between chaetigers 5–8 (dorsal view, stained with rose bengal); (D) thoracic/abdominal junction of M. johnstoni (lateral view), showing an anteriorly open pouch between chaetigers 10 and 11; (E) thoracic/abdominal junction of M. johnstoni (dorsal view). Abbreviations: DF—dorsal flap, Th—thorax, VF—ventral flap. Numbers indicate chaetiger.
Life span is unknown for most species. Although Magelona sacculata Hartman, 1961 is considered to be an annual species [54], animals of other species have been kept alive in tank environments for over two years (personal observations, KMJ). Magelonids are generally considered surface deposit-feeders [52], found in soft sediments, at a depth of less than 100 m [22]. However, several magelonid species have been recorded from deeper waters: 1000 to over 4000 m [55,56].
Regarding Oweniidae, there are around 60 species worldwide [57,58] included in four genera, namely Owenia Delle Chiaje, 1844, Myriochele Malmgren, 1867, Galathowenia Kirkegaard, 1959 and Myriowenia Hartman, 1960 [17].
In the last 50 years, there have been a number of studies on oweniid taxonomy in several regions across the world, namely Antarctica [59,60,61,62,63], Arctic and North Atlantic waters [64,65,66,67,68], Western Mediterranean [69], California [70,71], Gulf of Mexico and Caribbean Sea [72,73,74], Yellow Sea [75], Japan [76] and Australia [17,77,78]. However, updated information including revisions of old records are still lacking in many areas, such as eastern African coasts, across the Indian Ocean and most of the Pacific. Furthermore, some species, such as Owenia fusiformis Delle Chiaje, 1844 and Myriochele heeri Malmgren, 1867, have been for many years considered as having a large, cosmopolitan distribution (e.g., [67,71,79]). However, further morphological studies, focusing mainly on chaetal and crown tentacle morphology for Owenia species have revealed these are complexes of morphologically homogenous species [66,75,77,78,80,81].
The body length of oweniids usually ranges from 20 to 30 mm (e.g., Myriochele, Galathowenia) but species of Owenia may reach up to 10 cm [57]. Life span may be up to about 2–4 years [82]; development includes the planktonic larva mitraria that is unusual in having monociliated bands in comparison to the typical annelid trochophore [83]. Oweniids are deposit-feeders, tube dwellers usually found in sandy sediments, from shallow coastal habitats to the deep sea (e.g., [47,84]).
In this contribution we aim to provide an updated revision on current biodiversity knowledge in Palaeoannelida, focusing on species richness and distribution in different world sea regions, updates in taxonomy, classification, and systematics after recent advances in the knowledge of their anatomy, and evolutionary relationships and consequent classifications. We would like to emphasise major gaps in knowledge and where efforts should be made in terms of biodiversity surveys, analytical efforts, and the strengthening of taxonomic skills in order to increase our knowledge about the species inhabiting the planet.
2. Methods
A thorough literature review was performed in order to acquire information about species diversity, type locality, and geographic, bathymetric and ecological constrains of members of Palaeoannelida. Moreover, some recent advances in their anatomical study after Capa et al. [57] were incorporated due to their systematic significance. To accomplish this aim, the World register of Marine Species [58,85] database has been of great use, and some amendments herein made, (e.g., accepted synonymies according to latest references) have also been coordinated with WoRMS for its update. Tables with all considered currently valid nominal species alongside their type localities, depth (from original description) and marine realms (sensu Spalding et al. [86]) are provided (Table A1 and Table A2). Additionally, a revision about methodologies and techniques used for species identification and characterisation is made, with the latest trends.
3. Results
3.1. Magelonidae Cunningham and Ramage, 1888
3.1.1. Systematics
There have been many difficulties relating magelonids to other annelid groups; a problem mirrored in the Oweniidae. For many years, magelonids have been considered among the spioniform polychaetes [19] and after phylogentic analyses of morphological data were placed in the Spionida (together with Apistobranchidae, Chaetopteridae, Longosomatidae, Poecilochaetidae, Spionidae, Trochochaetidae, Uncispionidae clades [87]), principally based on the presence of a pair of grooved palps and spiomorph parapodia [87], or related to Oweniidae [17]. Various other placements have since been suggested, for example, related to Polydora Bosc, 1802 (Spionidae), Cirriformia Hartman, 1936 and Dodecaceria Örsted, 1843 (Cirratulidae), or Fauveliopsis McIntosh, 1922 (Fauveliopsidae) [10] utilising combined morphological and DNA sequences of nuclear markers. Further, phylogenomic analyses placed Magelonidae as sister to Oweniidae branching off at the base of the annelid tree [4,6,88].
Although a sister-group relationship of Magelonidae and Oweniidae has been outlined, this and their position within Annelida are still the subject of intense debate [57]. Clarification is needed in regard to the extent to which the prostomium is fused to the peristomium in magelonids (see below) and also in the nature of the ‘buccal organs’ in the two families (see below in relation to the burrowing organ). It has been suggested that the head structures in the Palaeoannelida clade are heterogeneous, with Magelona possessing papillated peristomial palps whilst grooved prostomial palps are present in Owenia [89]. Further work is clearly needed, but perhaps further studies assessing similarities and differences between the two families may shed light on the subject.
There is only one cladistic analysis on the Magelonidae to date [21], which confirmed the monophyly of the group but from which no further proposals were made. A forth coming account of inferences of phylogenetic hypotheses within the Magelonidae (Mortimer et al., in preparation A) may add clarity. A fossil polychaete from the early Cambrian (Dannychaeta tucolus Chen, Parry, Vinther, Zhai, Hou and Ma, 2020) has recently been described within the Magelonidae based on phylogenetic analyses [16].
3.1.2. Taxonomic History
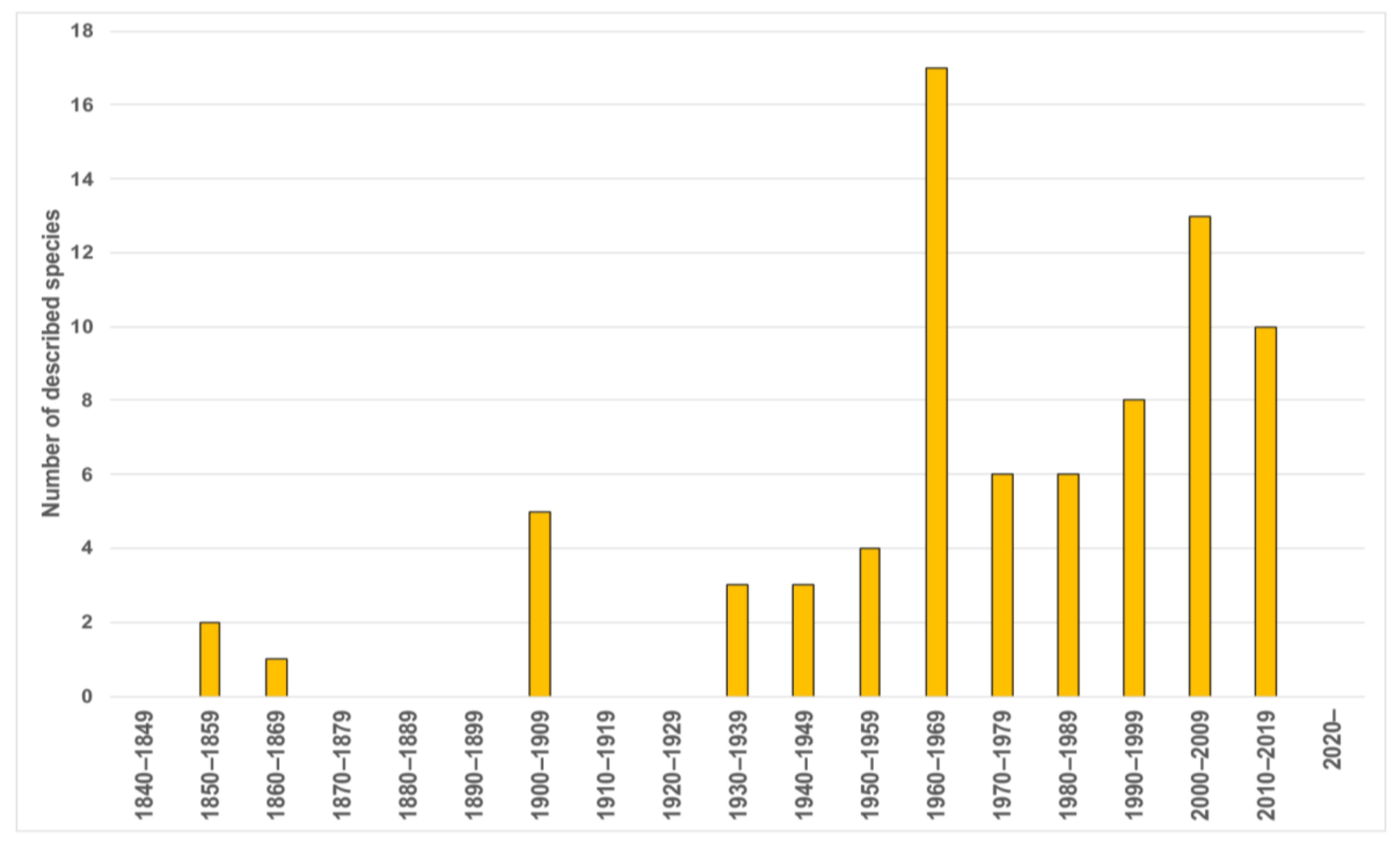
Taxonomically, Magelonidae received little attention prior to the 1930s (Figure 4), however, the number of taxonomic papers increased in the 1940s and 1950s with works by prolific polychaete researchers such as Hartman [90,91]. With the 1960s, the group saw a rapid expansion of taxonomic work, not only by notable magelonid workers such as Jones [29] but works by Gallardo [92], Eliason [93], Hartman [94,95], Glémarec [96], Kitamori [97], Reish [98], Hartmann-Schröder [99], Day [100] and Harmelin [101] (Table A1). This increase is likely to have been influenced by the publication of Jones, which set out a standard to which magelonid works should attain. The 1970s largely saw works by Jones, who by that time had been established as the magelonid expert [31,32,33]. His research on magelonids continued until 1979 where he switched his attention to the Vestimentifera (now Siboglinidae) [48]. The major taxonomic works of the 1980s and 1990s were those concentrating on Southeastern Brazil [46] and Thailand [34]. Since 2000, the number of researchers producing taxonomic works on the group, and the number of species being described has once again increased. Workers such as Mortimer and Mackie [35,36], Fiege et al. [48], Hernández-Alcántara and Solís-Weiss [43], Mortimer et al. [38,49] and Magalhães et al. [102] (see Table A1 for all references) contributing further to the taxonomic knowledge of the group. Certainly, with renewed interest in the group, it is likely that this trend in increasing publications will continue. However, outside of the key works by Jones there have been very few major revisions of the family [21,22,48]. A forthcoming account of inferences of phylogenetic hypotheses within Magelonidae (Mortimer et al., in preparation A) will add further information on the key morphological characters.
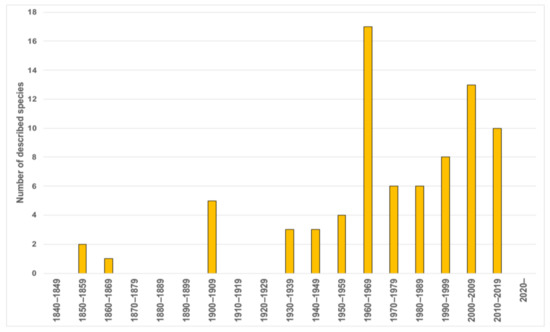
Figure 4.
Number of magelonid species described per decade.
The only worldwide key to species was produced in 1963 [29]. However, over 50 species have been described since that time (WoRMS, Table A1). Whilst keys to local regions can be found in several papers: California [45], Eastern USA [95], Gulf of Mexico [42,43,44], Brazil [46], Europe [18,48,49], Japan [97,103] Viet Nam [92], Thailand [34], Western Indian Ocean [37,38], Seychelles [36], South Africa [104], updated versions are warranted in most regions.
3.1.3. Taxonomic Characters and External Morphology
Magelonids are generally fragile, being long and slender and for this reason they can be extremely difficult to collect whole. Over 60% of species have been described from posteriorly incomplete specimens. They are often pale in colour (white to cream), although the gut, which frequently has a darkish green hue, can often be seen through the body wall. Several species (M. alleni, Magelona cincta Ehlers, 1908, Magelona equilamellae Harmelin, 1964, Magelona japonica Okuda, 1937 and Magelona variolamellata Bolívar and Lana, 1986) are known to carry dark pigmentation occurring as a distinct band between chaetigers 5–8 [52,53] (Figure 3C), and pigmentation in other parts of the body such as the palps has additionally been reported, e.g., Magelona mirabilis (Johnston, 1865) [48,51]. In addition, specimens belonging to two undescribed species recently found off West Africa carry dark pigmentation of the parapodia, and distinct stripy pigmentation from head to toe, respectively (Mortimer et al., in preparation B).
The magelonid body is divided into distinct regions: the head (comprising the prostomium and the peristomium), the thorax (an achaetous first segment, and either eight or nine chaetigers), the abdomen comprising many chaetigers (approximately 50–160 chaetigers, see [22], although for most species this is unknown), and the pygidium (Figure 2C,D). The precise location and organisation of the peristomium has been debated [10,19]. Early works suggested the prostomium “merges posteriorly with the achaetous peristomium” [30], suggesting that the peristomium is represented by the achaetous first segment. However, magelonid larvae have been shown to possess two anterior segments bearing chaetae which subsequently become achaetous [105]. Subsequent workers regarded the peristomium to be limited to the buccal region [87]. Personal observations (KMJ) suggest that the peristomium is visible on the ventrum (see [22]: figure 4.2.5, posterior to buccal region) and this and the achaetous ‘first segment’ (Figure 1) warrant further investigation. Magelonids have long been regarded to possess an eversible ventral proboscis (e.g., [30,87]), however this heart shaped sac is not connected to the buccal region, plays no part in feeding and is involved only in burrowing (Figure 2B). The term burrowing organ has now been applied to this structure to avoid future confusions [18,22]. Between the thorax and abdomen, there is an often-marked constriction in the body (Figure 3D,E), and the two flanking regions are additionally discernible by a change in chaetal type (Figure 2C,D and Figure 3) from capillary chaetae (Figure 5) to hooded hooks (Figure 6). Magelonids possess biramous parapodia, often carrying foliaceous flattened structures, for which the term ‘lateral lamellae’ was coined [29]. However, these structures may be filiform to foliaceous and may be prechaetal, postchaetal, subchaetal or lateral in position. It is the identification of these features, particularly in the thoracic region which is a major diagnostic feature in separating species, and it is for this reason that all thoracic parapodia must be fully described and illustrated [21,31].
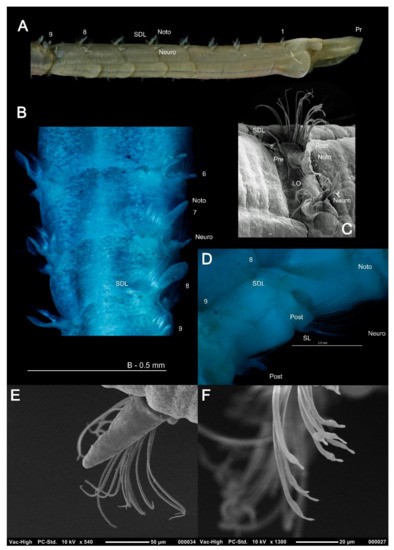
Figure 5.
Thoracic parapodia and chaetae: (A) anterior of Magelona filiformis (lateral view) showing filiform lamellae; (B) M. crenulifrons from chaetiger 6–9 (dorsal view, stained with methyl green) showing foliaceous notopodial lamellae and superior dorsal lobes; (C) left-hand parapodia of chaetiger 8 of M. johnstoni (lateral view); (D) right-hand parapodia of chaetigers 8 and 9 of M. wilsoni (E) thoracic subchaetal neuropodial lamellae of M. johnstoni showing capillary chaetae; (F) mucronate capillary chaetae of chaetiger 9 of M. johnstoni. Abbreviations: LO—lateral organ, Noto—notopodia, Neuro—neuropodia, Post—postchaetal, Pr—prostomium, Pre—prechaetal, SDL—superior dorsal lobe, SL – subchaetal lamellae. Numbers indicate chaetiger.
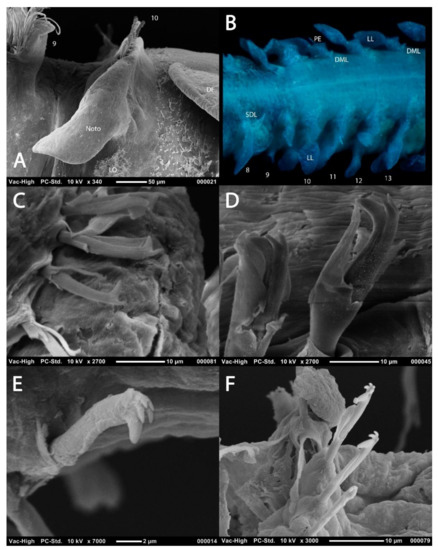
Figure 6.
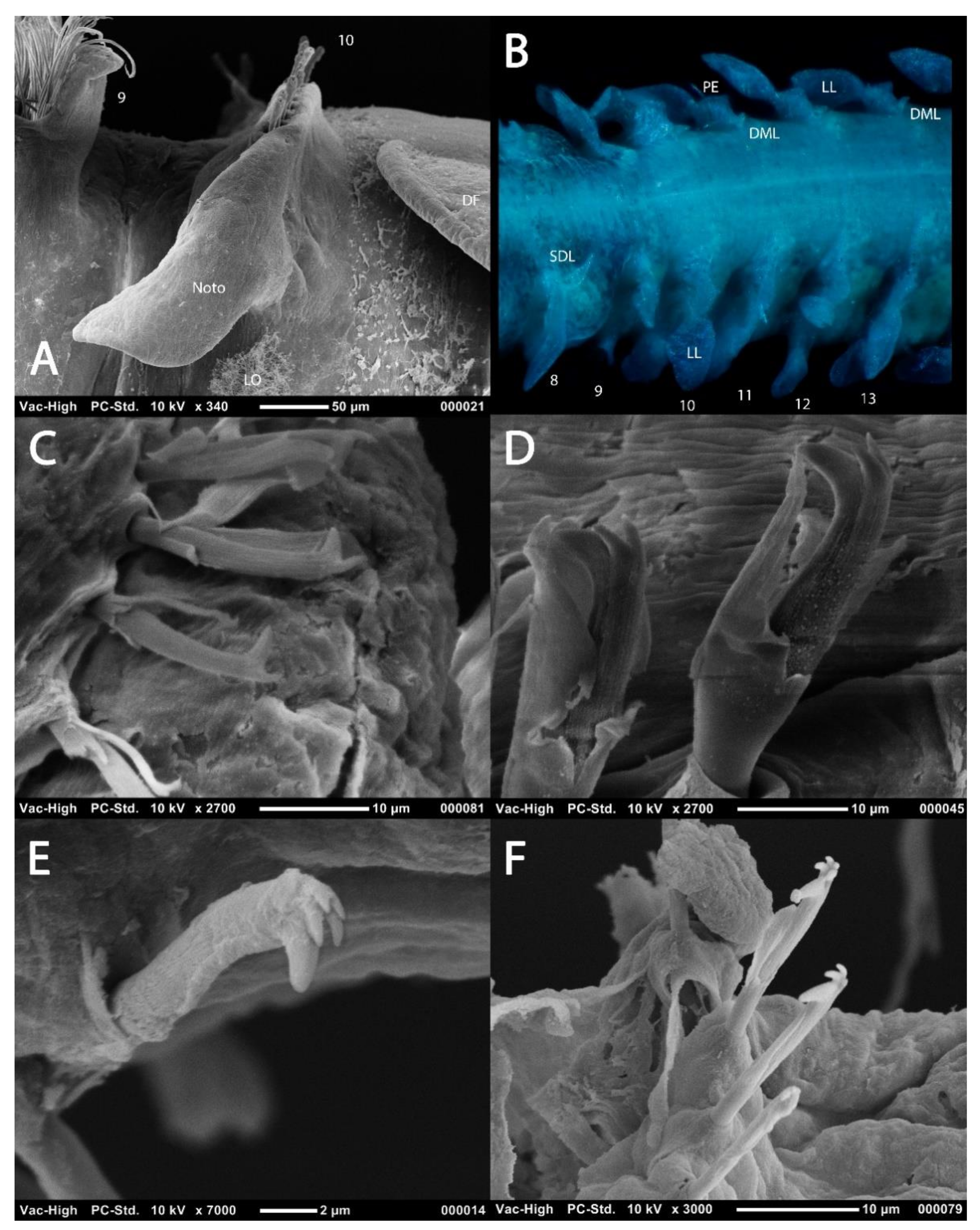
Abdominal parapodia and chaetae: (A) notopodia of chaetiger 10 of Magelona johnstoni showing abdominal hooded hoods in a vis-à-vis orientation (lateral view); (B), chaetigers 8–13 of M. crenulifrons (dorsal view, stained with methyl green); (C) bidentate abdominal hooded hook of M. minuta (lateral view); (D) tridentate abdominal hooded hook of M. alleni (lateral view); (E) pentadentate abdominal hooded hook of M. fauchaldi (oblique lateral view); (F) quadridentate and pentadentate abdominal hooded hooks of M. fauchaldi (oblique lateral view). (C–F), hoods broken via sonication prior to SEM. Abbreviations: DF—dorsal flap of the lateral pouch, DML—dorsal medial lobe, LL—lateral lamella, Noto—notopodia, PE—postchaetal expansion, SDL—superior dorsal lobe, VML—ventral medial lobe. Numbers indicate chaetiger. Figure F sourced from [39].
Several ‘crucial morphological’ characters for separation of species have been suggested [29,45]: (1) dentition of abdominal hooded hooks; (2) presence or absence of prostomial horns (distal projections of the anterior margin); (3) presence or absence of medial lamellae (DML, VML) in the posterior region (cirriform lobes adjacent to hooded hooks see Figure 6B and Figure 7B,C); (4) presence or absence of specialised chaetae of the ninth chaetiger (mucronate, pennoned); (5) morphology of thoracic lamellae; (6) the relative dimensions of the prostomium (L:W ratio); (7) presence or absence of superior dorsal lobes on thoracic chaetigers; (8) presence and distribution of lateral abdominal pouches and (9) the presence or absence of postchaetal expansions behind chaetal rows in the abdomen. With the description of further taxa, several other characters are additionally noted: (10) pigmentation patterns and (11) overall body size and proportions (Figure 3).
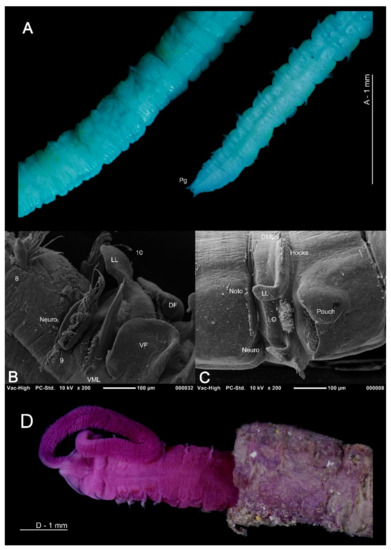
Figure 7.
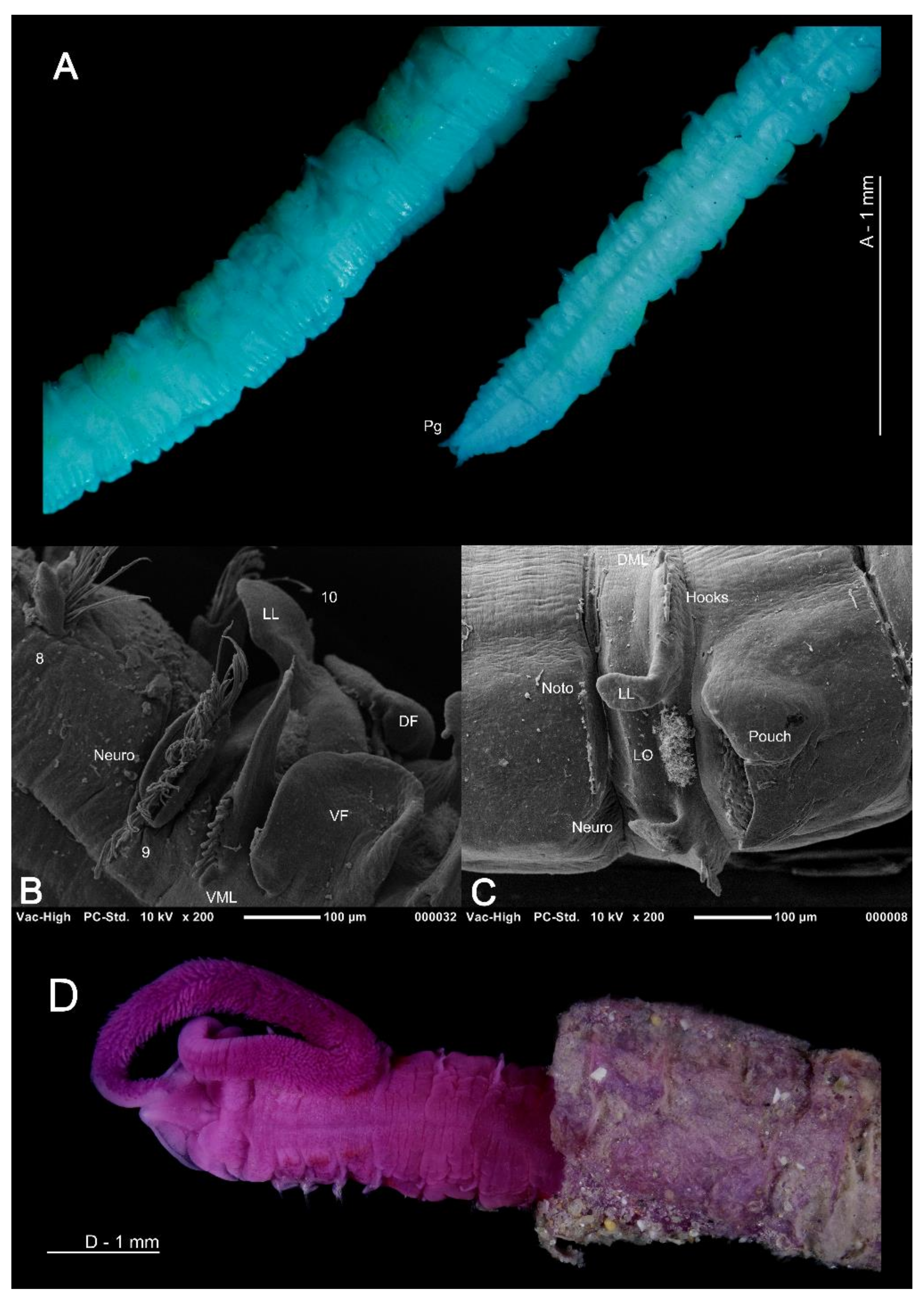
(A) Posterior abdomen and pygidium of Magelona mahensis (lateral and ventral views respectively), specimen ovigerous; (B) anteriorly open lateral pouch between chaetigers 10–11 of M. johnstoni (ventral view); (C) posteriorly open lateral pouch from posterior abdomen of M. johnstoni (lateral view); (D) anterior of M. alleni protruding from multi-layered sediment covered tube (dorsal view). (A) is stained with methyl green, (D) is stained with rose bengal. Abbreviations: DF—dorsal flap of the lateral pouch, DML—dorsal medial lobe, LL—lateral lamellae, LO—lateral organ, Neuro—neuropodia, Noto—notopodia, Pg—pygidium, VF—ventral flap of the lateral pouch, VML—ventral medial lobe. Numbers indicate chaetiger.
Magelonid prostomia can be broadly divided into three categories: length approximately equal to width (Figure 1B,C), length greater than width (Figure 1A), or width greater than length (Figure 1E). The overall shape can aid identification, but care must be taken as the appearance can be modified if lateral or anterior margins become compressed. Prostomial horns may be present or absent. When present, they may be distinct (Figure 1B,E) such as in Magelona montera Mortimer, Cassà, Martin and Gil, 2012, or ‘rudimentary’ (Figure 2D and Figure 3C) in which the anterior margin is straight and square, such as in M. alleni. The prostomial anterior margin may be smooth (e.g., M. mirabilis (Figure 1A), medially indented (e.g., Magelona symmetrica Mortimer and Mackie, 2006, Figure 1D) or crenulated (e.g., Magelona crenulifrons Gallardo, 1968) (Figure 1B,E). The prostomium may carry one (Figure 2D) or two pairs of dorsal muscular ridges (Figure 1A,B), and distinct patterned areas either side of the ridges (often as raised oblong or arched lines) may be present or absent (Figure 1).
The biramous parapodia of the thoracic region carry lamellae which may be equal in terms of size and shape in both rami, or noticeably larger in the notopodia. In some species the neuropodial lamellae may be marginally larger, however, this situation is generally infrequent. The lamellae may be filiform (e.g., Magelona filiformis Wilson, 1959) (Figure 5A) to foliaceous (e.g., notopodia of Magelona sinbadi Mortimer, Cassà, Martin and Gil, 2012) in shape (Figure 5B,D). The notopodial lamellae are generally postchaetal to subchaetal and encircle the chaetal bundle, confluent with a much lower prechaetal lamellae, almost cuff-like (Figure 5C). The upper edges of the lamellae may be smooth (e.g., M. equilamellae), crenulate (e.g., M. johnstoni) or bi-lobed (e.g., Magelona obockensis Gravier, 1905). At the top of the notopodia and in a slightly prechaetal position superior dorsal lobes (SDL) may be present (Figure 5B–D). These are smaller than the lamellae, and often digitiform in shape, although in some taxa they may be foliaceous. When present, they may occur on all thoracic chaetigers, from chaetigers 1–8 or only present in the posterior thorax (e.g., chaetigers 4–8). The neuropodial lamellae of the thoracic region may be prechaetal, subchaetal or postchaetal, the edges of which are generally smooth. Many species have filiform subchaetal lamellae attached to low pre- and post-chaetal ridges (Figure 5A,E). In these species the lamellae may occur in the same position along the thorax, or vary, often starting in a slightly prechaetal position, becoming ventral in the mid thorax and becoming postchaetal by the posterior thorax. The postchaetal portion may be more expanded in some species, particularly on the eighth and ninth chaetigers, where it is often triangular and of a similar length to the subchaetal lamellae (Figure 5D). The parapodia of the eighth and ninth chaetigers are particularly important to observe. In some species, the thoracic lamellae are similar on all thoracic chaetigers (e.g., M. symmetrica), whilst in others the first seven are similar with the lamellae of chaetigers eight and nine varying (e.g., M. montera), and in others it is the lamellae of chaetiger nine which varies in comparison to the remaining thoracic lamellae (e.g., M. mirabilis).
The thorax has unilimbate (e.g., M. minuta) or bilimbate capillary chaetae (e.g., M. equilamellae), the latter of which may have irregular blades [21,45,46]. More variation within thoracic chaetae may exist [18,50]. In most species, the ninth chaetiger has gently tapering chaetae like those on preceding chaetigers, however, several species have specialised chaetae on the 9th chaetiger. These may be mucronate (Figure 3D,E, Figure 5F and Figure 7B) or pennoned, in which the limbations broaden distally, culminating in an acute tip (see [33], p. 340).
The abdomen which starts at the 9th chaetiger for Octomagelona (Figure 2D) and the 10th chaetiger for Magelona (Figure 2C) is comprised of numerous chaetigers (~50–160 chaetigers). Parapodia are biramous, and as in the thorax, carry lateral lamellae (e.g., LL in Figure 7C) which are generally symmetrical in terms of size and shape between the two rami. However, in a few species such as M. alleni and Magelona korena Okuda, 1937 the notopodial lamellae are somewhat larger than those of the neuropodia. Lamellar shape varies but can be generally separated into those which are rounded (Figure 6B), carrying a basal constriction and those which are slender triangular, with no basal constriction (Figure 3D, Figure 5A and Figure 7C). Above the lamellae in the notopodia (Figure 6A), and below the lamellae in the neuropodia (Figure 7B), hooded hooks occur in a single row (N.B. the hooks of M. equilamellae were noted to occur in two rows towards the middle of the ramus [18]). In each ramus, hooks may be orientated in one direction, laterally towards the lamella (Figure 3D and Figure 6A), or in two groups vis-à-vis (Figure 6B). Behind the chaetal row, a postchaetal expansion of the lamella may be present (Figure 6B). At the inner margins of the chaetal rows, small triangular to digitiform processes (dorsal (DML) and ventral medial lobes (VML) of Jones) may be present (Figure 6B and Figure 7B,C).
Abdominal hooded hooks may be bidentate (with one secondary tooth above the main fang) (Figure 6C), tridentate (with two secondary teeth) (Figure 6D) or polydentate (Figure 6E,F). Quadridentate, pentadentate and hexodont hooks have all been reported to occur within the family. Enlarged hooks may be present, the form of which varies from spines, re-curved hooks or enlarged ‘ordinary’ hooks (e.g., Magelona falcifera Mortimer and Mackie, 2003 or Magelona spinifera (Hernández-Alcántara and Solís-Weiss, 2000)). Some species have a small hook emerging at the base of the lateral lamellae (e.g., M. filiformis see [18]: 104), whilst several curved support chaetae (aciculae) may also be present (e.g., Magelona conversa Mortimer and Mackie, 2003).
Lateral abdominal pouches are recorded in approximately half of all known species, although their function is currently unknown [51]. Whilst pouches may be divided roughly into two types [48], greater variation in morphology has been noted [22,37,51]. Pouches may be anteriorly (comprising of a dorsal and ventral flap, with a convoluted membrane in between) (Figure 3D and Figure 7B) or posteriorly open (simple and pocket-like flaps) (Figure 7C). The former type generally occurs in pairs on either side of segments at the start of the abdomen, whilst the latter can be paired or unpaired, and may occur from median abdominal chaetigers, or only in the extreme posterior of the animal [51]. Posteriorly open pouches may occur on consecutive segments or alternating from segment to segment and from one side of the body to the other. They may be smooth or medially split.
For the 40% of species in which the pygidium is described, morphology is relatively uniform, possessing two slender cirri, placed laterally, with the anus being ventral (Figure 7A). However, variation has recently been highlighted [18,52] (Mortimer et al., in preparation B) with M. alleni and M. equilamellae possessing more robust projections either side of a terminal anus.
Several species have shown species-specific staining patterns using methyl green or methyl blue [34,39,40,41,50] and these methods can be extremely useful in identifying specimens in bulk samples.
3.1.4. Internal Morphology
In comparison to observations on the external morphology of magelonids the internal morphology has received less attention. Much of the early work on the anatomy of magelonids came with the publications of McIntosh [23,24,25,26,27,28] and Jones [30]. The latter author providing details of early studies, including the circulatory system [106], lateral organs [29], musculature [107,108], nervous system [109] and including his own observations on the nervous system, muscular system, septa, and circulatory system. Monociliated epidermal cells in the larvae of M. mirabilis were reported [110], although figure 1A of that publication is unlikely to be M. mirabilis.
The closed circulatory system consists of an anterior dorsal vessel, divided into a series of chambers set apart by valves. A heavily muscularised portion of the dorsal vessel is largely responsible for the movement of blood around the animal [30]. The circulatory system is vital in the eversion of the burrowing organ ([30], although note comment above about terminology) which can be clearly observed in live animals ([22]: figure 4.2.4). Notes on the unusual pale pink blood which contains numerous corpuscles have been made [111], the blood pigment is hemerythrin [112]. Palps contain a single blood vessel, clearly observed in live material ([51]: figure 1F) and it has been suggested that the palps have a secondary respiratory function [26,30].
Various authors have reviewed the structure and evolution of the nervous system [2,113,114]. The central nervous system of the Magelonidae has been considered to be simple in comparison to those of errant polychaetes [114]. The brain is composed of an anterior compact neuropil and posteriorly encircles the prostomial coelomic cavities. Thereafter, two lateral medullary cords branch off and fuse caudally. The ventral nerve cord comprises of two parallel cords of neurite bundles and paired neuropils which fuse between the 9th and 10th chaetigers [2]. Nuchal organs, ganglia and mushroom bodies are all considered to be absent [114], although the former are considered to occur in larvae [19]. Although eyes have been recorded as absent in adult magelonids, a species recently described from Hawaii, Magelona cinthyae Magalhães, Bailey-Brock and Watling, 2018 has distinct eyespots on the posterior prostomium.
The musculature of a species approaching M. mirabilis was investigated by phalloidin labelling and confocal laser scanning microscopy [115]. The prostomial muscles are anterior extensions of the ventral longitudinal muscles of the body and a complex of circumbuccal muscles are described ventral to the mouth opening. However, it is perhaps worth noting that this region is at the base of the burrowing organ rather than connected to the buccal region itself and the terminology should be reviewed. The palps possess muscles along their entire lengths, comprised of longitudinal fibres, which the authors [115] suggested would restrict movement to contraction and slight coiling. Whilst some authors have noted this to be the case for some species, including M. mirabilis [51,116] motile palps have been reported for other species [51,52] and will be discussed below. Each palp is moved by two palp retractor muscles. Dorsally and ventrally muscle strands run longitudinally, however, there is a distinct change in musculature between the thoracic and abdominal regions. Oblique and cross-striated muscles in M. papillicornis (likely to be M. mirabilis or M. johnstoni, see [48]) were investigated by Wissocq [117].
3.1.5. Species Diversity and Distribution
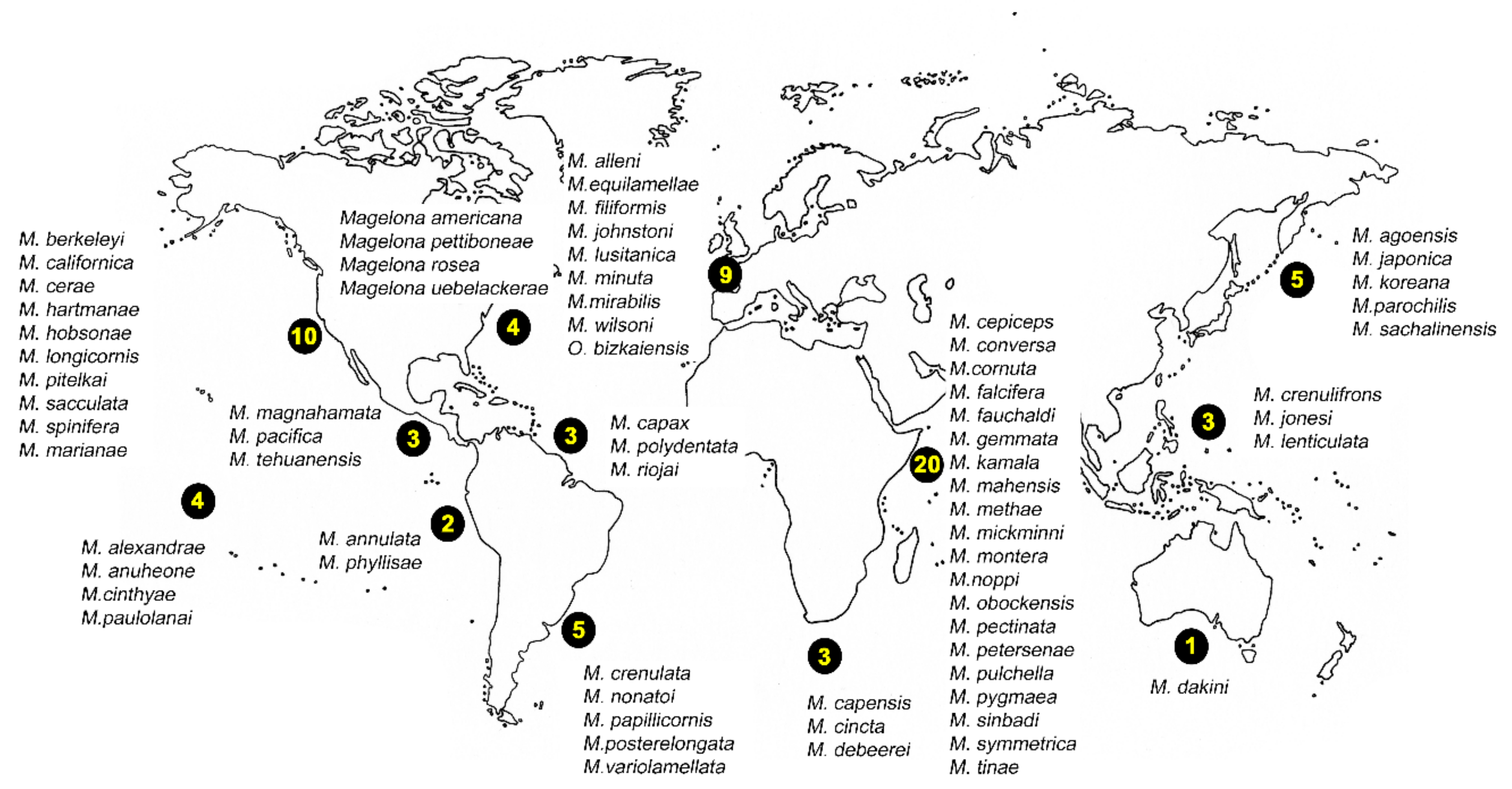
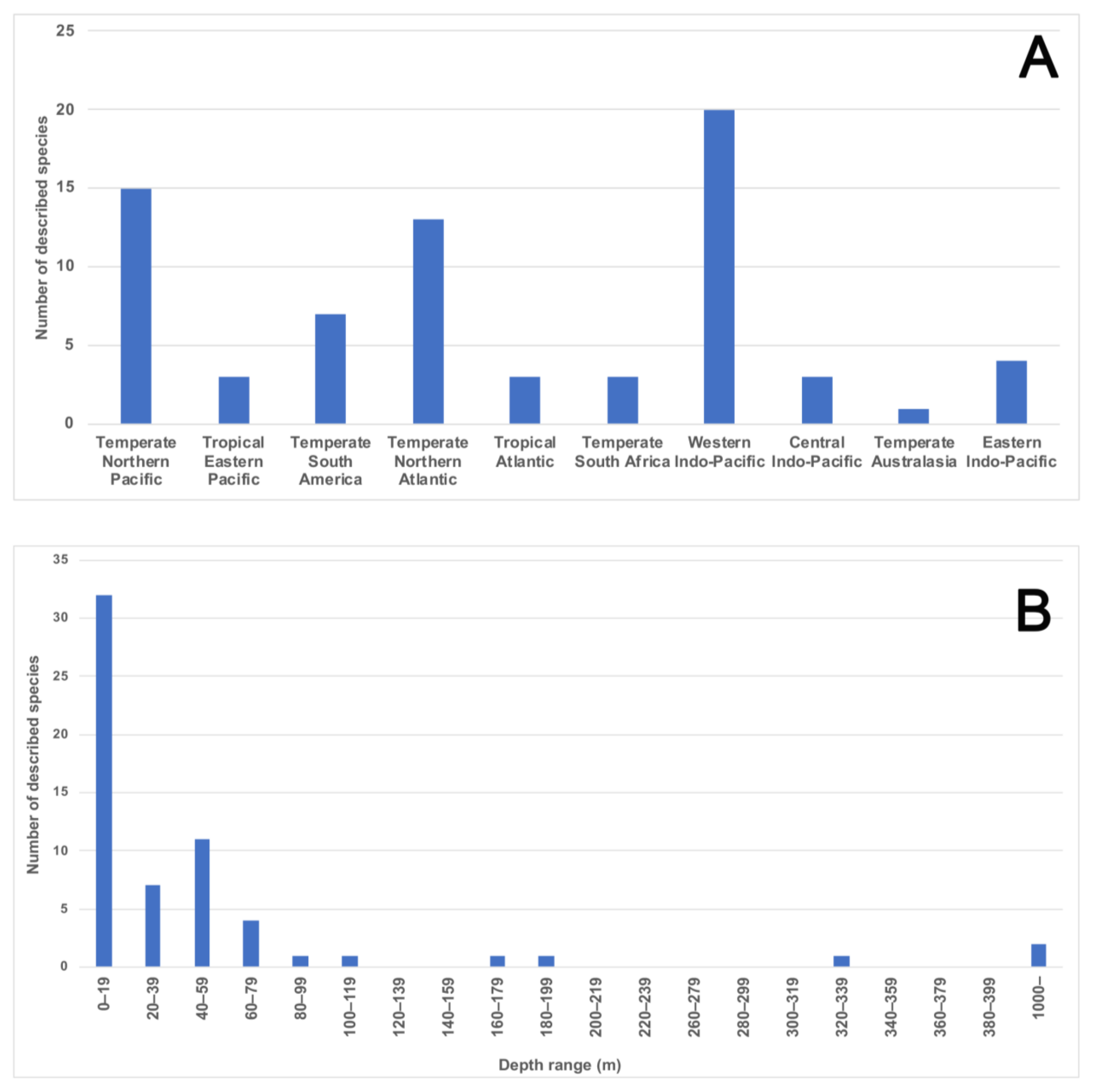
There are 72 magelonid species currently considered to be valid ([58], Table A1). Species have mainly been described from temperate and tropical environments, and the number described from the Temperate Northern Pacific, Temperate Northern Atlantic and Western Indo-Pacific is comparably larger than any other marine realm (Table A1, Figure 8 and Figure 9A). There are no species described from the Arctic or the Southern Ocean (Table A1, Figure 8 and Figure 9) although records from the Global Biodiversity Information Facility (GBIF [118] suggest magelonids have at least been found in these two regions but remain unverified at this time. These type locality occurrences perhaps reflect the influence of workers such as Jones [29], Hartman [95] and Wilson [119,120] in the Temperate North Atlantic, Hartman [90,94], and Jones [31,33] in the Temperate North Pacific, and Nateewathana and Hylleberg [34], Mortimer and Mackie [35,36] and Mortimer et al. [38] in the Western Indo-Pacific (Table A1), rather than differences in actual species numbers occurring in each region. However, further work is needed to corroborate this hypothesis.
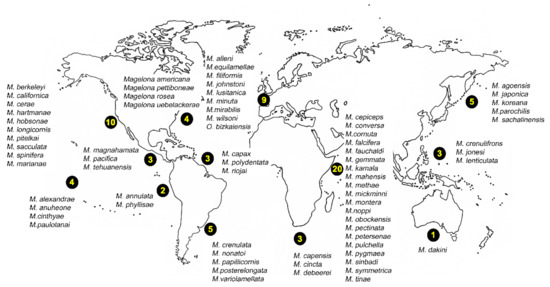
Figure 8.
World map showing number of magelonid species described per realms (sensu [86]).
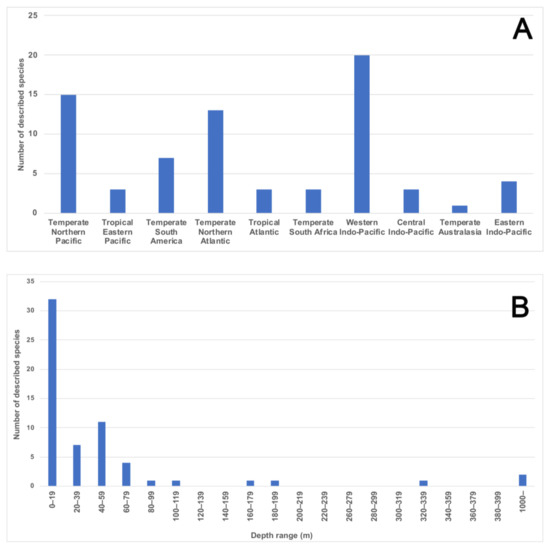
Figure 9.
(A) All 72 magelonid species currently consider valid [58] listed under the marine realms (sensu [86]) from which they were described. Numbers indicate the number of species described from each bioregion; (B) number of species described from each depth range (depth in meters, data from the original descriptions of 61 species for which the data was provided).
The diversity of magelonid species is generally high in relatively small geographical areas, e.g., 13 species in the Gulf of Mexico [42], nine species in European waters [18], 11 in the Arabian Gulf [38] and over 20 off west African waters (Mortimer et al., in preparation B). It was suggested that the high diversity of magelonid species observed off Phuket Island, Andaman Sea may be a direct result of sediment disturbance associated with monsoons [121], the authors hypothesising that catastrophic events affect interspecific competition by removing dominant species. However, areas with similar species diversities can be seen in non-monsoon affected areas. Abundance data on populations of Magelona in Monterey Bay indicate that population densities can be extremely variable form year to year [122].
Magelonids show a preference for shallow waters and data taken from the original descriptions of 61 magelonid species (for which data was provided) shows the number of species at depths of less than 20 m to be much greater than at any other depth range, with the number of species dropping off substantially after 60 m (Figure 9B). Although this information only includes data from the original descriptions, it does indicate the group’s preference for shallower waters. Whilst the data may be biased by the ease of sampling onshore or in shallower waters it is likely that these depth patterns contrast with other annelid groups. However, further work is certainly needed to clarify depth ranges of individual species. Whilst some magelonid species are found both intertidally and subtidally, some are known to be distinct offshore species (e.g., M. minuta; [22]). Far fewer deep-water species have been recorded: O. bizkaiensis (1000–1040 m; [56]), M. minuta (1000 m; [48]), M. capax Hartman, 1965 (4769 m) and Magelona spp. (3753–5000 m) [55]. Magelona phyllisae Jones, 1963 (note incorrect spelling M. physilia) has been noted to occur in low oxygen habitats [123] in northern Chile. Sensitivity to hypoxic conditions was also noted in the “dead zone” of the Gulf of Mexico for a species approaching M. phyllisae [124].
Very little information about the distribution ranges of magelonid species exists, and those already documented need validation. Magelona papillicornis was believed to be a wide-spread species, occurring from regions such as America, Africa, Europe, New Zealand, and India [32]. It is now recognised that many of these records are erroneous and have since been referred to several other species such as Magelona debeerei Clarke, Paterson, Florence and Gibbons, 2010, M. mirabilis and M. johnstoni [32,48]. Current maps from GBIF [118] highlight many of these erroneous records still and should be verified to prevent further confusion. Recent work off the western coast of Africa (Mortimer et al., in preparation B) involving morphological and molecular work suggests that whilst some species have quite restricted distributions others may be far greater. Certainly, further work is needed but this relies heavily on increased taxonomic work to resolve the identity of species in many areas. Undoubtedly, species recorded large distances from the type locality should be treated with caution at this time, e.g., American and Brazilian species recorded off India [125].
3.1.6. Biology and Ecology
Magelonids are common in sands and muds, both intertidally and subtidally; most species occurring in waters less than 100 m deep. The group’s preference for fine sediments has been linked to avoidance of sharp fragments that might damage the burrowing organ so vital for moving within sediments [27]. Whilst the broad sediment preferences are recognised for the family, very little is known about specific species habitats. Investigations into the distribution of four species (M. alleni, M. filiformis, M. mirabilis and M. johnstoni) in the German Bight showed sediment (median grain size diameter or mud content) to be the most important predictor in habitat suitability, but with salinity and water depth also of importance [126]. However, variations in habitat preference were observed between species (e.g., M. filiformis in sediments with less than 10% mud, whilst M. alleni occurred in sediments with elevated mud contents), something mirrored in observations in the UK, with M. minuta and M. alleni (personal observations, KMJ). Within the waters off the Arabian Peninsula individual species were noted to occur in sediments with distinct granulometric characteristics [38].
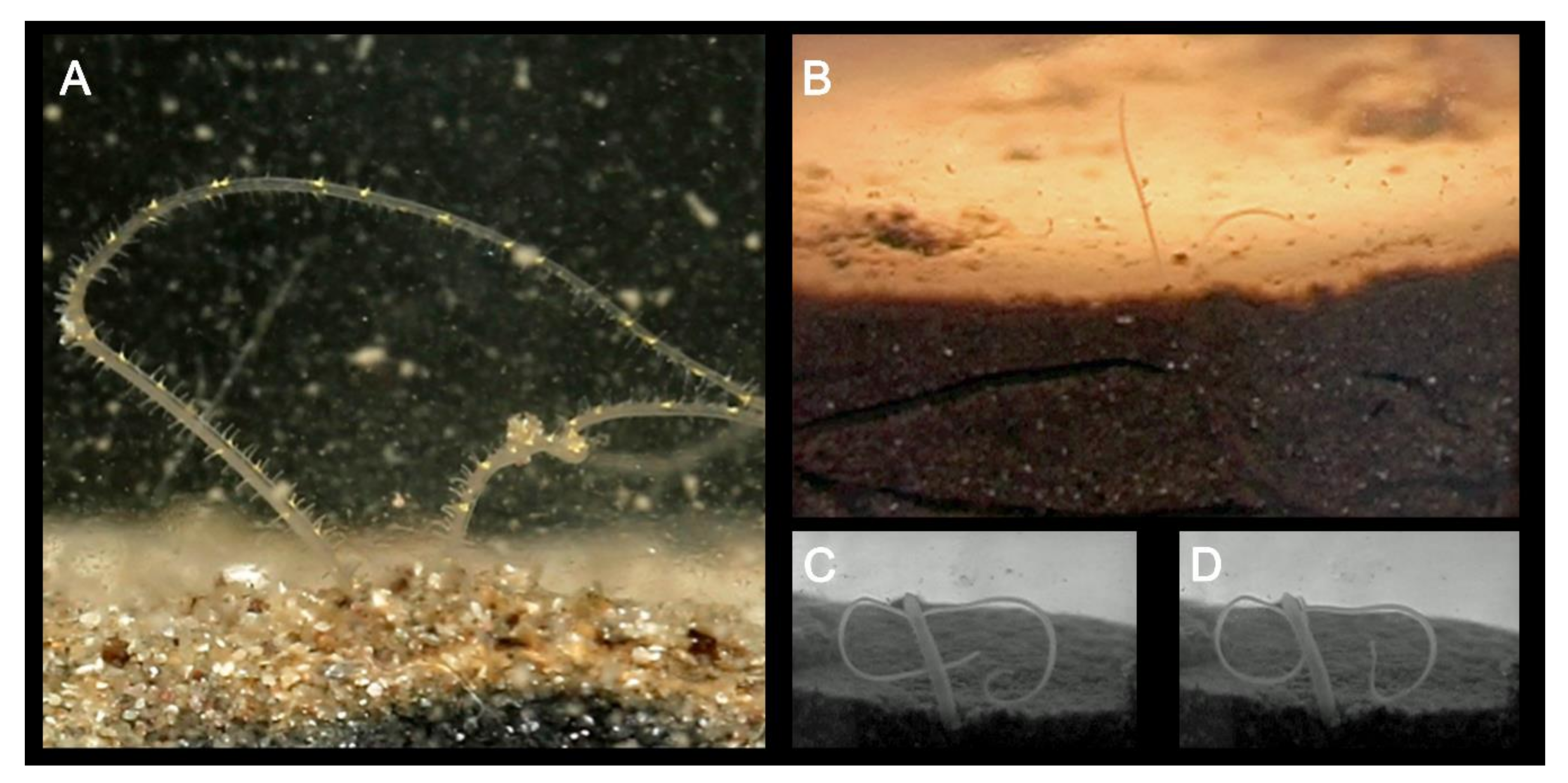
Whilst most magelonid species are believed to burrow more or less continuously through sediments [51,127], at least 10 species (e.g., M. alleni, M. cincta, Magelona polydentata Jones, 1963, M. variolamellata) are known to build distinct tubes (Figure 7D), which are often multi-layered paper-like tubes covered in sediment [22,52,53]. Individuals of M. alleni, have been observed to live for over a year in tube-lined burrows under tank conditions, being highly sedentary in comparison to previously observed species (Personal observations, KMJ).
Magelonids have been primarily described as surface deposit feeders [30,42,51,52], although suspension feeding [19,51,128,129], subsurface feeding and carnivory [116] have additionally been suggested. Varying feeding modes have been observed to predominate in different species observed simultaneously in the same laboratory setting [52]. Fauchald and Jumars [127] considered feeding to be selective, with selectivity increasing in nutrient-poor conditions. However, in contrast M. alleni was reported to be predominately non-selective [52] and magelonids feeding upon large volumes of sediment have been reported [116]. The feeding process has been described in detail for several species [30,51,52,130] and involves palps above the sediment surface, collecting particles (Figure 10A,B), which are then transferred along the palp to the mouth by cooperative movements of the papillae. A mucus thread has been postulated to aid transport of particles along the palps [30,130] and additionally reported to be involved in excretion in the tubicolous species M. alleni [52]. Given papillae involvement in particle transfer, differences in palp morphology between species, e.g., those species which possess slender palps with few papillae, in comparison to those that are broad carrying numerous rows of long papillae may indicate differences in feeding strategies and diet.
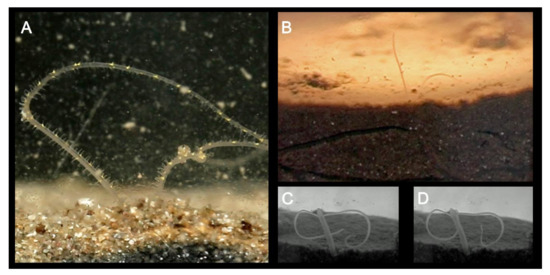
Figure 10.
(A) Distal portion of the palps of Magelona alleni within the water column collecting food particles. Particles are transferred to the mouth via cooperative movements of the palp papillae (from [52]); (B) distal portion of the palps of M. johnstoni above the sediment surface. Animal can be seen in the burrow below, as well other burrowing tracks made by another individual; (C,D) anterior of an individual M. johnstoni protruding into the water column prior to initiation of lateral sinuous movements thought to be linked to reproduction.
Magelonids have been reported to consume crustaceans, crustacean larvae, sediment, detritus, diatoms, algal cysts, spores, tintinnids, molluscs, worms and other small animals [26,30,51,52,128,129,131,132,133]. Despite the number of papers recording diet, knowledge of species-specific diets is generally lacking, although interspecific variation is likely [51,52].
Very little is known about the reproductive biology of the group [19,22], although sexes are separate. Eggs are easily observed in the abdomen of live animals and range from 50 to 150 µm in size [22]. Magelona sacculata was recorded to possess small eggs, with a high fecundity, varying from 8 to 12,000 eggs [122]. Eggs first occurred generally between chaetigers 10 and 20, and in the most fecund individuals extended to the last chaetiger. The family are believed to have ect-aquasperm and fertilisation is thought to be external [22,134,135]. Studies on the life history and population dynamics of M. sacculata suggest it reaches maturity in approximately two months, spawning in its first year with death occurring at or shortly after [122]. Lateral sinuous movements of the thorax within the water column are believed to be associated with reproduction (Figure 10C,D), although further evidence is needed. Both planktotrophic and lecithotrophic larvae have been recorded to occur within the group [105,135,136], and it has been suggested that the planktotrophic larvae of M. sacculata are long-lived, surviving for at least two months [122]. Although little is known about larval development, several species have been described and illustrated [22,105,137]. The dispersal potential of four species of Magelona in the Southern California Bight was estimated to be high based on known and inferred life-history information [138]. Magelonid larvae (Magelona sp.) have been observed to perform reverse diel vertical migrations off Central Peru [139].
Magelonids as potential indicator species of marine pollution, have been suggested [140], based primarily on the observation that non-polluted mangrove areas in Brazil were characterised by the magelonid M. papillicornis [141].
3.2. Family Oweniidae Rioja, 1917
3.2.1. Systematics
The monophyly of Oweniidae is supported by embryos which do not go through the typical spiralian development, by having a unique mitraria larvae, a pair of protonephridia resembling those of deuterostomes, lacking the typical external cuticle of most annelids, possessing a monociliated epidermis and a nerve cord that is intraepidermal in some species [17,57,65,142,143,144,145,146,147,148]. Although robust and unambiguous characters, some of the aforementioned attributes are not easily observed. As a practical aid, members of this family are easily recognised by their elongate and cylindrical bodies, without protruding parapodia or appendages (except for those of the head, present in some species), and by bearing oval patches of transverse irregular rows of packed uncini provided with two distal teeth.
Due to the morphological and ontogenic peculiarities of members of this family, the relationships between oweniids and other annelids have been object of debate for decades (see [57] for a summary about the systematic history of the family).
Oweniidae currently contains four genera: Owenia, Myriochele, Galathowenia, and Myriowenia; a fifth genus, Myrioglobula Hartman, 1967 was considered as valid until its recent synonymisation with Myriochele [17]. Other genera such as Ammochares Grube, 1844, Mitraria Müller, 1851 and Ops Carrington, 1865 are now considered subjective synonyms of Owenia, and Psammocollus Grube, 1866 of Myriochele [85]. Clymenia Örsted, 1844 is still considered as a nomen dubium with uncertain homonymy with any of the aforementioned oweniid genera [85].
Monophyly of Oweniidae and the four currently considered valid genera has been assessed based on morphological information [17] but a phylogeny including molecular data has never been published for members of the family. The monophyly of Owenia is supported by the presence of their characteristic ramified tentacular crown on the anterior end and neuropodial uncini with teeth arranged side by side [17]. The monophyly of Myriowenia is also based on the presence of head appendages, but in this case, corresponding to a pair of grooved palps, as well as the arrangement of uncinal teeth, one on top of the other [17]. Members of Galathowenia are characterised by an anteriorly truncated head and the presence of a ventral cleft (e.g., [17,59,62,68,80,149,150]). The monophyly of Myriochele, as currently circumscribed, is supported by the presence of acicular chaetae with an elongated tapering distal end and a smooth surface and by the shape of the head, which is similar in width to the segments with a rounded anterior margin [17].
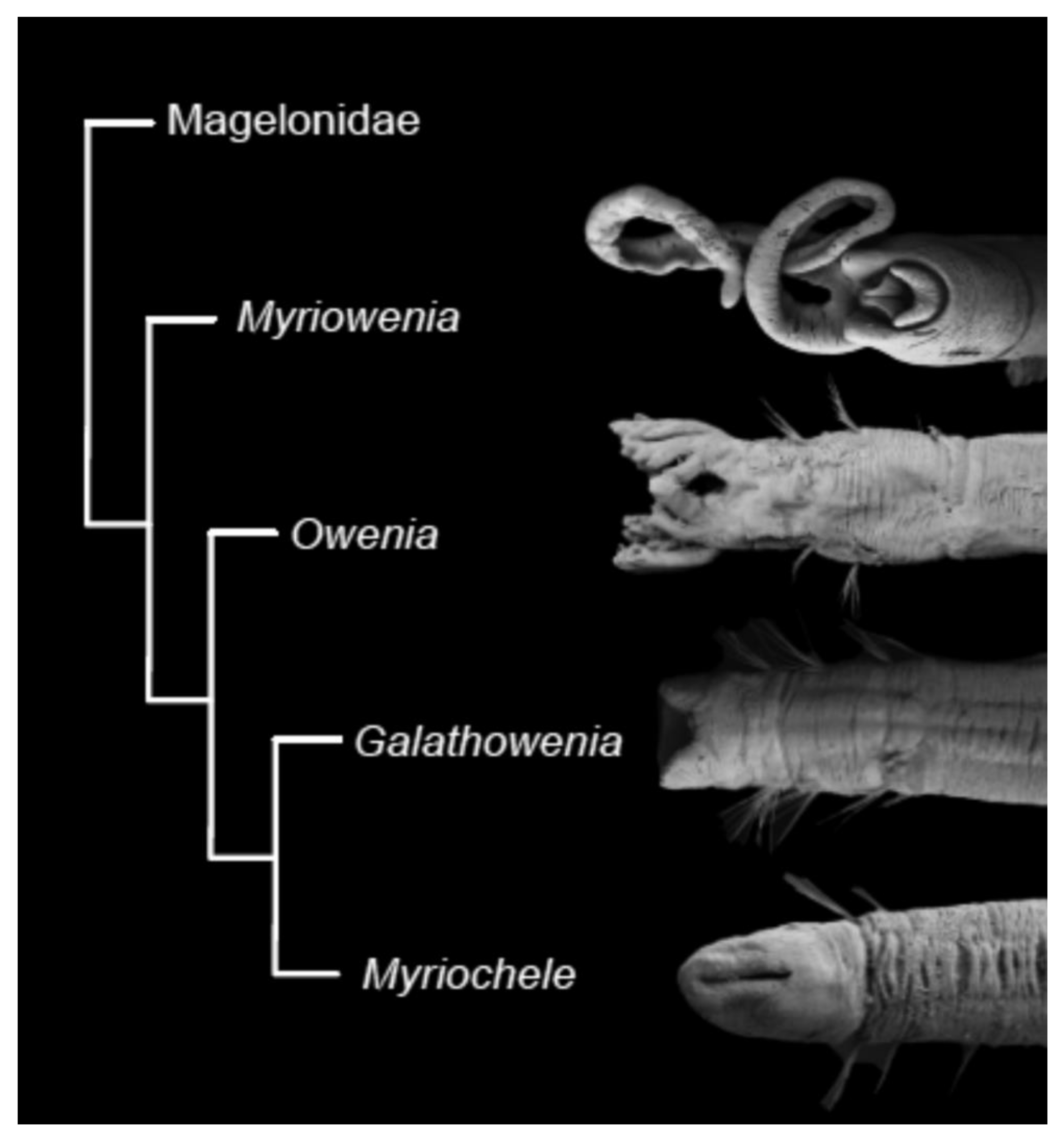
According to the latest phylogenetic hypothesis, oweniids possessing palps (Myriowenia and Owenia) branch off at the base of the family tree, with Galathowenia and Myriochele as sister groups [17,57] (Figure 11). If this hypothesis is corroborated, it would mean that the presence of palps is the plesiomorphic condition and have subsequently been lost in Galathowenia and Myriochele.
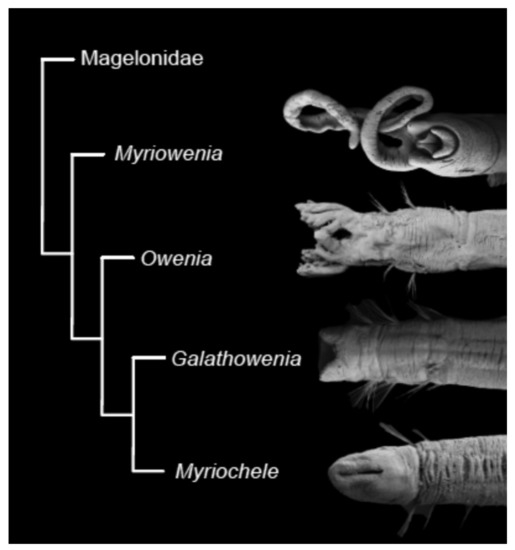
Figure 11.
Phylogenetic hypothesis, based on morphological data, of relationships between oweniid genera. Modified after Capa et al. [17].
3.2.2. Taxonomic History
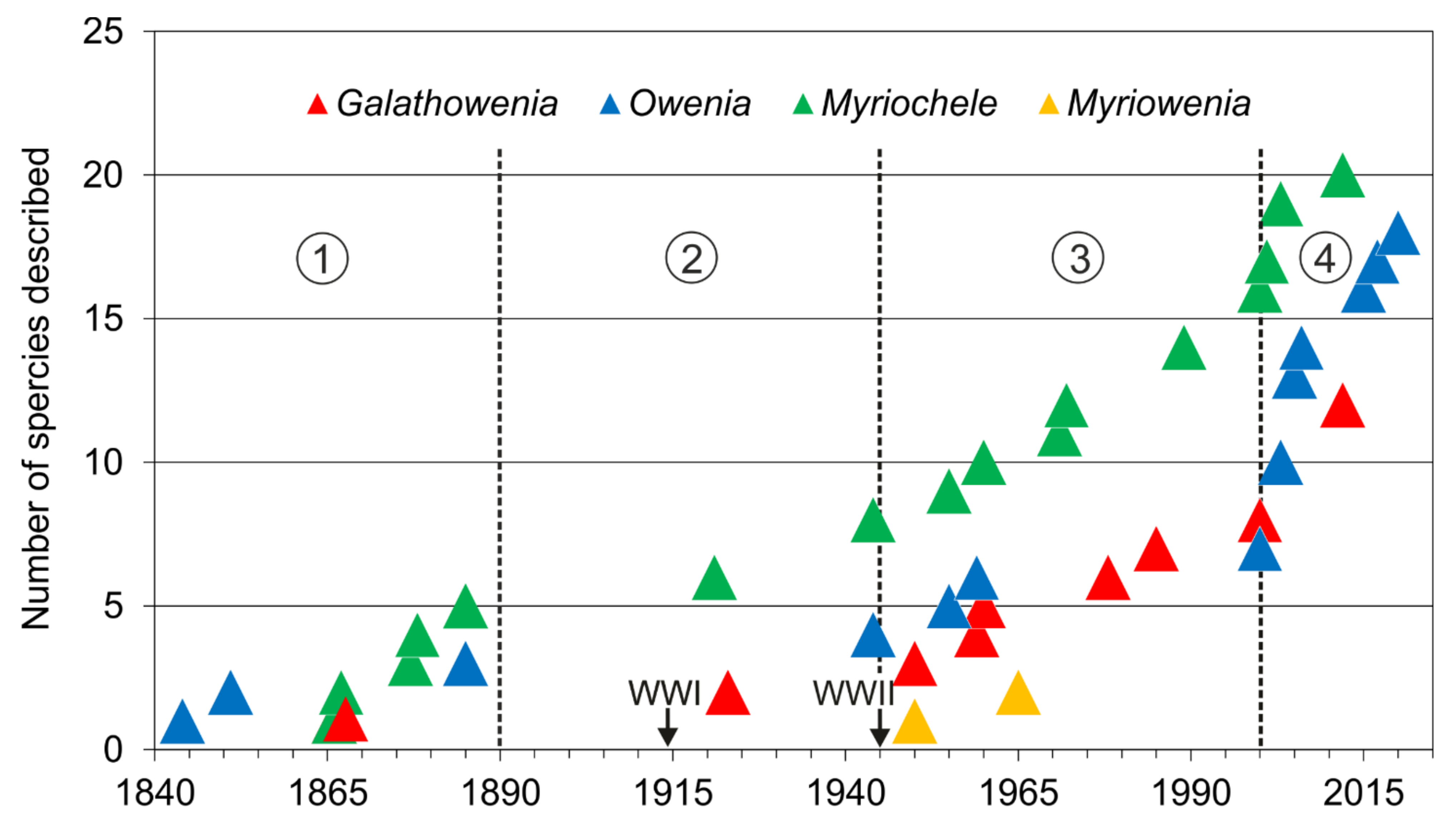
The first described oweniid was Owenia fusiformis Delle Chiaje, 1844. The genera Owenia and Myriochele are the most speciose containing 19 and 20 species respectively [85]. Galathowenia comprises 12 species with only one species described in the 19th century (Galathowenia australis (Grube, 1866), as Psammocollus). Finally, Myriowenia comprises only four species and was erected well into the 20th century.
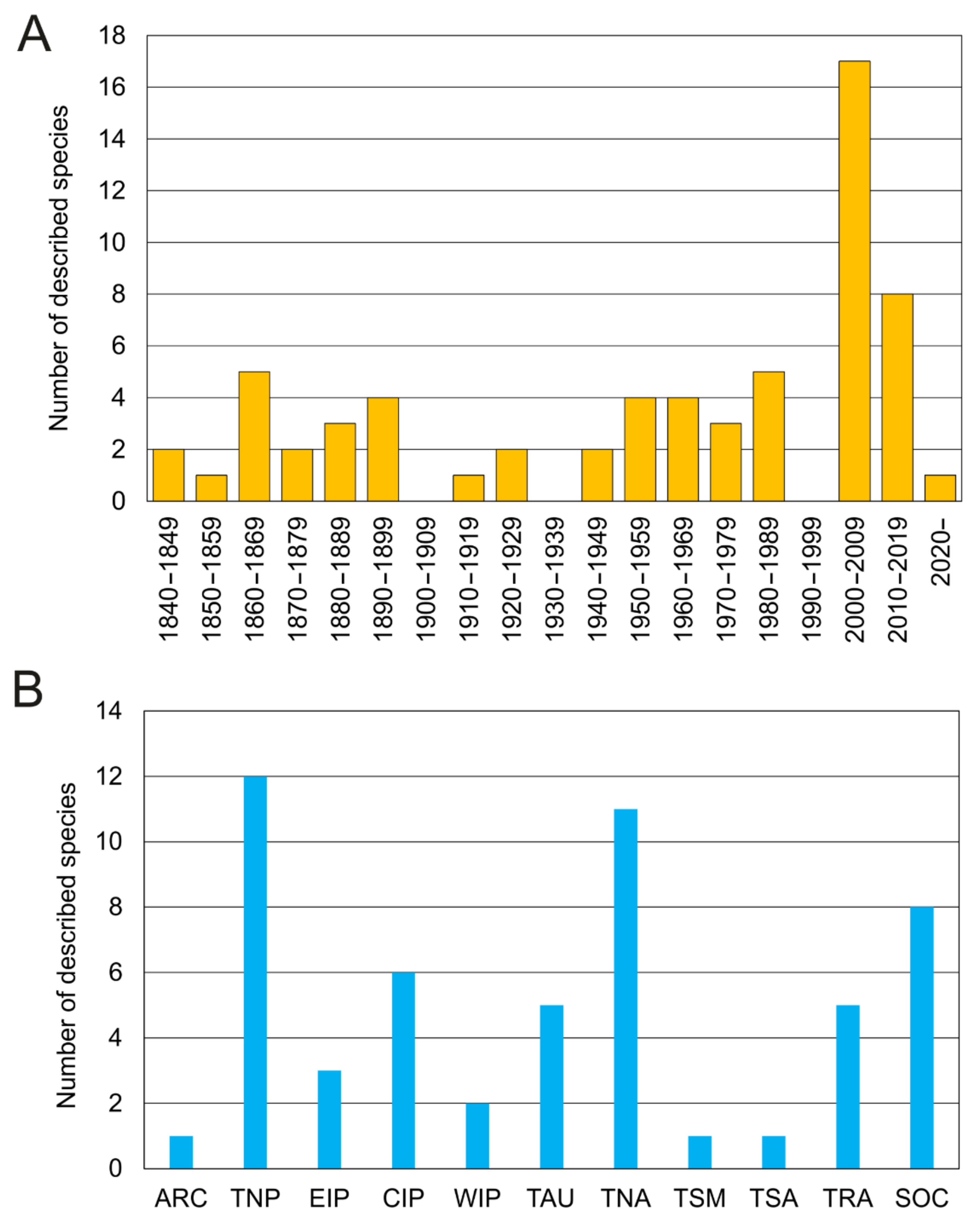
Taxonomic studies on the family are closely related to significant historical events (Figure 12A and Figure 13). For instance, a number of species were described from the mid-19th century to early-20th century (i.e., three Owenia, five Myriochele, one Galathowenia), followed by almost non-existent progress extending to the end of WWII (in which only Myriochele picta Southern, 1921 and Galathowenia oculata (Zachs, 1933) were described). From 1945, studies increased substantially; Myriowenia was erected by Hartman in 1960 and further species were described belonging to Myriochele and Galathowenia. However, the number of Owenia species did not increase in the same way and this is perhaps due to the belief that O. fusiformis was cosmopolitan. Finally, the 21st century is witnessing a substantial increase in number of new oweniids described, influenced heavily by the reconsideration of the status and past records of both O. fusiformis and M. heeri.
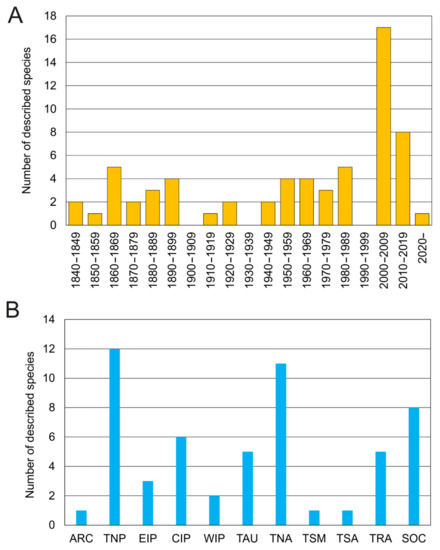
Figure 12.
(A) Number of oweniid species described per decade; (B) all oweniid species currently consider valid [85] listed under the bioregion (sensu [86]) of their type locality. Abbreviations: ARC—Arctic; TNP—temperate North Pacific; EIP—Eastern Indo-Pacific; CIP—Central Indo-Pacific; WIP—Western Indo-Pacific; TAU—temperate Australasia; TNA—temperate Northern Atlantic; TSM—temperate South America; TSA—temperate South Africa; TRA—tropical Atlantic; SOC—Southern Ocean.
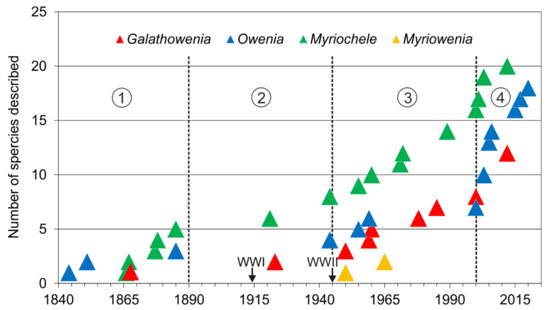
Figure 13.
Number of described species (accumulated) of each oweniid genus. Encircled numbers indicate relevant historic moments (separated by discontinuous lines): (1) from first described species to 1890; (2) 1890 to the end of WWII in 1945; (3) 1945 to 2000; (4) 2000 to the present.
The taxonomic bibliography of the group includes several regional studies that include information about genera/species, keys and/or comparative tables of distinguishing characters. Among the most relevant are Fauvel [151] on Atlantic and Mediterranean French coasts, Ushakov [152] in the USRR, Hartman [153] in Antarctica, Day [154] in South Africa, Milligan [72] in the Gulf of Mexico, Nilsen and Holthe [65] in Scandinavia, Imajima and Morita [76] in Japan, Hartmann-Schröder [155] in Germany, and more recently Blake [71] in California, Cantone and Di Pietro [61] and Parapar [62,63] in Antarctica, Parapar [67,68] in Iceland, Gil [156] from European coasts and Capa et al. [17] in Australia.
3.2.3. Taxonomic Characters
The shape of the body is consistent across members of the family, i.e., elongated, cylindrical and fairly rigid [71,157]. The prostomium and peristomium are fused forming the head [65,83] and its shape differs across the genera: distally truncated without palps (Galathowenia), distally rounded without palps (Myriochele), rounded and provided with a pair of grooved tentacles (Myriowenia) and with multi-lobed tentacles, in the shape of a tentacular crown (Owenia) (Figure 14 and Figure 15). These head structures are generally used for particle selection and tube building (e.g., [158,159,160,161]), and are homologous between them and seemingly also to the palps of magelonids [161]. However, we consider further work is needed to assess the homology of the head appendages of members of these two groups.

Figure 14.
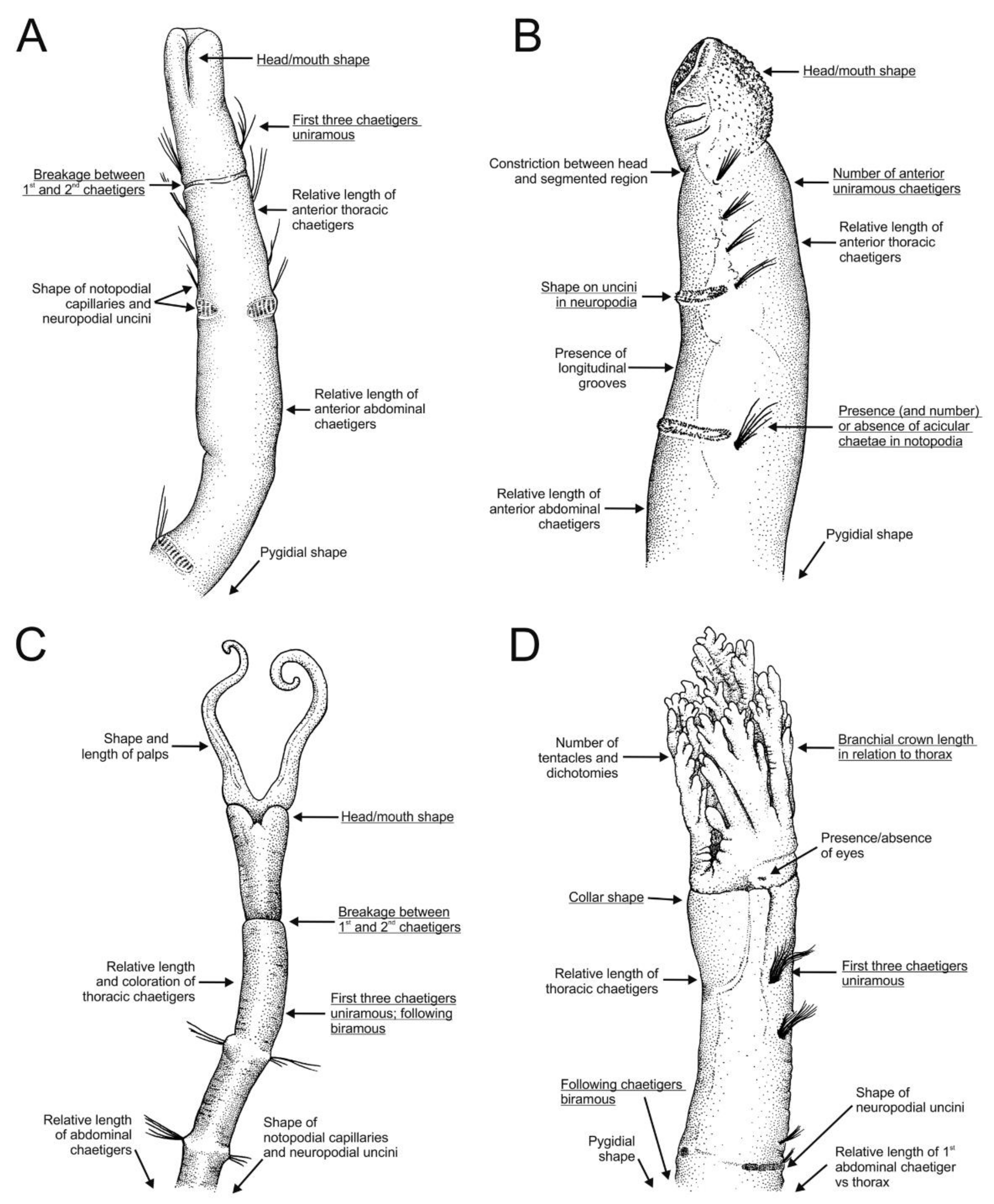
SEM micrographs of anterior end of representative species of all Oweniidae genera showing main diagnostic characters. (A) Myriochele olgae, ventral view; (B) M. heeri, lateral view; (C) Galathowenia quelis, ventral view; (D) Myriowenia sp., lateral view; (E) Owenia fusiformis, lateral view. (A,B) Modified after Parapar [68], (C,D) after Capa et al. [17]. Chaetigers number provided. Abbreviations: Clob—crown lobe, Co—collar, Gr—groove, H—head, Llg—longitudinal lateral groove, M—mouth, Neuro—neuropodium, Noto—notopodium, Pa—palps.
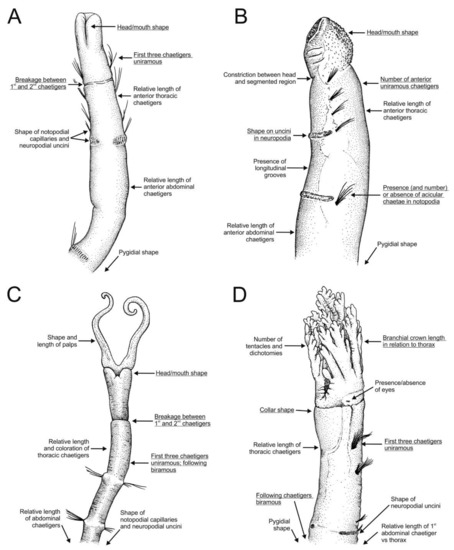
Figure 15.
Stylised drawings of anterior region of representative species of all Oweniidae genera showing main diagnostic characters both of genus (underlined) and species. (A) Galathowenia quelis Capa, Parapar and Hutchings, 2012 in ventral view; (B) Myriochele australiensis Hartman, 1955 in lateral view; (C) Myriowenia sp. in ventral view; (D) Owenia australis Ford and Hutchings, 2005 in ventro-lateral view. (A) Redrawn after Parapar and Moreira ([78]; (B) after Blake [71]; (C) after Hartman [95]; (D) after Ford and Hutchings [77].
The mouth and lips are differently developed among members of Oweniidae; Myriochele, Myriowenia and Owenia are provided with a button-hole mouth with shallow lips (Figure 14B–D); while Galathowenia bears a cleft directed backwards from the mouth, forming two large lateral lips (Figure 14A and Figure 15A) (see [17,56]). An epithelial fold between the head and the first segment, often referred to as a collar is common in some oweniid species [60,68,71,76,79] (Figure 14E and Figure 15D).
Posterior to the head, the trunk, consists of several chaetigers (up to 30 in species of Galathowenia and Myriochele, and up to 60 in species of Owenia and Myriowenia; [17]). The anterior three chaetigers are provided with uniramous parapodia whilst the following chaetigers are biramous (Figure 14 and Figure 15). These regions have been previously referred to as thorax and abdomen, respectively (e.g., [65,71]), names that have recently been discouraged (e.g., [17,67,80]). The number and relative lengths of uniramous chaetigers is species specific and is one of the main taxonomic features used for species discrimination, as was shown by Parapar et al. [68] in the review of Myriochele (and the then accepted Myrioglobula) in Icelandic waters. It is common to find species descriptions including a formula indicating the relative length of uniramous (or else three anterior) segments, for example, for Galathowenia annae Capa, Parapar and Hutchings, 2012 the following formula is given RLUS = 1:2:1, where the second chaetiger is twice the length of the first and third [17]. Genera specific patterns can also be observed, especially in members of Myriowenia and Owenia, which always possess three uniramous chaetigers, while Galathowenia have two or, more commonly, three uniramous segments. Species of Myriochele show a wide range of variability for this feature from species with a single uniramous chaetiger (e.g., Myriochele islandica (Parapar, 2003)), others with two (e.g., Myriochele olgae Blake, 2000) and others with even three uniramous chaetigers (e.g., M. heeri). In some oweniids additional segments are added before the pygidium, thus chaetiger number is highly dependent on age. Biramous chaetigers are unequal in length, the anteriormost being longer and usually those towards the pygidium becoming progressively shorter (e.g., [17,67,80]).
Oweniids possess ciliated, longitudinal lateral grooves, running between the noto- and neuropodia, homologous to the lateral organs of other annelids (Figure 14B, [17,161]). In addition, glandular longitudinal grooves (lacking cilia) are present on ventral body surfaces, while there is a non-glandular and non-ciliated groove running along the mid-dorsum [17,62], however, none of these characters are used in taxonomic descriptions.
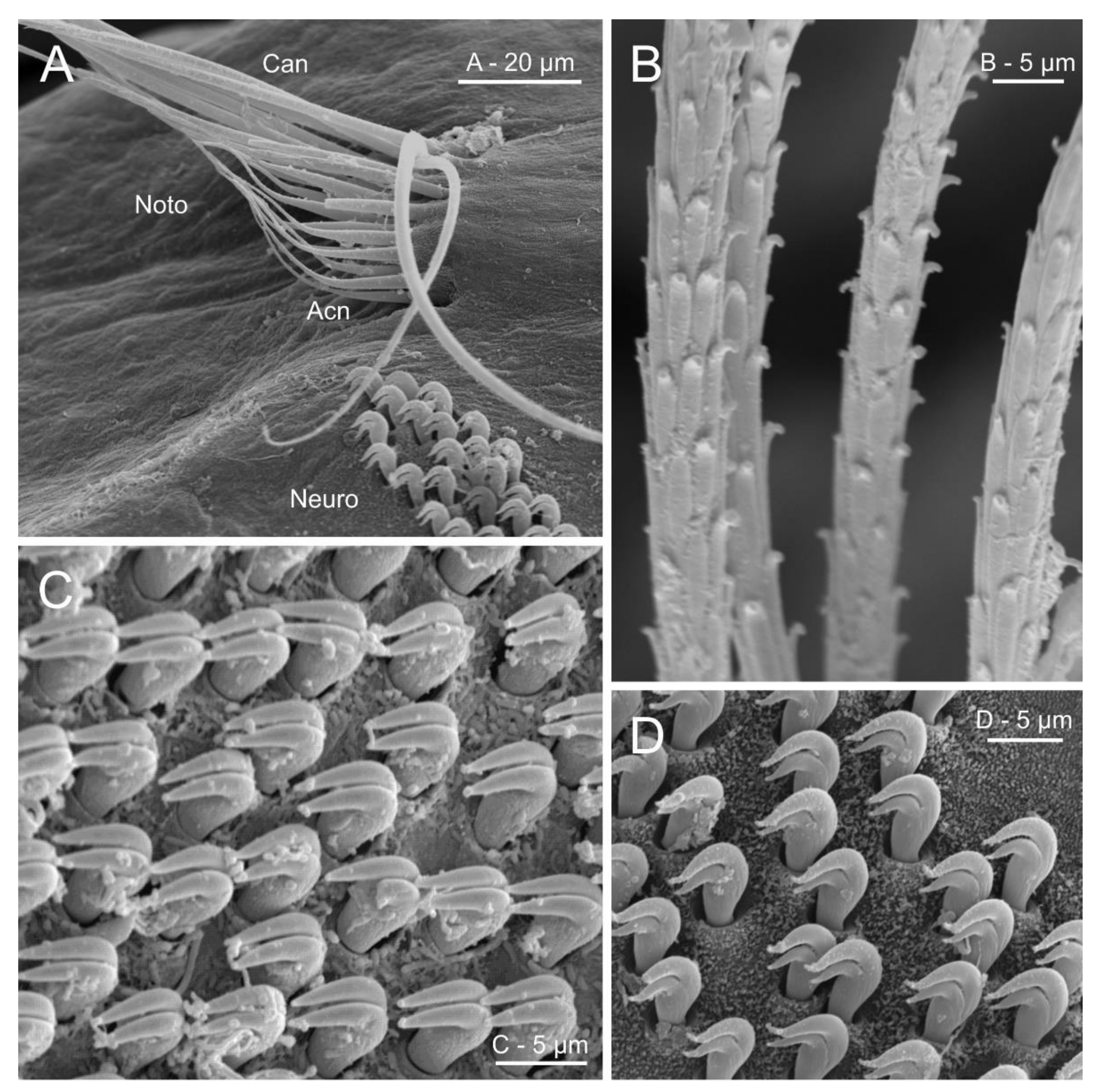
Parapodia are conspicuously reduced in oweniids compared to other annelids, including the sister group Magelonidae. They lack structures such as cirri, branchiae or epidermal papillae (Figure 16A). The notochaetae emerge directly from the body wall and neurochaetae from low glandular transverse tori [162]. Notochaetae include fine distally tapering capillaries, known as “capillary chaetae” in Galathowenia, Myriowenia and Owenia (Figure 16A,B) [17,77], that coexist with the shorter and comparatively more robust chaetae, with a narrow distal tip, known as “acicular chaetae ”, in Myriochele [17,62,65,67,68,76] (Figure 16A). Capillary chaetae show external structures under SEM like densely packed scales. Morphometric analyses of these scales have shown value for identification of species of Owenia (e.g., [163,164]), although some authors found the intra- and interspecific variation to overlap [77]. Neurochaetae come in the shape of dentate hooks with long shafts (i.e., uncini) provided with two distal teeth, homologous to the capitium of other annelids but lacking instead a true rostrum (Figure 14A,B, Figure 15A–C and Figure 16C,D) [57]. Uncini are arranged in multiple transverse rows per segments [146] forming the uncinal patches (Figure 16A). Uncinal morphology has been long used for generic assignment. Species of Galathowenia possess teeth arranged in an oblique row (with one tooth higher than the other). Teeth are generally arranged vertically (one on top of the other) in members of Myriochele and Myriowenia, and horizontally (teeth occurring side by side) in Owenia species [57]. Morphometric analyses with the aid of SEM have also been performed within members of Owenia in order to find distinct traits to separate species among this morphologically homogenous group [77,163,164].
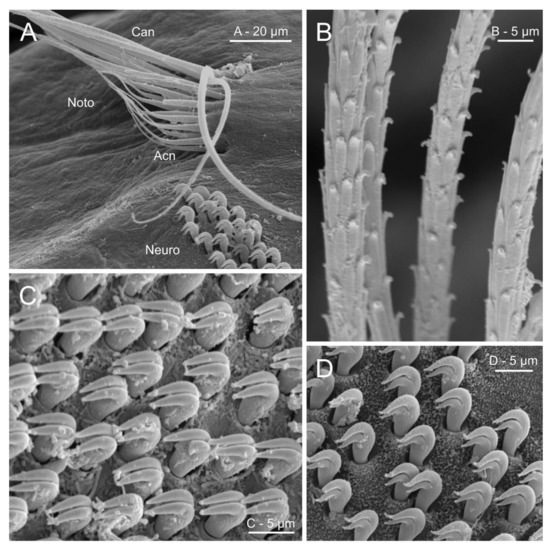
Figure 16.
SEM micrographs illustrating several chaetal characters in Oweniidae. (A) Anterior abdominal chaetiger in Myriochele olgae; (B) detail of thoracic notochaetae scale covering of Owenia fusiformis; (C) neuropodial uncini of O. fusiformis; (D) neuropodial uncini of M. olgae. Abbreviations: Acn—acicular notochaetae, Can—capillary notochaetae, Neuro—neuropodium, Noto—notopodium.
Oweniids display a variability of pygidial shapes. They may be simple, bilobed, multilobed or pointed (Figure 17) [57] showing intrageneric variability but being species specific [62]. Even if reported, the presence of true pygidial cirri has not been confirmed yet and the structures reported for Myriowenia gosnoldi Hartman, 1965 may be related to body regeneration after breakage [17].
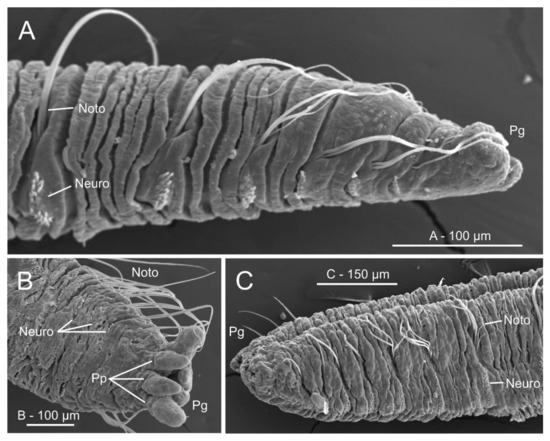
Figure 17.
SEM micrographs of posterior end of (A) Galathowenia oculata; (B) G. fragilis; (C) Myriochele heeri. (B,C) modified from [68]. Abbreviations: Neuro—neuropodium, Noto—notopodium, Pg—pygidium, Pp—pygidial papillae.
With genera being fairly homogenous morphologically there has been a reconsideration of the number and identity of morphological characters used in oweniid taxonomy and characterisation of species compared with early studies (Figure 18). For instance, the unique combination of approximately eight characters were needed to identify species prior to 1970, which were visible under a stereo or optic microscope (but see [165]). In contrast, there are roughly twice as many characters needed to correctly identify oweniids to species currently (e.g., [65]). While the same characters are considered across the family due to the relative homogeneity in the oweniid body shape, the anterior end shows more variation among genera and is therefore more useful for species characterisation. However, for all members of Oweniidae, the use of a SEM is essential, and as mentioned before, very often morphometric analyses are needed for species discrimination [77,81,163,164]. Branching patterns of the head tentacles have also been shown to bear taxonomic information for some Owenia species [78].
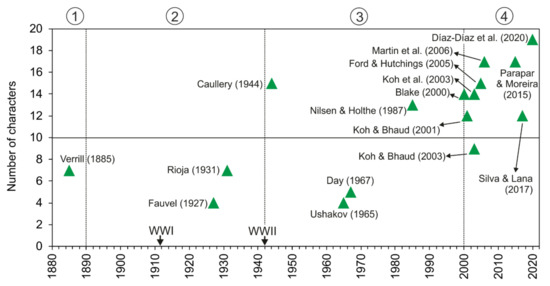
Figure 18.
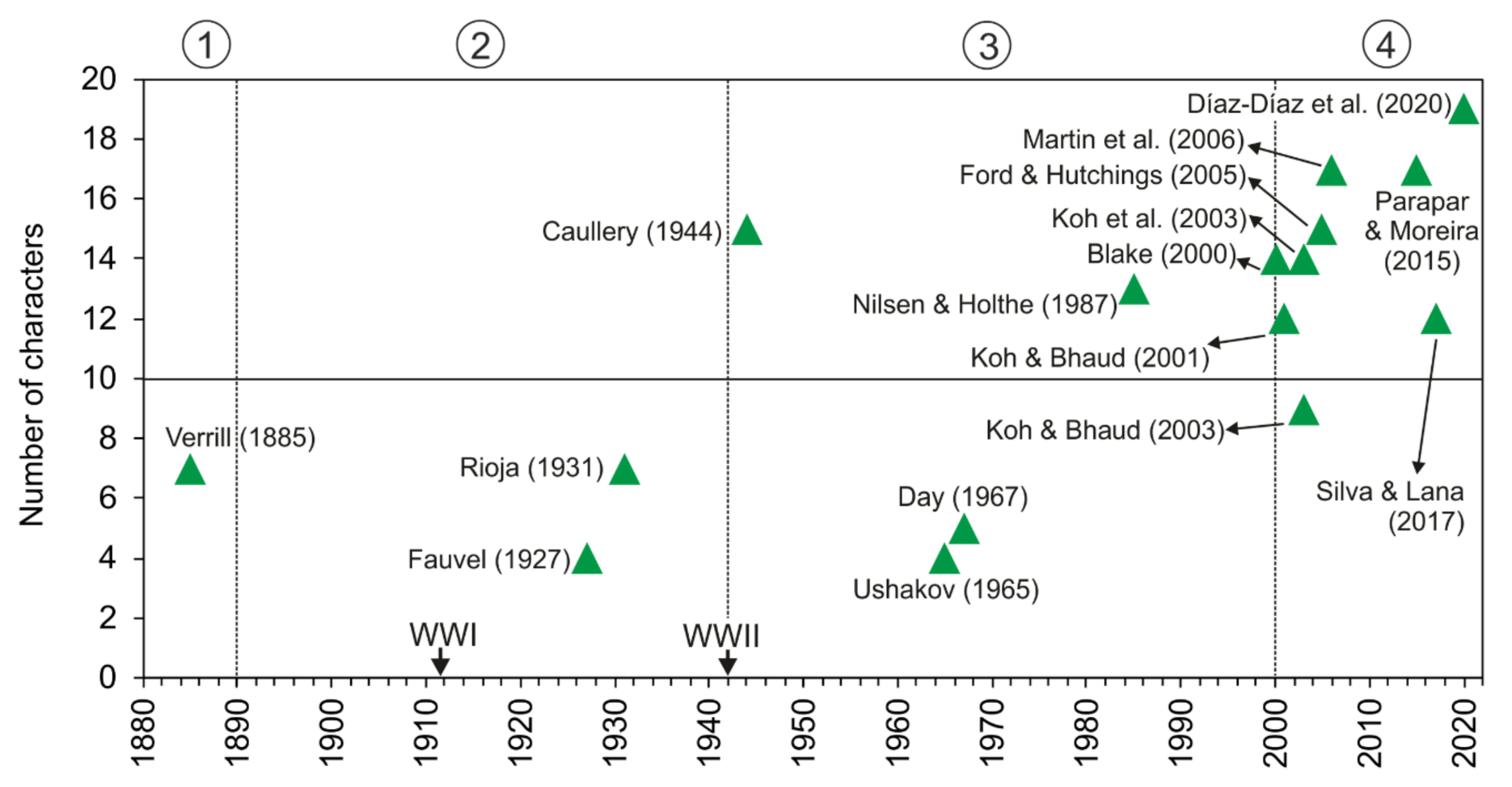
Number of characters (accumulated) used in the description of Owenia species. Encircled numbers denote relevant historic moments (separated by discontinuous lines): (1) from the first described species to 1890; (2) 1890 to the end of WWII in 1945; (3) 1945 to 2000; (4) 2000 to present. Species described in each paper corresponds to O. fusiformis or to the original description of the taxon. Verrill [166] (O. artifex); Fauvel [151] (O. fusiformis); Rioja [167] (O. fusiformis); Caullery [165] (O. assimilis); Uschakov [152] (O. fusiformis); Day [154] (O. fusiformis); Nilsen and Holthe [65] (O. fusiformis); Hartmann-Schröder [155] (O. fusiformis); Blake [71] (O. johnstoni); Koh and Bhaud [75] (O. gomsoni); Koh and Bhaud [66] (O. petersenae); Ford and Hutchings [77] (O. australis); Martin et al. [81] (O. persica); Parapar and Moreira [78] (O. picta); Silva and Lana [164] (O. caissara); Díaz-Díaz et al. [168] (O. vieitezi).
The use of methyl blue stain and the different colouration body patterns in Owenia is being increasingly used (e.g., [71,164]) although its use as a taxonomic character in the characterisation of the species is not generally used in recent works (e.g., [74,78]).
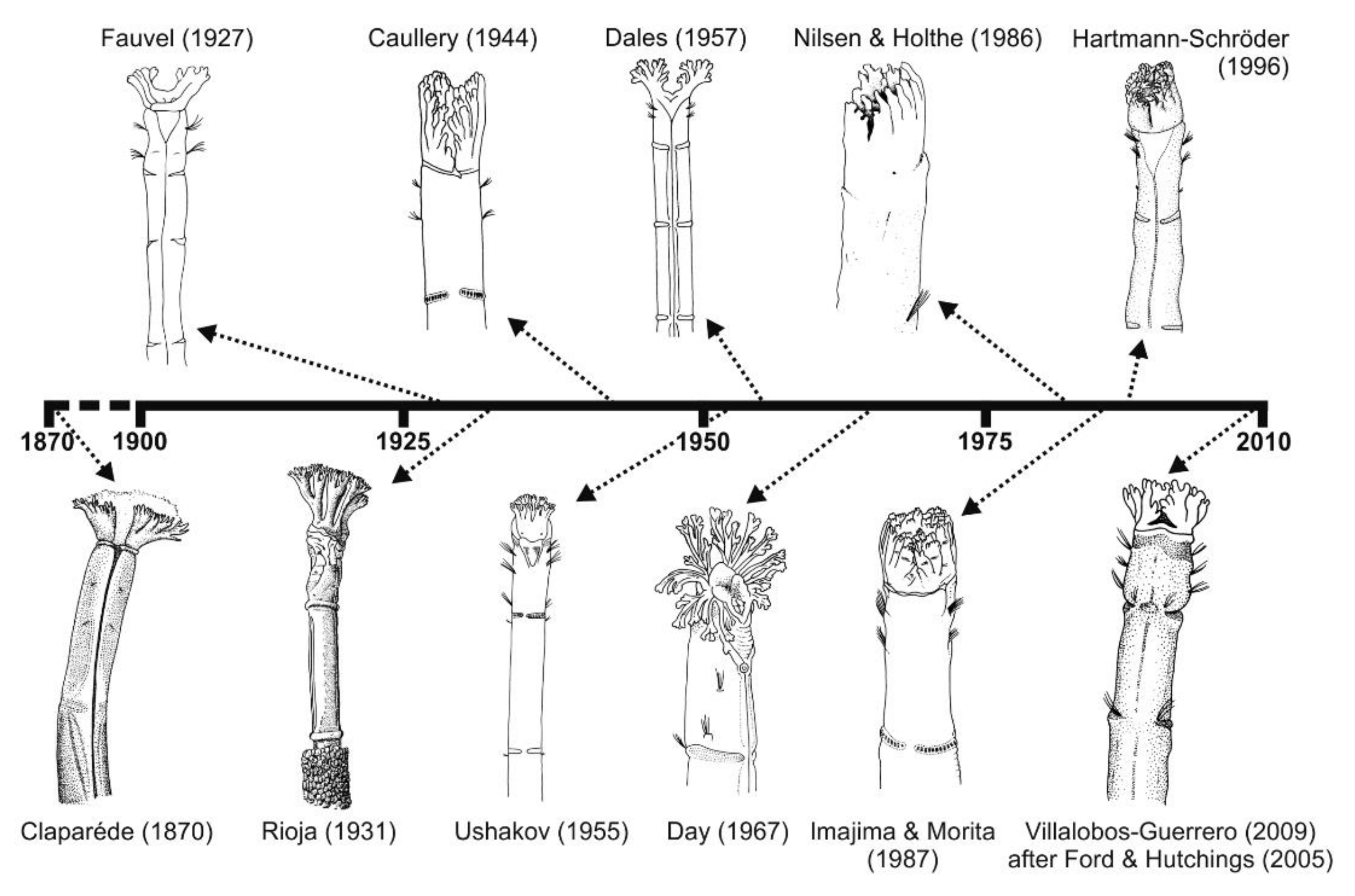
Iconography has also shown much variation across the literature through time (Figure 19), even showing evident differences in artistic quality. For instance, drawings and illustrations by Rioja [167] and Day [154] are generally superior to those found in some of the later works. In the case of M. heeri, accurate and artistic illustrations can, however, be found in early work (e.g., [169]).
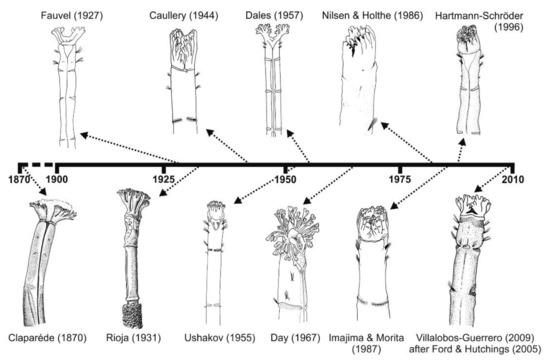
Figure 19.
Selection of illustrations of anterior body end of Owenia fusiformis through time. All redrawn from original.
3.2.4. Internal Morphology
The first studies on internal anatomy date back to the beginning of the 20th century [158] but are still mostly limited to a handful of species such as those referred as O. fusiformis and M. heeri [158,170,171,172], the microanatomy of the central nervous system has received more attention due to its relevance in understanding the phylogeny of this group, and of annelids in general (see below).
The body wall in Oweniidae is composed of longitudinal muscles only, which are developed and grouped into bundles that gradually decrease in thickness [173,174]. The coelomic lining of the body wall is composed of a layer of monociliated peritoneal cells that cover the muscle cells except near the ventral and dorsal mesenteries, where longitudinal muscles form the lining [173,175].
The use of histological sectioning (HIS) (Figure 20A,B) and micro-computed X-ray tomography (micro-CT, or µCT) (Figure 20C,E and Figure 21) has allowed for a re-examination and better assessment of the annelid internal anatomy [176]. Internal coelomic space is occupied mostly by the gut (Figure 20A,B and Figure 21B–D), which is composed of three regions: foregut (esophagus and stomach), midgut (intestine) and hindgut (rectum), which can be easily distinguished histologically [177]. The anteriormost region of the gut of Owenia, Myriochele and Myriowenia has been described or illustrated as having a ventral pharyngeal organ [59,65,68,155,158,159,160,161,178]; this organ has often been reported incorrectly in previous work as a proboscis (e.g., [71]). Correspondingly, protractible ciliary folds on the dorsolateral walls of the foregut were observed in Galathowenia, Myriochele and Myriowenia [17,68,178]. The buccal region and the esophagus are lined by ciliated epithelial cells interspersed with glandular cells that secrete digestive enzymes; stomach walls are thick and involuted (Figure 20B) containing two types of numerous glandular epithelial cells [160]. The intestine lacks glandular cells and serves purely for absorption. The rectum is thin-walled and less convoluted; faecal pellets are mostly composed of sand grains and other material that are released when the animal reverses into its tube [160]. The gut musculature is responsible for the movement of material through the gut and is helped by contraction of the body wall muscles [57].
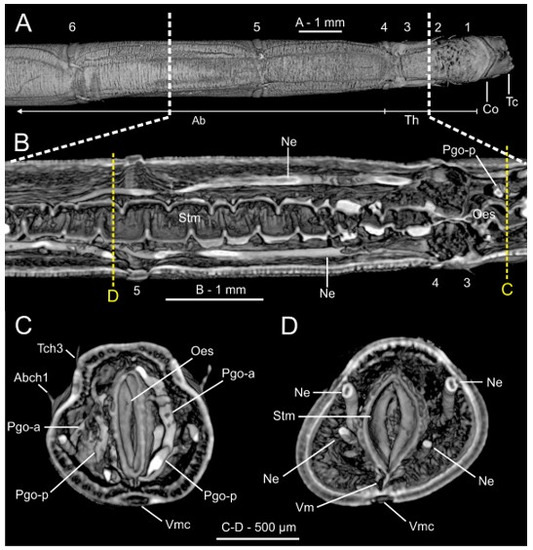
Figure 20.
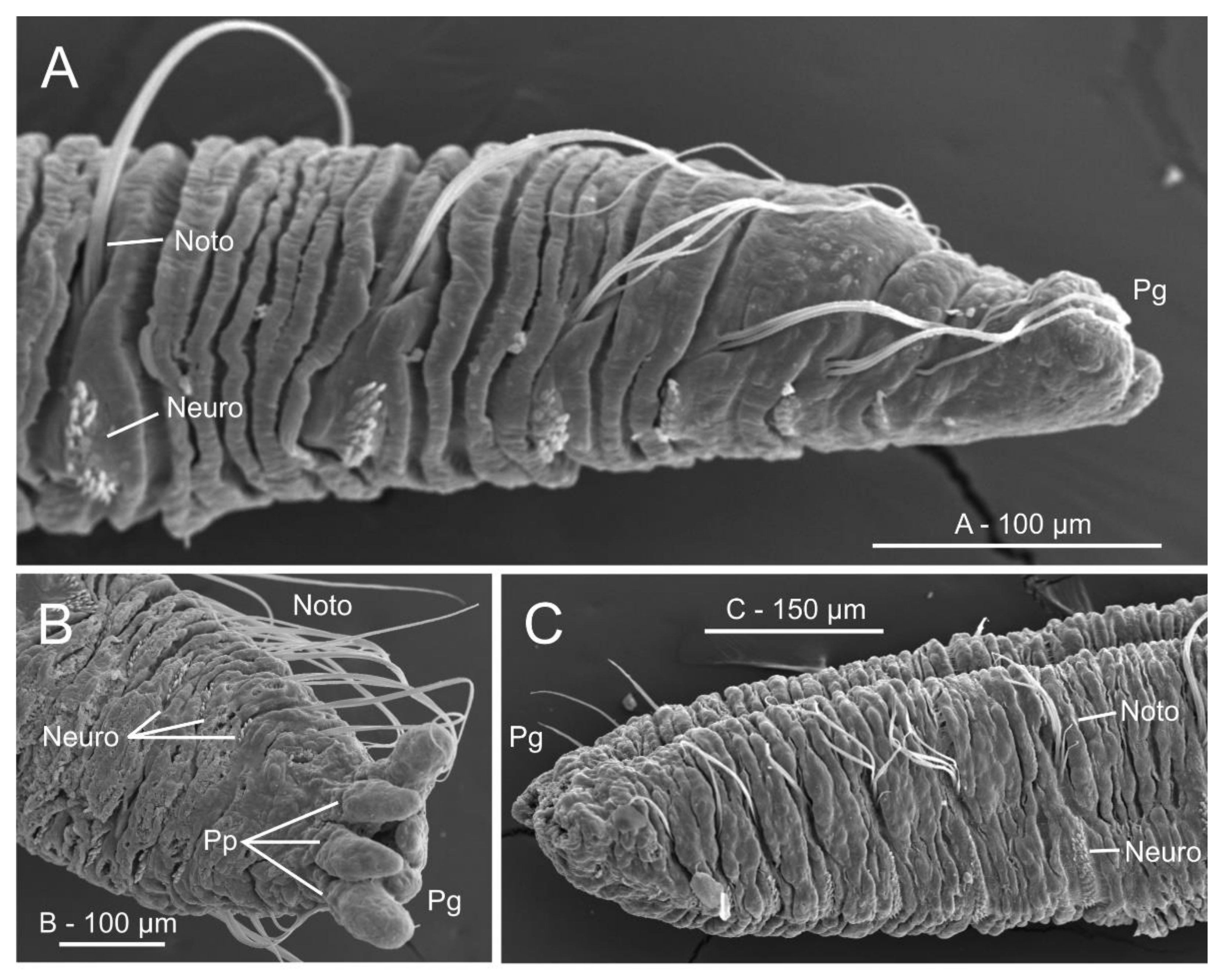
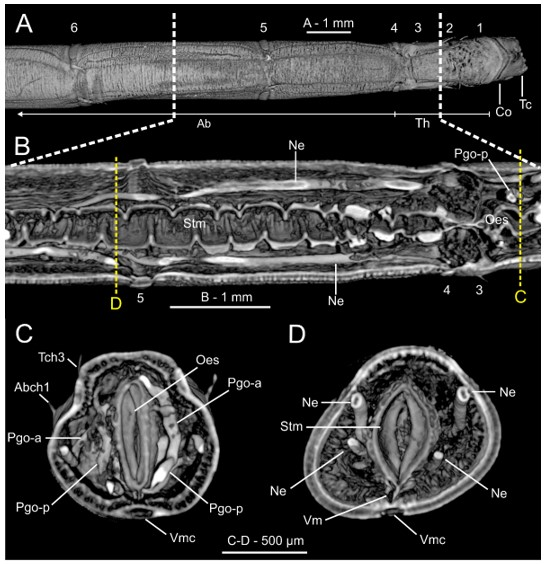
Micro-computed tomography (μCT) sections of Owenia fusiformis (MNCN 2866). (A) Dorsal view of body surface, (B) frontal and (C–D) transversal body sections showing internal anatomy. White discontinuous lines in (A) marking body region showed in (B). Yellow discontinuous lines in (B) marking position of transversal sections in (C) and (D) respectively. Chaetigers number provided. Abbreviations: Ab—abdomen, Abch1—abdominal chaetiger 1, Co—collar, Lml—longitudinal muscle layer, Ne—nephridial sac, Oes—oesophagus, Pgo-a/p—parapodial glandular organ—anterior/posterior, Stm—stomach, Tc—tentacular crown, Tch3—thoracic chaetiger 3, Th—thorax, Vm—ventral mesentery, Vmc—ventral medullary cord. Numbers indicate chaetigers.
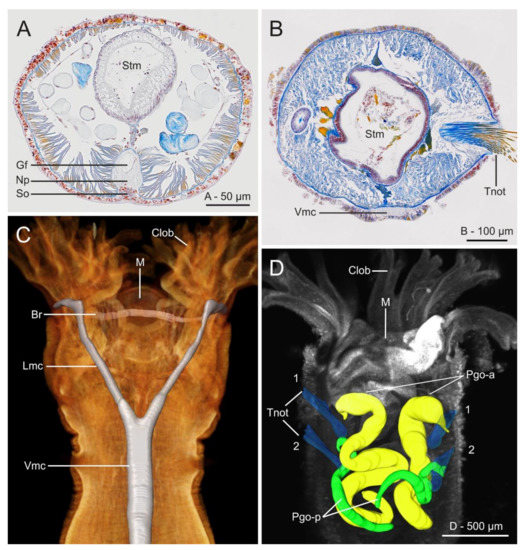
Figure 21.
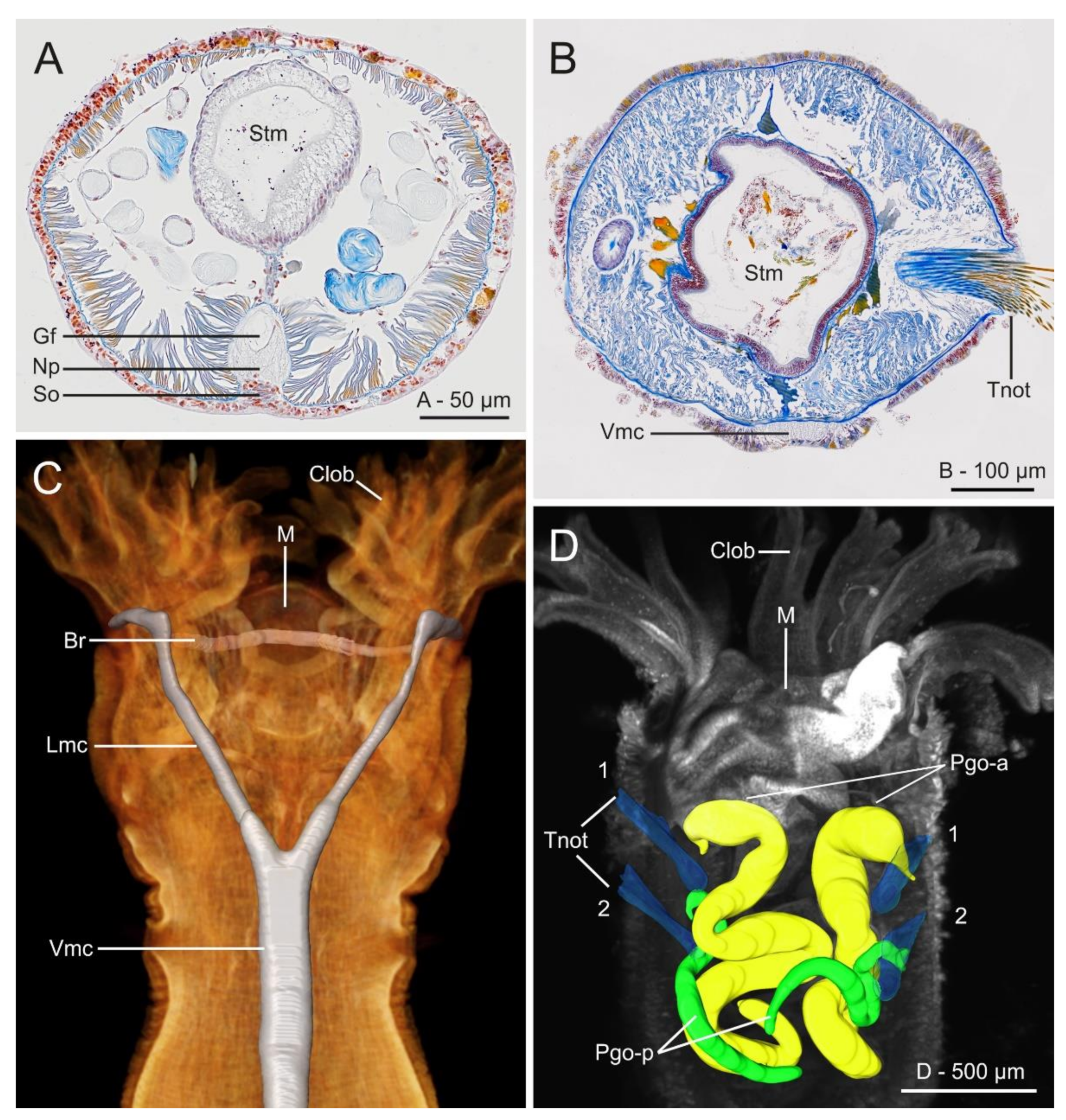
Histological cross sections (azan staining) at gut level of (A) Myriowenia sp. and (B) Owenia fusiformis; (C) micro-computed tomography (μCT) volume rendering and 3D-reconstruction of the nervous system of O. fusiformis; (D) 3D reconstruction of parapodial glandular organs (Pgo); anterior pair are in yellow, posterior ones are in green; notochaetae in blue. (A–C) From Beckers et al. [161]; (D) from Rimskaya-Korsakova et al. [148]. All modified from the original. Abbreviations: Br—brain ring, Clob—crown lobes, Gf—giant fibre, Lmc—lateral medullary cord, M—mouth, Np—neuropil, Pgo-a/p—parapodial glandular organ—anterior/posterior, So—somata, Stm—stomach, Tnot—thoracic notochaetae, Vmc—ventral medullary cord. In (D) first two thoracic chaetigers are indicated.
The nervous system shows some unusual features when compared to other annelids and is currently considered in phylogenetic studies [2]. It is largely intraepidermal with a well-developed nerve plexus, including many peripheral nerves but lacks segmental ganglia [179,180]. The absence of nuchal organs [172] a condition shared with the Magelonidae, could be regarded as plesiomorphic among the annelids and supporting their basal position at the base of the annelid tree [4]. However, further investigations are needed to truly verify their absence [57,181]. Eyespots are usually present in the head of adults and are closely associated to the brain [182] but it is still unclear whether they develop from the prostomium or the peristomium [17]. Helm et al. [2,183] compared the development of oweniid neuroarchitecture with that of other annelids based on histology, SEM and immunohistochemistry and found that development and metamorphosis of the mitraria larva is mostly similar to that of other annelids irrespective of the drastic changes in body shape during metamorphosis. The central nervous system in Oweniidae is medullary, with a ring-shaped and basiepidermal brain lacking higher brain centers, ganglia associated to ventral nerve cord or complex sensory organs (Figure 21A–C) [147,148,161]. In Owenia borealis Koh, Bhaud and Jirkov, 2003, the glandular structures located in the first two segments are parapodial organs (Figure 20B,C and Figure 21D) which contain secretory cells producing the tube materials. Their relationship with the nephridial sacs located in first chaetigers of the abdominal region remains obscure and warrants further investigation [148].
3.2.5. Species Diversity and Distribution
The family Oweniidae is currently composed of 55 valid species in four genera, 16 species in genus Galathowenia, 18 in Myriochele, 19 in Owenia (plus three taxon inquirendum) and two in Myriowenia [57,85]. The first species to be described was Owenia fusiformis Delle Chiaje, 1844 from Sicily and the group was raised to the rank of family by Rioja [184].
Oweniids have colonised a wide range of marine habitats across the world, from the intertidal fringe to the deep sea [185,186,187] (Figure 12B), occurring in all marine ecoregions (sensu [86]). Literature reviews show Galathowenia, Myriochele and Owenia to have broad distributions; Myriowenia appears restricted to the Atlantic and Pacific coasts of North and South America (Figure 22). There are, however, apparent gaps in the knowledge of diversity and distribution for all genera. According to the type localities of all described species, the temperate East Northern Atlantic (with nine species described thus far) appears to be as well-known as temperate Australasia (eastern coast of Australia); however, in contrast, data are still lacking for Eastern Indo-Pacific, Temperate South America and South Africa, and Western Indo-Pacific (Figure 22).
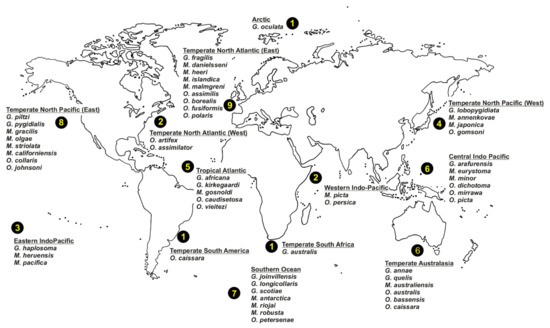
Figure 22.
World map showing number of valid oweniid species (sensu [85]) described per bioregion (sensu [86]).
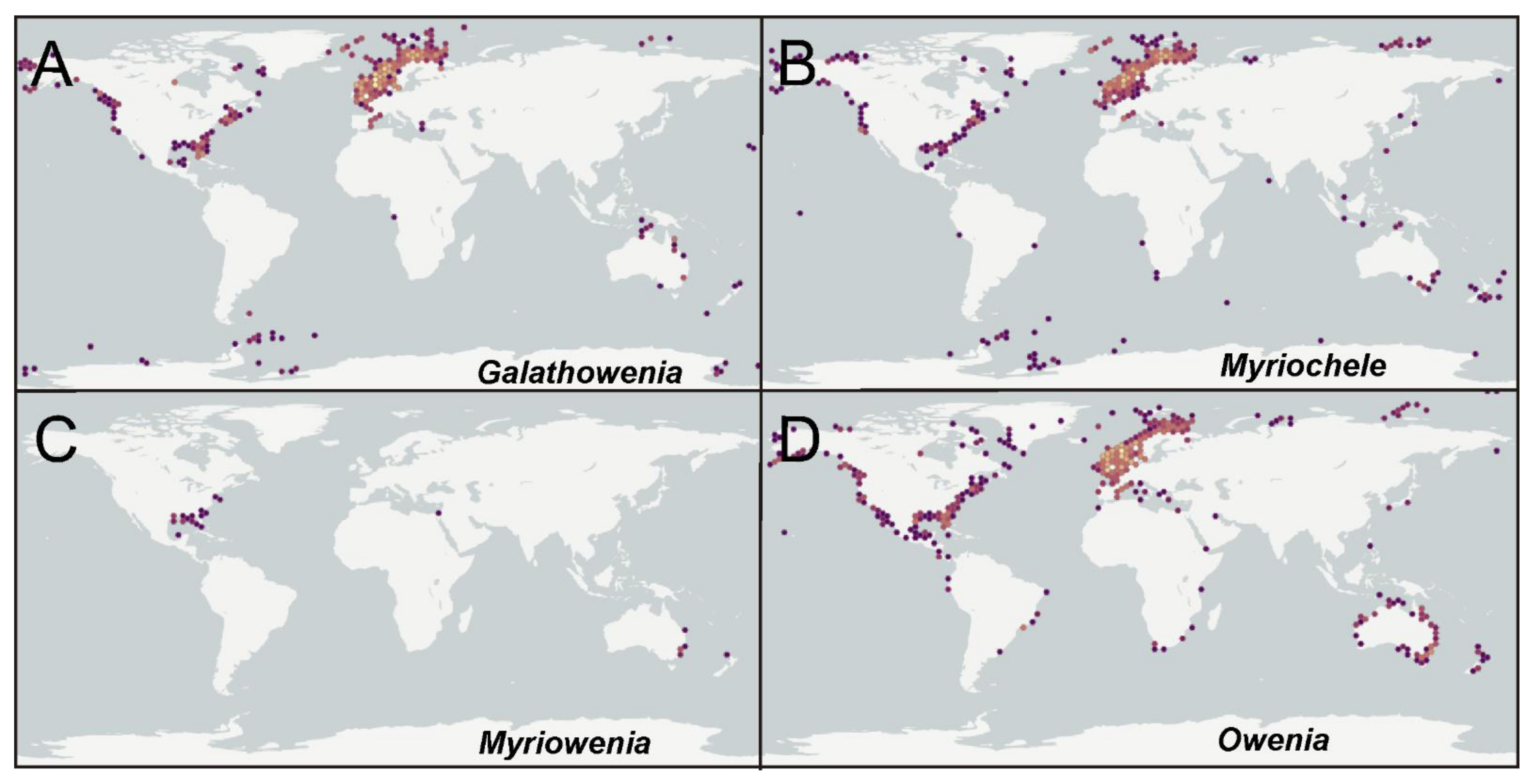
According to the international network and data infrastructure GBIF [118] there are up to 112,695 records for this family. Records are heterogeneously distributed, with many reports concentrated in Atlantic and Pacific North America, the Arctic and European Atlantic, the Australian Indo-Pacific and New Zealand; reports are fewer in comparison for the Subantarctic Scotia Arc, the Mediterranean Sea and some European and North American areas. These records correspond mostly to the genera Galathowenia (47%), Myriochele (17%) and Owenia (34%) (Figure 23A–D), and particularly to a few numbers of species considered previously as cosmopolitan (see below). Myriowenia records account for just 0.1% of all references and those are restricted to North American Atlantic and Australian Pacific (Figure 23C). Regarding Galathowenia, the most reported species are Galathowenia fragilis (Nilsen and Holthe, 1985) and G. oculata, particularly from North America and Europe (Figure 23A); a similar distribution pattern is found for Myriochele, mainly represented (83% of records) by M. heeri, Myriochele danielsseni Hansen, 1879 (both mostly in Europe), and, to a lesser extent, by M. olgae. Furthermore, Galathowenia and Myriochele are the only genera reported so far from subantarctic latitudes. Owenia is also often reported from the northern Hemisphere but also shows many records from Australia and New Zealand (Figure 21D); O. fusiformis accounts for up to 80% of the reports while Owenia collaris Hartman, 1955 is the most reported taxon from the North America Pacific. However, it is likely that further sampling and examination of oweniid collections from across Australasia and Pacific regions would reveal new undescribed taxa.
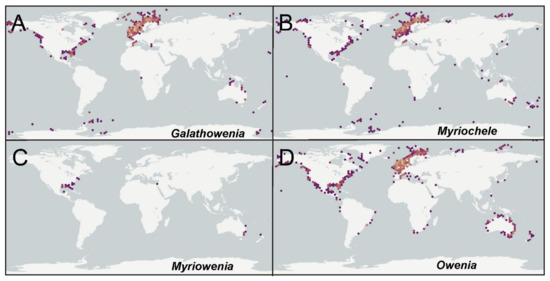
Figure 23.
World distribution of all oweniid genera. (A) Galathowenia; (B) Myriochele; (C) Myriowenia; (D) Owenia. Source: GBIF [118] (https://www.gbif.org).
Several species such as O. fusiformis, G. oculata and M. heeri, have been traditionally reported as cosmopolitan or broadly distributed; recent available data suggests that most of these species fit better within the concept of complexes of morphologically similar species showing narrow ranges of distribution in regard to environmental features (e.g., [63,66,67,75,77,79,80,188,189,190,191,192,193]). However, the only study that has included molecular information to assess this hypothesis was that of Jolly et al. [194] who analysed several populations of Owenia in the northeast Atlantic, considering COI sequences and concluding that several species exist there that are, in turn, distinct from those occurring in the Mediterranean Sea.
3.2.6. Biology and Ecology
Knowledge about reproductive and developmental strategies is restricted to species of Owenia (summarised in [57]). Mature animals are dioecious [83]. It has been indicated that reproduction occurs once or several times a year depending on the geographical location and environmental conditions. For instance, some populations of O. fusiformis in NE Atlantic were found showing discrete seasonal spawning (e.g., [159,195]) while others breed throughout the year [196]. However, this disparity of reproductive modes should be considered with caution as some of the studied animals could have been misidentified as O. fusiformis and, in fact, belong to several different Owenia species inhabiting these waters (e.g., [66,194]). Maturity was reported after the first year of benthic life [195] and fecundity seems correlated with the size of females, but number of spawned eggs can reach up to 85,000 [195,197].
Owenia collaris and O. fusiformis embryos undergo holoblastic spiral cleavage to form a typical coeloblastula [83,198], where the anus is developed from the blastopore and the mouth is later formed from a secondary opening [83,159]. This development pattern shares more similarities to that seen in deuterostomes [83]. In addition, the larval cilial bands are formed later in the ontogenetic stages compared to other annelids, these are also short and simple and are differently arranged to the typical trochophore [83]. This mitraria larva develops completely 24 h after fertilisation in Owenia, having already a defined mouth and anus and the ability to swim. Several morphological changes take place in the larvae for about four weeks, and then metamorphosis starts with the protrusion of the trunk segments from the larval hyposphere, the reduction of the episphere as part of the head, and the disintegration of the ciliary bands [83]. Early juveniles have a head (fused prostomium and peristomium), a uniramous segment with two sets of chaetae, six or seven biramous segments each with a single pair of chaetae and one pair of uncinal tori, and a pygidium. Soon after metamorphosis the juvenile readily builds the tube by gathering materials with the anterior end; after two weeks, pairs of buds start to form the prostomial tentacles typical of adult Owenia. As noted above, Helm et al. [183] show that development and metamorphosis of the mitraria has many parallels to other annelids irrespective of the drastic changes in body shape during metamorphosis.
Asexual reproduction has been suggested in several species of Galathowenia, Myriochele and Owenia due to the presence of breakage grooves in anterior segments [17,57,62,67,199,200], bodies already broken at this level [62,63] or anterior/posterior ends showing different degrees of regeneration [57].
All oweniids are tube dwellers; the tube is flexible and made after mucous secretions binding foreign particles (i.e., sediment grains, foraminiferan thecae, shell fragments) [160,201,202]. Branchial tentacles and buccal lips are involved in tube building (e.g., [158,159,160]). It has been argued as to whether the materials utilised for the tube are preselected, and whether there is any preference for type and size of particles used [64,66,164] or whether the animal simply relies on any available material [67,68]. It appears that tube-building patterns depend on the species under consideration, development and the availability of materials. Experimental work with Owenia caissara Silva and Lana, 2017 has demonstrated that the animal is unable to rebuild the tube if completely released from it [164]; in turn, those partly removed were able to rebuild it using a wide range of sediment particles including filamentous debris, at least in laboratory conditions.
Oweniids are suspension or surface deposit feeders that collect particles at the sediment-water interface [71,160]. Species of Owenia, with a tentacular crown provided with cilia, create water currents that direct suspended particles into the crown and the mouth; Owenia and other oweniids bend over the sediment and collect sand and detritus directly from the bottom surface. Particles are sorted by the highly muscular lips helped by mucus [203]; the shape of the upper lip can be modified in order to facilitate sorting of materials, similarly to that observed for terebellids [204]. Feeding behaviour in Myriowenia has not been studied, though they may gather particles from sediment surface using their long palps [71].
Oweniids are quite common in subtidal soft seabeds, occurring across a wide range of depth, but very little is known about specific species habitats. Data taken from original descriptions of all oweniid species (i.e., Table A2) shows that: (1) only two species have been reported from the intertidal and the number of species at depths of less than 200 m is about 50% of the total; (2) about 20% of species show a wide depth range, from shallow waters to the continental slope realm, reaching 2000 m deep or more; (3) few species (e.g., Myriochele malmgreni) seem to be typical of deep waters. The second group includes the widely distributed G. fragilis, G. oculata, Galathowenia scotiae (Hartman, 1978) and M. heeri, but also recently described species such as O. borealis (Table A2); this suggests again the existence of species complexes or rather frequent misidentifications attributed to those species. In contrast to oweniids (see above), magelonids show a preference for shallow waters, with only handful of species that occur from 400–1000 m. Further work is certainly warranted to clarify depth ranges for most species.
Most ecological knowledge comes from studies done at European latitudes and about O. fusiformis, a species that has been attributed in the past to a cosmopolitan distribution but actually represents a complex of sibling species (e.g., [82,205,206,207]). Owenia fusiformis may reach high densities in sandy sediments, of up to 1000 indiv./m2 [82] while those of the small-sized G. oculata range up to 500–700 indiv./m2 [205,208]; an increase in the abundance of the latter has also been reported during early stages of eutrophication related to mariculture [209]. In the Northeast Atlantic, O. fusiformis may also be the dominant species in terms of biomass in shallow sandy-muddy sediments [210]. In general, the very presence of dense aggregations of oweniid tubes may serve for sediment stabilisation [211,212,213], and allows for an increase in local benthic diversity by favouring larval settlement and providing shelter for other invertebrates as other tube-building polychaetes do [213]. The ability of O. fusiformis to switch between deposit- and filter-feeding has been suggested as being a key factor for successful colonisation of habitats subjected to variations in hydrodynamism [206,214]. Finally, oweniids may serve as a relevant source of food for demersal fishes and flatfishes [155]; for instance, Owenia is among usual prey items for opportunistic preying fishes depending on the season [215].
4. Conclusions and Future Perspectives
Despite some efforts to increase the known biodiversity information of Magelonidae and Oweniidae (especially since the 60s in the former group and since 2000 in the latter), there are still large gaps in our taxonomic knowledge and the number of species world-wide, is most likely under reported, and in need of reappraisal. Additionally, the evolutionary relationships and subsequent classification for members of these two groups need to be assessed combining different sources of data.
Several marine realms and world seas should be further explored and sampled. Thus, Australia, South America, the Western Pacific and Africa at the very least for Magelonidae, while in East Atlantic, North West and West Indo-Pacific and also South America for Oweniidae. Whilst the forthcoming review of magelonids of Western Africa (Mortimer et al., in preparation B) should help in part to resolve the situation in that region, more work is needed, such as the description of the aforementioned undescribed Octomagelona species.
Most of our understanding of the biology, reproductive biology, anatomy and behaviour of both families have come from a relatively small number of studies on one or two species. Further work based on traditional and modern microscopy imaging technique (e.g., micro-CT, SEM) in combination with molecular methods (e.g., molecular phylogenies and species delimitation analyses) are needed to look at both assessing current species diversity and intraspecific variability. This is especially useful in morphologically homogenous groups, such as magelonids and oweniids, and crucial for a precise and effective assessment of their diversity, their distribution patterns and specific ecological preferences, and relevant for assessing putative cosmopolitan or broadly distributed species.
Although the latest molecular analyses suggest both families are part of the basal radiation of the Annelida (Palaeoannelida), morphological characters do not support a close relationship. Further investigation of potential homologies, understanding the morphological diversity found in each group. Assessment of the relationships within both Oweniidae and Magelonidae are also warranted, ideally integrating different sources of data.
Author Contributions
Conceptualisation and supervision, J.P., K.M., M.C. and J.M.; methodology, investigation, writing—original draft preparation, review and editing, illustrations, J.P., K.M., M.C. and J.M; funding acquisition, J.P. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by FAUNA IBÉRICA research project Polychaeta VII, Palpata, Canalipalpata II (PGC2018–095851–B–C64) funded by the Agencia Estatal de Investigación and coordinated by JP. MC was funded by the Ramón y Cajal program (RYC-2016-20799) funded by Spanish MINECO, Agencia Estatal de Investigación, Comunidad Autónoma de las Islas Baleares and the European Social Fund. Pat Hutchings (Australian Museum) also provided some funds to cover the publication costs.
Acknowledgments
Authors would thank Patrick Beckers (Institute of Evolutionary Biology, University of Bonn, Germany) and Elena Temereva (Department of Invertebrate Zoology, Moscow State University, Russia) for Figure 21A–D respectively. Also to Ada Castro and Catalina Sueiro (Servizos de Apoio á Investigación, Universidade da Coruña) and Sue Lidnsay (Australian Museum, Sydney) for SEM assistance, and to María Candás (Estación de Bioloxía Mariña da Graña–Ferrol, Universidade de Santiago de Compostela, Spain) for assistance with the micro-CT. Antón Taboada is thanked for help with line drawings. We would additionally like to thank Kimberley Mills (Amgueddfa Cymru and Cardiff University) for providing the image of magelonid palps feeding utilised in this paper. Authors deeply thank reviewers’ comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Magelonid valid species (according to WoRMS), type locality, assigned marine realms (sensu [86]), and depth of type locality. N.d.= no data.
Table A1.
Magelonid valid species (according to WoRMS), type locality, assigned marine realms (sensu [86]), and depth of type locality. N.d.= no data.
| SPECIES | TYPE LOCALITY | REALM | DEPTH (m) |
|---|---|---|---|
| Magelona agoensis Kitamori, 1967 | Ago Bay, Japan | Temperate Northern Pacific | 5 |
| Magelona alexandrae Magalhães, Bailey-Brock and Watling, 2018 | Hawaii, USA | Eastern Indo-Pacific | 39.6 |
| Magelona alleni Wilson, 1958 | Plymouth, England | Temperate Northern Atlantic | Intertidal |
| Magelona americana Hartman, 1965 | off New England, USA | Temperate Northern Atlantic | N.d. |
| Magelona annulata Hartmann-Schröder, 1962 | Isla Blanca, Chimbote Bay, Peru | Temperate South America | 8 |
| Magelona anuheone Magalhães, Bailey-Brock and Watling, 2018 | Easter Island | Eastern Indo-Pacific | Shallow |
| Magelona berkeleyi Jones, 1971 | Puget Sound, Washington, USA | Temperate Northern Pacific | 32–40 |
| Magelona californica Hartman, 1944 | Mission Bay, California, USA | Temperate Northern Pacific | ? |
| Magelona capax Hartman, 1965 | off the mouth of the Amazon River, Brazil | Tropical Atlantic | 1500 |
| Magelona capensis Day, 1961 | Agulhas Bank, South Africa | Temperate South Africa | 86 |
| Magelona cepiceps Mortimer and Mackie, 2006 | Mahé, Seychelles | Western Indo-Pacific | 33–45 |
| Magelona cerae Hartman and Reish, 1950 | Coos Bay, Oregon, USA | Temperate Northern Pacific | 37–73 |
| Magelona cincta Ehlers, 1908 | Algoa Bay, South Africa | Temperate South Africa | 40 |
| Magelona cinthyae Magalhães, Bailey-Brock and Watling, 2018 | Hawaii, USA | Eastern Indo-Pacific | 31–39 |
| Magelona conversa Mortimer and Mackie, 2003 | Mahé, Seychelles | Western Indo-Pacific | 26–42 |
| Magelona cornuta Wesenberg-Lund, 1949 | E by N of Ras Jagin, Gulf of Oman | Western Indo-Pacific | 12 |
| Magelona crenulata Bolívar and Lana, 1986 | Paranaguá Bay, Brazil | Temperate South America | 10 |
| Magelona crenulifrons Gallardo, 1968 | Nha Trang Bay, Viet Nam | Central Indo-Pacific | 6–48 |
| Magelona dakini Jones, 1978 | Careel Bay, New South Wales, Australia | Temperate Australasia | Shallow |
| Magelona debeerei Clarke, Paterson, Florence and Gibbons, 2010 | Beverly Hill, Namibia | Temperate South Africa | 37 |
| Magelona equilamellae Harmelin, 1964 | Rade de Villefranche and Golfe de Marseille, France | Temperate Northern Atlantic | 13–18 |
| Magelona falcifera Mortimer and Mackie, 2003 | Mahé, Seychelles | Western Indo-Pacific | 10–56 |
| Magelona fauchaldi Shakouri, Mortimer and Dehani, 2017 | Chabahar Bay, Iran, Gulf of Oman | Western Indo-Pacific | 1.5–20 |
| Magelona filiformis Wilson, 1959 | Mill Bay, Salcombe, England | Temperate Northern Atlantic | Intertidal |
| Magelona gemmata Mortimer and Mackie, 2003 | Mahé, Seychelles | Western Indo-Pacific | 48–62 |
| Magelona hartmanae Jones, 1978 | near Port Hueneme and Oxnard, California, USA | Temperate Northern Pacific | 12.7 |
| Magelona hobsonae Jones, 1978 | Eagle Cove, Puget Sound, Washington, USA | Temperate Northern Pacific | Intertidal |
| Magelona japonica Okuda, 1937 | Jinsen, Korean Archipelago | Temperate Northern Pacific | N.d. |
| Magelona johnstoni Fiege, Licher and Mackie, 2000 | St Andrews, Scotland | Temperate Northern Atlantic | N.d. |
| Magelona jonesi Hartmann-Schröder, 1980 | Western Australia | Central Indo-Pacific | N.d. |
| Magelona kamala Nateewathana and Hylleberg, 1991 | Kamala Bay, Phuket Island, Thailand | Western Indo-Pacific | 10–20 |
| Magelona koreana Okuda, 1937 | Jinsen, Korean Archipelago | Temperate Northern Pacific | N.d. |
| Magelona lenticulata Gallardo, 1968 | Nha Trang Bay, Viet Nam | Central Indo-Pacific | N.d. |
| Magelona longicornis Johnson, 1901 | West Seattle, Puget Sound, USA | Temperate Northern Pacific | N.d. |
| Magelona lusitanica Mortimer, Gil and Fiege, 2011 | SW Portuguese continental shelf | Temperate Northern Atlantic | 105–327 |
| Magelona magnahamata Aguado and San Martín, 2004 | Granito de Oro, Panamá | Tropical Eastern Pacific | N.d. |
| Magelona mahensis Mortimer and Mackie, 2006 | Mahé, Seychelles | Western Indo-Pacific | 11–48 |
| Magelona marianae Hernández-Alcántara and Solís-Weiss, 2000 | Punta Mita, Mexico | Temperate Northern Pacific | 23.5–46.4 |
| Magelona methae Nateewathana and Hylleberg, 1991 | Kamala Bay, Phuket Island, Thailand | Western Indo-Pacific | 8.4–10 |
| Magelona mickminni Nateewathana and Hylleberg, 1991 | Patong Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona minuta Eliason, 1962 | Öresund, Sweden | Temperate Northern Atlantic | 15–18 |
| Magelona mirabilis (Johnston, 1865) | St Andrews, Scotland, UK. | Temperate Northern Atlantic | N.d. |
| Magelona montera Mortimer, Cassà, Martin and Gil, 2012 | Eilat, Gulf of Aqaba, Red sea | Western Indo-Pacific | Intertidal |
| Magelona nonatoi Bolívar and Lana, 1986 | Rio de Janeiro, Brazil | Temperate South America | N.d. |
| Magelona noppi Nateewathana and Hylleberg, 1991 | Nopparat-Tara Beach, Krabi, Thailand | Western Indo-Pacific | Intertidal |
| Magelona obockensis Gravier, 1905 | Obock, Gulf of Aden, Red Sea | Western Indo-Pacific | Intertidal |
| Magelona pacifica Monro, 1933 | Gorgona Island, Panamá | Tropical Eastern Pacific | Intertidal |
| Magelona papillicornis F. Müller, 1858 | Santa Catarina Island, Brazil | Temperate South America | 0–0.8 |
| Magelona parochilis Zhou and Mortimer, 2013 | Yellow Sea China | Temperate Northern Pacific | Intertidal |
| Magelona paulolanai Magalhães, Bailey-Brock and Watling, 2018 | Hawaii, USA | Eastern Indo-Pacific | 30–40 |
| Magelona pectinata Nateewathana and Hylleberg, 1991 | Kamala Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona petersenae Nateewathana and Hylleberg, 1991 | Bang Tao Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona pettiboneae Jones, 1963 | St Andrew Bay, Bay County, Florida, USA | Temperate Northern Atlantic | 30 |
| Magelona phyllisae Jones, 1963 | Lamont Geological Observatory Station V-15-68, Santa, Peru | Temperate South America | 181 |
| Magelona pitelkai Hartman, 1944 | Tomales Bay, California | Temperate Northern Pacific | Shallow |
| Magelona polydentata Jones, 1963 | Bimini Lagoon, Bahamas | Tropical Atlantic | Intertidal |
| Magelona posterelongata Bolívar and Lana, 1986 | Paraná Bay, Brazil | Temperate South America | Shallow |
| Magelona pulchella Mohammad, 1970 | Kuwait, Arabian Gulf | Western Indo-Pacific | Intertidal |
| Magelona pygmaea Nateewathana and Hylleberg, 1991 | Kamala Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona riojai Jones, 1963 | Antón Lizardo, Veracruz, Mexico | Tropical Atlantic | Intertidal |
| Magelona rosea Moore, 1907 | Buzzard’s Bay, Wood’s Hole, Massachusetts | Temperate Northern Atlantic | Intertidal |
| Magelona sacculata Hartman, 1961 | San Pedro shelf area, California, USA | Temperate Northern Pacific | 20–40 |
| Magelona sachalinensis Buzhinskaja, 1985 | Sakhalin Island, Sea of Japan | Temperate Northern Pacific | 30–175 |
| Magelona sinbadi Mortimer, Cassà, Martin and Gil, 2012 | Iran, Persian Gulf | Western Indo-Pacific | 20 |
| Magelona spinifera (Hernández-Alcántara and Solís-Weiss, 2000) | Santa María Bay, Mexico | Temperate Northern Pacific | 75 |
| Magelona symmetrica Mortimer and Mackie, 2006 | Mahé, Seychelles | Western Indo-Pacific | 20 |
| Magelona tehuanensis Hernández-Alcántara and Solís-Weiss, 2000 | Western Salina Cruz, Mexico | Tropical Eastern Pacific | 70 |
| Magelona tinae Nateewathana and Hylleberg, 1991 | Bang Tao Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona uebelackerae (Hernández-Alcántara and Solís-Weiss, 2000) | Texas, Gulf of Mexico | Temperate Northern Atlantic | 3 |
| Magelona variolamellata Bolívar and Lana, 1986 | Paraná, São Paulo and Rio de Janeiro, Brazil | Temperate South America | Shallow |
| Magelona wilsoni Glémarec, 1966 | South of Brittany, ‘Grande Vasière’, France | Temperate Northern Atlantic | 60–110 |
| Octomagelona bizkaiensis Aguirrezabalaga, Ceberio and Fiege, 2001 | Capbreton Canyon, Bay of Biscay | Temperate Northern Atlantic | 984–1040 |

Table A2.
Oweniid valid species (partially according to WoRMS), type locality, assigned marine realms (sensu [86]), and depth of type locality. N.d.= No data.
Table A2.
Oweniid valid species (partially according to WoRMS), type locality, assigned marine realms (sensu [86]), and depth of type locality. N.d.= No data.
| SPECIES | TYPE LOCALITY | REALMS | DEPTH (m) |
|---|---|---|---|
| Galathowenia africana Kirkegaard, 1959 | West Africa | Tropical Atlantic | 30–42 |
| Galathowenia annae Capa, Parapar and Hutchings, 2012 | Botany Bay, Sydney, Australia | Temperate Australasia | 16 |
| Galathowenia arafurensis Capa, Parapar and Hutchings, 2012 | Arafura Sea, Australia | Central Indo-Pacific | 88 |
| Galathowenia australis (Grube, 1866) | Saint Paul Island, Indian Ocean | Temperate Southern Africa | N.d. |
| Galathowenia eurystoma (Caullery, 1944) | Indonesia | Central Indo-Pacific | 31–1570 |
| Galathowenia fragilis (Nilsen and Holthe, 1985) | Norwegian Sea | Temperate Northern Atlantic | 800–2600 |
| Galathowenia haplosoma (Gibbs, 1972) | Cook Islands | Eastern Indo-Pacific | 1–5 |
| Galathowenia joinvillensis Hartmann-Schröder and Rosenfeldt, 1989 | Antarctic Ocean | Southern Ocean | 68–458 |
| Galathowenia kirkegaardi De León-González and Sanchez-Hernández, 2012 | Gulf of Mexico | Tropical Atlantic | 1–4.1 |
| Galathowenia lobopygidiata (Uschakov, 1950) | Okhotsk Sea | Temperate Northern Pacific | 110–1366 |
| Galathowenia longicollaris Hartmann-Schröder and Rosenfeldt, 1989 | Antarctic Ocean | Southern Ocean | 68 |
| Galathowenia oculata (Zachs, 1923) | White Sea | Arctic | 12–2500 |
| Galathowenia piltzi Blake, 2000 | Santa Maria Basin, California | Temperate Northern Pacific | 92 |
| Galathowenia pygidialis (Harman, 1960) | California and Baja California | Temperate Northern Pacific | >2000 |
| Galathowenia quelis Capa, Parapar and Hutchings, 2012 | North of Sydney, Australia | Temperate Australasia | 1–60 |
| Galathowenia scotiae (Hartman, 1978) | Weddell Sea | Southern Ocean | 42–1592 |
| Myriochele annenkovae Hartman, 1960 (1) | Northern Sea of Japan | Temperate Northern Pacific | 45 |
| Myriochele antarctica (Hartman, 1967) | South Orkney Islands | Southern Ocean | 604–3816 |
| Myriochele antarctica Cantone and Di Pietro, 2001 | Ross Sea, Terra Nova Bay | Southern Ocean | 126–136 |
| Myriochele australiensis Capa, Parapar and Hutchings, 2012 | East of Long Reef, Sydney, Australia | Temperate Australasia | 60 |
| Myriochele danielsseni Hansen, 1878 | Norwegian Sea | Temperate Northern Atlantic | 1187 |
| Myriochele gracilis Hartman, 1955 | Pacific Ocean, Southern California | Temperate Northern Pacific | 115 |
| Myriochele heeri Malmgren, 1867 | Spitsbergen, Norway | Temperate Northern Atlantic | 120–2600 |
| Myriochele heruensis Gibbs, 1971 | Solomon Islands | Eastern Indo-Pacific | 16 |
| Myriochele islandica (Parapar, 2003) | West Iceland | Temperate Northern Atlantic | 1187–1407 |
| Myriochele japonica (Imajima and Morita, 1987) | Japan | Temperate Northern Pacific | 62–125 |
| Myriochele malmgreni (Parapar, 2006) | Faroe Passage, Iceland | Temperate Northern Atlantic | 1187–1407 |
| Myriochele minor Caullery, 1944 | Indonesia | Central Indo-Pacific | 462–959 |
| Myriochele olgae Blake, 2000 | off Half Moon Bay, California | Temperate Northern Pacific | 100–200 |
| Myriochele pacifica McIntosh, 1885 | Central Pacific Ocean | Eastern Indo-Pacific | 4754 |
| Myriochele picta Southern, 1921 | Chilka Lake, India | Western Indo-Pacific | 1–3 |
| Myriochele riojai Parapar, 2003 | Bransfield Strait, Antarctic Ocean | Southern Ocean | 647–1416 |
| Myriochele robusta Parapar, 2003 | Bransfield Strait, Antarctic Ocean | Southern Ocean | 640–1500 |
| Myriochele striolata Blake, 2000 | Santa Maria Basin, California | Temperate Northern Pacific | 91–400 |
| Myriowenia californiensis Hartman, 1960 | Southern California | Temperate Northern Pacific | 106 |
| Myriowenia gosnoldi Hartman, 1965 | off Suriname | Tropical Atlantic | 520–550 |
| Owenia artifex (Verrill, 1885) | Massachusetts | Temperate Northern Atlantic | 122 |
| Owenia assimilator Caullery, 1944 (2) | n.d. | n.d. | N.d. |
| Owenia assimilis (Sars, 1851) | Norwegian Sea | Temperate Northern Atlantic | 2–460 |
| Owenia australis Ford and Hutchings, 2005 | New South Wales, Australia | Temperate Australasia | <100 |
| Owenia bassensis Ford and Hutchings, 2005 | Victoria, East Bass Strait, Australia | Temperate Australasia | <100 |
| Owenia borealis Koh, Bhaud and Jirkov, 2003 | North East Atlantic | Temperate Northern Atlantic | 41–1350 |
| Owenia caissara Silva and Lana, 2017 | Santa Catarina State, Brazil | Temperate South America | 0–5 |
| Owenia caudisetosa Hartmann-Schröder, 1959 | El Salvador | Tropical Atlantic | N.d. |
| Owenia collaris Hartman, 1955 | Santa Catalina Island | Temperate Northern Pacific | 0–150 |
| Owenia dichotoma Parapar and Moreira, 2015 | Lizard Island, Australia | Central Indo-Pacific | 12 |
| Owenia fusiformis Delle Chiaje, 1844 | Sicily, Mediterranean | Temperate Northern Atlantic | N.d. |
| Owenia gomsoni Koh and Bhaud, 2001 | South Korea, Yellow Sea | Temperate Northern Pacific | 0 |
| Owenia johnsoni Blake, 2000 | Tomales Bay, California | Temperate Northern Pacific | 3–20 |
| Owenia mirrawa Ford and Hutchings, 2005 | Northern Territory, Australia | Central Indo-Pacific | <100 |
| Owenia persica Martin, Koh, Bhaud, Dutrieux and Gil, 2006 | Persian Gulf | Western Indo-Pacific | 16 |
| Owenia petersenae Koh and Bhaud, 2003 | Wellington, New Zealand | Southern Ocean | 20–30 |
| Owenia picta Parapar and Moreira, 2015 | Lizard Island, Australia | Central Indo-Pacific | 2–12 |
| Owenia polaris Koh, Bhaud and Jirkov, 2003 | East Iceland | Temperate Northern Atlantic | 12–930 |
| Owenia vieitezi Díaz-Díaz, Parapar and Moreira, 2020 | Gulf of Venezuela, Caribbean Sea | Tropical Atlantic | 6–18 |
- (1)
- Hartman [216] (p.150) propose to rename Myriochele pacifica Annenkova [217] (p. 183) as Myriochele annenkovae for distinguishing it from M. pacifica McIntosh [218] (p. 413).
- (2)
- Read and Fauchald [85] name it as Owenia assimilator Caullery, 1944 but Caullery [165] reports it as O. assimilator Andrews. Gil [156] affirms that Caullery was mistaken, because he was referring to Ammochares aedificator Andrews, 1891, a species that Read and Fauchald [85] report as synonym of Owenia fusiformis.
References
- Weigert, A.; Bleidorn, C. Current Status of Annelid Phylogeny. Org. Divers. Evol. 2016, 16, 345–362. [Google Scholar] [CrossRef]
- Helm, C.; Beckers, P.; Bartolomaeus, T.; Drukewitz, S.H.; Kourtesis, I.; Weigert, A.; Purschke, G.; Worsaae, K.; Struck, T.H.; Bleidorn, C. Convergent Evolution of the Ladder-like Ventral Nerve Cord in Annelida. Front. Zool. 2018, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Struck, T. Phylogeny. In Handbook of Zoology. Annelida. Basal Groups and Pleistoannelida, Sedentaria I; Purschke, G., Böggemann, M., Westheide, W., Eds.; DeGruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 37–68. [Google Scholar]
- Weigert, A.; Helm, C.; Meyer, M.; Nickel, B.; Arendt, D.; Hausdorf, B.; Santos, S.R.; Halanych, K.M.; Purschke, G.; Bleidorn, C.; et al. Illuminating the Base of the Annelid Tree Using Transcriptomics. Mol. Biol. Evol. 2014, 31, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.C.S.; Novo, M.; Kawauchi, G.Y.; Worsaae, K.; Pleijel, F.; Giribet, G.; Rouse, G.W. Articulating “Archiannelids”: Phylogenomics and Annelid Relationships, with Emphasis on Meiofaunal Taxa. Mol. Biol. Evol. 2015, 32, 2860–2875. [Google Scholar] [CrossRef]
- Struck, T.H.; Golombek, A.; Weigert, A.; Franke, F.A.; Westheide, W.; Purschke, G.; Bleidorn, C.; Halanych, K.M. The Evolution of Annelids Reveals Two Adaptive Routes to the Interstitial Realm. Curr. Biol. 2015, 25, 1993–1999. [Google Scholar] [CrossRef]
- Kojima, S. Paraphyletic Status of Polychaeta Suggested by Phylogenetic Analysis Based on the Amino Acid Sequences of Elongation Factor 1-Alpha. Mol. Phylogenet. Evol. 1998, 9, 255–261. [Google Scholar] [CrossRef]
- Eeckhaut, I.; McHugh, D.; Mardulyn, P.; Tiedemann, R.; Monteyne, D.; Jangoux, M.; Milinkovitch, C. Myzostomida: A Link between Trochozoans and Flatworms? P. Roy. Soc. B Biol. Sci. 2000, 267, 1383–1392. [Google Scholar] [CrossRef]
- Bleidorn, C.; Vogt, L.; Bartolomaeus, T. New Insights into Polychaete Phylogeny (Annelida) Inferred from 18S RDNA Sequences. Mol. Phylogenet. Evol. 2003, 29, 279–288. [Google Scholar] [CrossRef]
- Rousset, V.; Rouse, G.W.; Siddall, M.E.; Tillier, A.; Pleijel, F. The Phylogenetic Position of Siboglinidae (Annelida) Inferred from 18S RRNA, 28S RRNA and Morphological Data. Cladistics 2004, 20, 518–533. [Google Scholar] [CrossRef]
- Rousset, V.; Pleijel, F.; Rouse, G.W.; Erséus, C.; Siddall, M.E. A Molecular Phylogeny of Annelids. Cladistics 2007, 23, 41–63. [Google Scholar] [CrossRef]
- Struck, T.H.; Schult, N.; Kusen, T.; Hickman, E.; Bleidorn, C.; McHugh, D.; Halanych, K.M. Annelid Phylogeny and the Status of Sipuncula and Echiura. BMC Evol. Biol. 2007, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Struck, T.H.; Nesnidal, M.P.; Purschke, G.; Halanych, K.M. Detecting Possibly Saturated Positions in 18S and 28S Sequences and Their Influence on Phylogenetic Reconstruction of Annelida (Lophotrochozoa). Mol. Phylogenet. Evol. 2008, 48, 628–645. [Google Scholar] [CrossRef] [PubMed]
- Struck, T.H. The Impact of Paralogy on Phylogenomic Studies—A Case Study on Annelid Relationships. PLoS ONE 2013, 8, e62892. [Google Scholar] [CrossRef] [PubMed]
- Zrzavý, J.; Říha, P.; Piálek, L.; Janouškovec, J. Phylogeny of Annelida (Lophotrochozoa): Total-Evidence Analysis of Morphology and Six Genes. BMC Evol. Biol. 2009, 9, 189. [Google Scholar] [CrossRef]
- Chen, H.; Parry, L.A.; Vinther, J.; Zhai, D.; Hou, X.; Ma, X. A Cambrian Crown Annelid Reconciles Phylogenomics and the Fossil Record. Nature 2020, 583, 249–252. [Google Scholar] [CrossRef]
- Capa, M.; Parapar, J.; Hutchings, P. Phylogeny of Oweniidae (Polychaeta) Based on Morphological Data and Taxonomic Revision of Australian Fauna. Zool. J. Linn. Soc. 2012, 166, 236–278. [Google Scholar] [CrossRef]
- Mortimer, K.; Mills, K.; Jordana, E.; Pinedo, S.; Gil, J. A Further Review of European Magelonidae (Annelida), Including Redescriptions of Magelona equilamellae and Magelona filiformis. Zootaxa 2020, 4767, 89–114. [Google Scholar] [CrossRef]
- Rouse, G.W. Magelona Müller, 1858. In Polychaetes; Rouse, G.W., Pleijel, F., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 261–263. [Google Scholar] [CrossRef]
- Cunningham, J.T.; Ramage, G.A. The Polychaeta Sedentaria of the Firth of Forth. Trans. Roy. Soc. Edinburg 1888, 33, 635–684. [Google Scholar] [CrossRef]
- Dos Santos Brasil, A.C. Filogenia de Magelonidae Cunningham & Ramage, 1888 (Annelida–Polychaeta) com Base na Morfologia Externa. PhD Thesis, Universidade Federal do Paraná, Curitiva, Brazil, 2003. [Google Scholar]
- Mortimer, K. 4.2 Magelonidae. In Handbook of Zoology. Annelida. Basal Groups and Pleistoannelida, Sedentaria I; Purschke, G., Böggemann, M., Westheide, W., Eds.; DeGruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 112–131. [Google Scholar]
- McIntosh, W.C. On the Structure of Magelona. Proc. R. Soc. Lond. 1877, 25, 559–564. [Google Scholar]
- McIntosh, W.C. Beiträge zur Anatomie von Magelona. Z. Wiss. Zool. 1878, 31, 401–472. [Google Scholar]
- McIntosh, W.C. On the Circulatory System of Magelona. J. Anat. Physiol., London 1879, 13, 331–345. [Google Scholar]
- McIntosh, W.C. On the Structure of Magelona. Ann. Mag. Nat. Hist. (Ser. 8) 1911, 7, 417–457. [Google Scholar] [CrossRef]
- McIntosh, W.C. A monograph of the British marine annelids. In Polychaeta. Opheliidae to Ammocharidae; The Ray Society: London, UK, 1915; Volume 3, Part I. Text; pp. 1–368. [Google Scholar]
- McIntosh, W.C. A monograph of the British marine annelids. In Polychaeta. Opheliidae to Ammocharidae; The Ray Society: London, UK, 1916; Volume 3, Part II. Plates. [Google Scholar]
- Jones, M.L. Four New Species of Magelona (Annelida, Polychaeta) and a Redescription of Magelona longicornis Johnson. Am. Mus. Novit. 1963, 2164, 1–29. [Google Scholar]
- Jones, M.L. On the Morphology, Feeding, and Behavior of Magelona sp. Biol. Bull. 1968, 134, 272–297. [Google Scholar] [CrossRef]
- Jones, M.L. Magelona berkeleyi n. sp. from Puget Sound (Annelida: Polychaeta), with a Further Redescription of Magelona longicornis Johnson and a Consideration of Recently Described Species of Magelona. J. Fish. Res. Bd. Can. 1971, 28, 1445–1454. [Google Scholar] [CrossRef]
- Jones, M.L. Redescription of M. papillicornis F. Müller. In Essays on Polychaetous Annelids in Memory of Dr. Olga Hartman; Reish, D.J., Fauchald, K., Eds.; Allan Hancock Foundation: University of Southern California: Los Angeles, CA, USA, 1977; pp. 247–266. [Google Scholar]
- Jones, M.L. Three New Species of Magelona (Annelida, Polychaeta) and a Redescription of Magelona pitelkai Hartman. Proc. Biol. Soc. Wash. 1978, 91, 336–363. [Google Scholar]
- Nateewathana, A.; Hylleberg, J. Magelonid Polychaetes from Thailand, the Andaman Sea, with Descriptions of Eight New Species. Ophelia 1991, 5, 169–184. [Google Scholar]
- Mortimer, K.; Mackie, A.S.Y. The Magelonidae (Annelida: Polychaeta) from the Seychelles, with the Description of Three New Species. Hydrobiologia 2003, 496, 163–173. [Google Scholar] [CrossRef]
- Mortimer, K.; Mackie, A.S.Y. The Magelonidae (Annelida: Polychaeta) from the Seychelles. 2. Description of Four Additional Species, Three New to Science. Sci. Mar. 2006, 70, 125–137. [Google Scholar] [CrossRef][Green Version]
- Mortimer, K. Magelonidae (Polychaeta) from the Arabian Peninsula: A Review of Known Species, with Notes on Magelona tinae from Thailand. Zootaxa 2010, 2628, 1–26. [Google Scholar] [CrossRef]
- Mortimer, K.; Cassà, S.; Martin, D.; Gil, J. New Records and New Species of Magelonidae (Polychaeta) from the Arabian Peninsula, with a Re-Description of Magelona pacifica and a Discussion on the Magelonid Buccal Region. Zootaxa 2012, 3331, 1–43. [Google Scholar] [CrossRef]
- Shakouri, A.; Mortimer, K.; Dehani, E. A New Species and New Records of Magelona (Annelida: Magelonidae) from Chabahar Bay, Gulf of Oman, South-Eastern Iran. J. Mar. Biol. Ass. UK 2017, 97, 1537–1552. [Google Scholar] [CrossRef]
- Mortimer, K.; Mackie, A.S.Y. Magelonidae (Polychaeta) from Hong Kong, China; with Discussions on Related Species and Redescriptions of Three Species. Zoosymposia 2009, 2, 179–199. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Mortimer, K. A New Species of Magelona (Polychaeta: Magelonidae) from Chinese Coastal Waters. J. Mar. Biol. Ass. UK 2013, 93, 1503–1510. [Google Scholar] [CrossRef]
- Uebelacker, J.M.; Jones, M.L. Family Magelonidae. In Taxonomic Guide to the Polychaetes of the Northern Gulf of Mexico; Uebelacker, J.M., Johnson, P.G., Eds.; Barry A. Vittor & Associates: Mobile, AL, USA, 1984; Volume 2, pp. 7.1–7.29. [Google Scholar]
- Hernández-Alcántara, P.; Solís-Weiss, V. Magelonidae from the Mexican Pacific and Northern Gulf of Mexico, with the Description of a New Genus (Meridithia) and Four New Species. Bull. Mar. Sci. 2000, 67, 625–644. [Google Scholar]
- Hernández-Alcántara, P.; Solís-Weiss, V. Magelonidae Cunningham & Ramage, 1888. In Poliquetos (Annelida: Poychaeta) de México y América Tropical; León-González, J.A., Bastida-Zavala, J.R., Carrera Parra, L.F., García-Garza, M.E., Peña-Rivera, A., Salazar-Vallejo, S.I., Solís-Weiss, V., Eds.; Universidad Autónoma de Nuevo León: Nuevo León, Mexico, 2009; Volume 2, pp. 277–289. [Google Scholar]
- Blake, J.A. Family Magelonidae Cunnigham & Ramage, 1888. In Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel; Blake, J.A., Hilbig, B., Scott, P.H., Eds.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1996; Volume 6, pp. 253–261. [Google Scholar]
- Bolívar, G.A.; Lana, P.C. Magelonidae (Annelida: Polychaeta) do Litoral Sudeste do Brasil. Neritica 1986, 1, 131–147. [Google Scholar] [CrossRef][Green Version]
- Almeida, T.C.M.; Vivan, J.M.; Pesserl, B.H.; Lana, P. Polychaetes of the North-Central Santa Catarina State, Brazil. Check List 2012, 8, 204–206. [Google Scholar] [CrossRef][Green Version]
- Fiege, D.; Licher, F.; Mackie, A.S.Y. A Partial Review of the European Magelonidae (Annelida: Polychaeta): Magelona mirabilis Redefined and M. johnstoni sp. nov. Distinguished. J. Mar. Biol. Assoc. UK 2000, 80, 215–234. [Google Scholar] [CrossRef]
- Mortimer, K.; Gil, J.; Fiege, D. Portuguese Magelona (Annelida: Magelonidae) with a Description of a New Species, a Re-Description of Magelona wilsoni Glémarec, and a Key to Adult Magelonidae from European Waters. Ital. J. Zool. 2011, 78, 124–139. [Google Scholar] [CrossRef]
- Mills, K.; Mortimer, K. Redescription of Magelona minuta Eliason, 1962 (Annelida), with discussions on the validity of Magelona filiformis minuta. Zootaxa 2018, 4527, 541–559. [Google Scholar] [CrossRef]
- Mortimer, K.; Mackie, A.S.Y. Morphology, Feeding and Behaviour of British Magelona (Annelida: Magelonidae), with Discussions on the Form and Function of Abdominal Lateral Pouches. Mem. Mus. Vic. 2014, 71, 177–201. [Google Scholar] [CrossRef][Green Version]
- Mills, K.; Mortimer, K. Observations on the Tubicolous Annelid Magelona alleni (Magelonidae), with Discussions on the Relationship between Morphology and Behaviour of European Magelonids. J. Mar. Biol. Ass. UK 2019, 99, 715–727. [Google Scholar] [CrossRef]
- Mills, K.; Mortimer, K. Habitat and Distribution of the Shovelhead Worm Magelona equilamellae Harmelin, 1964 with Notes on the Morphologically Similar Magelona alleni Wilson, 1958. Bull. Porcupine Mar. Nat. Hist. Soc. 2019, 12, 63–67. [Google Scholar]
- Fauchald, K. Life Diagram Patterns in Benthic Polychaetes. Proc. Biol. Soc. Wash. 1983, 96, 160–177. [Google Scholar]
- Hartman, O. Abyssal Polychaetous Annelids from the Mozambique Basin off Southeast Africa, with a Compendium of Abyssal Polychaetous Annelids from World-Wide Areas. J. Fish. Res. Board Can. 1971, 28, 1407–1428. [Google Scholar] [CrossRef]
- Aguirrezabalaga, F.; Ceberio, A.; Fiege, D. Octomagelona bizkaiensis (Polychaeta: Magelonidae) a New Genus and Species from the Capbreton Canyon (Bay of Biscay, North-East Atlantic). J. Mar. Biol. Ass. UK 2001, 81, 221–224. [Google Scholar] [CrossRef]
- Capa, M.; Parapar, J.; Hutchings, P. 4.1. Oweniidae. In Handbook of Zoology. Annelida. Basal Groups and Pleistoannelida, Sedentaria I; Purschke, G., Böggemann, M., Westheide, W., Eds.; DeGruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 91–112. [Google Scholar] [CrossRef]
- Read, G.; Fauchald, K. (Eds.) World Polychaeta Database. Magelonidae Cunningham & Ramage, 1888. 2021. Available online: http://marinespecies.org/aphia.php?p=taxdetails&id=975 (accessed on 12 January 2021).
- Blake, J.A. Polychaeta Oweniidae from Antarctic Seas Collected by the United States Antarctic Research Program. In Proceedings of the First International Polychaete Conference; Hutchings, P.A., Ed.; Linnean Society of New South Wales: Sydney, Australia, 1984; pp. 112–117. [Google Scholar]
- Hartmann-Schröder, G.; Rosenfeldt, P. Die Polychaten Der “Polarstern”- Reise ANT III/2 in Die Antarktis) 1984. Teil 2: Cirratulidae Dis Serpulidae. Mitt. Hamb. Zool. Mus. Inst. 1989, 86, 65–106. [Google Scholar]
- Cantone, G.; Di Pietro, N. A New Species of Myriochele (Polychaeta, Oweniidae) from Antarctica, with Considerations on Antarctic Oweniids. Polar Biol. 1998, 19, 421–423. [Google Scholar] [CrossRef]
- Parapar, J. Revision of Five Species Referred to Myriochele and Galathowenia (Polychaeta: Oweniidae) from the Antarctic Seas Based upon Type Material. Proc. Biol. Soc. Wash. 2001, 114, 403–413. [Google Scholar]
- Parapar, J. Two New Species of Myriochele (Polychaeta: Oweniidae) from the Bransfield Strait (Antarctica). Antarct. Sci. 2003, 15, 219–226. [Google Scholar] [CrossRef]
- Blake, J.A.; Dean, D. Polychaetous Annelids Collected by the R/V Hero from Baffin Island, Davis Strait, and West Greenland in 1968. Bull. S. Calif. Acad. Sci. 1973, 72, 31–39. [Google Scholar]
- Nilsen, R.; Holthe, T. Arctic and Scandinavian Oweniidae (Polychaeta) with a Description of Myriochele fragilis sp.n., and Comments on the Phylogeny of the Family. Sarsia 1985, 70, 17–32. [Google Scholar] [CrossRef]
- Koh, B.S.; Bhaud, M.R.; Jirkov, I.A. Two New Species of Owenia (Annelida: Polychaeta) in the Northern Part of the North Atlantic Ocean and Remarks on Previously Erected Species from the Same Area. Sarsia 2003, 88, 175–188. [Google Scholar] [CrossRef]
- Parapar, J.O. Oweniidae (Annelida, Polychaeta) from Icelandic Waters, Collected by the BIOICE Project, with the Description of Myrioglobula islandica n. sp. Sarsia 2003, 88, 274–290. [Google Scholar] [CrossRef]
- Parapar, J. The Genera Myriochele and Myrioglobula (Polychaeta, Oweniidae) in Icelandic Waters with the Revision of Type Material of Myriochele heeri Malmgren, 1867, and the Description of a New Species. J. Nat. Hist. 2006, 40, 523–547. [Google Scholar] [CrossRef]
- Martin, D. Revisión de las especies de Owenidae (Annelida, Polychaeta) de la Peninsula Ibérica. Sci. Mar. 1989, 53, 47–52. [Google Scholar]
- Hartman, O. Atlas of the Sedentariate Polychaetous Annelids from California; Allan Hancock Foundation, University of Southern California: Los Angeles, CA, USA, 1969. [Google Scholar]
- Blake, J.A. Chapter 5. Family Oweniidae Rioja, 1917. In Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and the Western Santa Barbara Channel; Blake, J.A., Hilbig, B., Scott, P.H., Eds.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 2000; Volume 7, pp. 97–127. [Google Scholar]
- Milligan, M.R. Family Oweniidae Rioja, 1917. In Taxonomic Guide to the Polychaetes of the Northern Gulf of Mexico; Uebelacker, J.M., Johnson, P.G., Eds.; Barry A. Vittor & Associates: Mobile, AL, USA, 1984; Volume 6, pp. 46.1–46.12. [Google Scholar]
- Villalobos-Guerrero, T.F. Oweniidae, Rioja 1917. In Poliquetos (Annelida: Poychaeta) de México y América Tropical; León-González, J.A., Bastida-Zavala, J.R., Carrera Parra, L.F., García-Garza, M.E., Peña-Rivera, A., Salazar-Vallejo, S.I., Solís-Weiss, V., Eds.; Universidad Autónoma de Nuevo León: Nuevo León, Mexico, 2009; Volume 2, pp. 391–402. [Google Scholar]
- Díaz-Díaz, O.; Parapar, J.; Moreira, J. A New Species of Genus Owenia Delle-Chiaje, 1844 (Annelida; Oweniidae) from the Coast of Venezuela. Cah. Biol. Mar. 2018, 59, 589–597. [Google Scholar]
- Koh, B.; Bhaud, M. Description of Owenia gomsoni n. sp. (Oweniidae, Annelida Polychaeta) from the Yellow Sea and Evidence That Owenia fusiformis Is Not a Cosmopolitan Species. Vie Milieu 2001, 51, 77–87. [Google Scholar]
- Imajima, M.; Morita, Y. Oweniidae (Annelida, Polychaeta) from Japan. Bull. Natl. Sci. Mus. 1987, 13, 85–102. [Google Scholar]
- Ford, E.; Hutchings, P. An Analysis of Morphological Characters of Owenia Useful to Distinguish Species: Description of Three New Species of Owenia (Oweniidae: Polychaeta) from Australian Waters. Mar. Ecol. 2005, 26, 181–196. [Google Scholar] [CrossRef]
- Parapar, J.; Moreira, J. The Oweniidae (Annelida; Polychaeta) from Lizard Island (Great Barrier Reef, Australia) with the Description of Two New Species of Owenia Delle Chiaje, 1844. Zootaxa 2015, 4019, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Dauvin, J.-C.; Thiébaut, E. Is Owenia fusiformis Delle Chiaje a Cosmopolitan Species? Mém. Mus. Nat. Paris 1994, 162, 383–404. [Google Scholar]
- Parapar, J. Resurrection of Galathowenia australis (Polychaeta, Oweniidae) Based upon Type Material. Cah. Biol. Mar. 2003, 44, 249–255. [Google Scholar]
- Martin, D.; Koh, B.-S.; Bhaud, M.; Gil, J.; Dutrieux, E. The Genus Owenia (Annelida, Polychaeta) in the Persian Gulf, with Description of a New Species, Owenia persica. Org. Divers. Evol. 2006, 6, 325–326. [Google Scholar] [CrossRef]
- Ménard, F.; Gentil, F.; Dauvin, J.-C. Population Dynamics and Secondary Production of Owenia fusiformis Delle Chiaje. Polychaeta from the Bay of Seine Eastern English Channel France. J. Exp. Mar. Biol. Ecol. 1989, 133, 151–168. [Google Scholar] [CrossRef]
- Smart, T.I.; Von Dassow, G. Unusual Development of the Mitraria Larva in the Polychaete Owenia collaris. Biol. Bull. 2009, 217, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Kirkergaard, J.B. Benthic Polychaeta from Depths Exceeding 6000 m. Galathea Rep. 1956, 2, 63–78. [Google Scholar]
- Read, G.; Fauchald, K. (Eds.) World Polychaeta Database. Oweniidae Rioja, 1917. 2021. Available online: http://marinespecies.org/aphia.php?p=taxdetails&id=975 (accessed on 12 January 2021).
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Rouse, G.W.; Fauchald, K. Cladistics and Polychaetes. Zool. Scr. 1997, 26, 139–204. [Google Scholar] [CrossRef]
- Weigert, A.; Golombek, A.; Gerth, M.; Schwarz, F.; Struck, T.H.; Bleidorn, C. Evolution of Mitochondrial Gene Order in Annelida. Mol. Phylogenet. Evol. 2016, 94, 196–206. [Google Scholar] [CrossRef]
- Parry, L.; Vinther, J.; Edgecombe, G.D. Cambrian stem-group annelids and a metameric origin of the annelid head. Biol. Lett. 2015, 11, 20150763. [Google Scholar] [CrossRef] [PubMed]
- Hartman, O. Polychaetous Annelids from California, Including the Descriptions of Two New Genera and Nine New Species. Allan Hancock Pac. Exp. 1944, 10, 239–307. [Google Scholar]
- Hartman, O.; Reish, D.J. The Marine Annelids of Oregon. Or. State Monogr. Stud. Zool. 1950, 6, 1–64. [Google Scholar]
- Gallardo, V.A. Polychaeta from the Bay of Nha Trang, South Viet Nam. Naga Report 1968, 4, 35–279. [Google Scholar]
- Eliason, A. Undersökningar Över Öresund. XXXXI. Weitere Untersuchungen Über Die Polychaetenfauna Des Öresunds. Acta Univ. Lund. Avd. 2 1962, 58, 1–98. [Google Scholar]
- Hartman, O. Polychaetous Annelids from California. Allan Hancock Pac. Exp. 1961, 25, 1–226. [Google Scholar]
- Hartman, O. Catalogue of the Polychaetous Annelids of the World. Supplement 1960-1965 and Index. Allan Hancock Found. Publ. Occas. Pap. 1965, 23, 1–197. [Google Scholar]
- Glémarec, M. Les Magelonidae des Coates de Bretagne. Description de Magelona wilsoni n. sp. Vie Milieu 1966, 17, 1077–1085. [Google Scholar]
- Kitamori, R. Magelonidae (Polychaetous Annelids) from Japan, Including the Description of a New Species. Bull. Tokai Reg. Fish. Res. Lab. 1967, 50, 49–54. [Google Scholar]
- Reish, D.J. Benthic Polychaetous Annelids from Bering, Chukchi, and Beaufort Seas. Proc. US Nat. Mus. 1965, 117, 131–157. [Google Scholar] [CrossRef][Green Version]
- Hartmann-Schröder, G. Zweiter Beitrag Zur Polychaetenfauna von Peru. Kiel. Meeresforsch. 1962, 18, 109–147. [Google Scholar]
- Day, J.H. The Polychaet [Sic] Fauna of South Africa. Part 6. Sedentary Species Dredged off Cape Coasts with a Few New Records from the Shore. J. Linn. Soc. London 1961, 44, 463–560. [Google Scholar] [CrossRef]
- Harmelin, J.G. Étude de l’endofaune des “mattes” d’herbiers de Posidonia oceanica Delile. Recl. Trav. Stn. Mar. Endoume 1964, 35, 43–105. [Google Scholar]
- Magalhães, W.F.; Bailey-Brock, J.; Watling, L. Four New Species of Magelona (Annelida: Magelonidae) from Easter Island, Guam and Hawaii. Zootaxa 2018, 4457, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S. Spioniform Polychaetes from Japan. J. Fac. Sci. Hokkaido Univ. Ser. 1937, 6, 217–254. [Google Scholar]
- Clarke, D.T.; Paterson, G.L.J.; Florence, W.K.; Gibbons, M.J. A New Species of Magelona (Polychaeta: Magelonidae) from Southern Namibia. Afr. Nat. Hist. 2010, 6, 77–82. [Google Scholar]
- Wilson, D.P. The Larval Development of Three Species of Magelona (Polychaeta) from Localities near Plymouth. J. Mar. Biol. Assoc. UK 1982, 62, 385–401. [Google Scholar] [CrossRef]
- Mésnil, F. Études de morphologie externe chez les annélides. I. Les spionidens des côtes de la Manche. Bull. Sci. Fr. Belg. 1896, 29, 110–287. [Google Scholar] [CrossRef]
- Romieu, M. Contribution à l’histologie comparée du muscle strié. C. R. Acad. Sci. Paris 1923, 176, 864–867. [Google Scholar]
- Orrhage, L. Über das Vorkommen von Muskelzellen vom “Nematoden-Typus” bei Polychaeten als phylogenetisch-systematisches Merkmal. Zool. Bidr. Upps. 1962, 35, 321–327. [Google Scholar]
- Orrhage, L. Über Die Anatomie Des Zentralen Nervensystemes Der Sedentären Polychaeten. Ark. Zool. 1966, 19, 99–133. [Google Scholar]
- Bartolomaeus, T. Secondary Monociliarity in the Annelida: Monociliated Epidermal Cells in Larvae of Magelona mirabilis (Magelonidae). Microfauna Mar. 1995, 10, 327–332. [Google Scholar]
- Benham, W.B. The Blood of Magelona. Q. J. Microsc. Sci. 1896, 39, 1–17. [Google Scholar]
- Wells, R.M.; Dales, R.P. Oxygenational Properties of Haemerythrin in the Blood of Magelona papillicornis Muller (Polychaeta: Magelonidae). Comp. Biochem. Physiol. 1974, 49A, 57–64. [Google Scholar] [CrossRef]
- Purschke, G. Annelida: Basal groups and Pleistoannelida. In Structure and Evolution of Invertebrate Nervous Systems; Schmidt-Rhaesa, A., Harzsch, S., Purschke, G., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 254–312. [Google Scholar]
- Beckers, P.; Helm, C.; Bartolomaeus, T. The Anatomy and Development of the Nervous System in Magelonidae (Annelida)—Insights into the Evolution of the Annelid Brain. BMC Evol. Biol. 2019, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Filippova, A.; Purschke, G.; Tzetlin, A.B.; Müller, M.C.M. Reconstruction of the Musculature of Magelona cf. mirabilis (Magelonidae) and Prionospio cirrifera (Spionidae) (Polychaeta, Annelida) by Phalloidin Labeling and CLSM. Zoomorphology 2005, 124, 1–8. [Google Scholar] [CrossRef]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of Worms Emended: An Update of Polychaete Feeding Guilds. Annu. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef]
- Wissocq, J.-C. Muscles à striation oblique et à striation transversale chez les Annélides Polychètes. Ital. J. Zool. 1981, 48, 57–74. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Home Page. 2021. Available online: https://www.gbif.org (accessed on 13 January 2021).
- Wilson, D.P. The Polychaete Magelona alleni n. sp. and a Re- Assessment of Magelona cincta Ehlers. J. Mar. Biol. Assoc. UK 1958, 37, 617–626. [Google Scholar] [CrossRef]
- Wilson, D.P. The Polychaete Magelona filiformis n. sp. and Notes on Other Species of Magelona. J. Mar. Biol. Assoc. UK 1959, 38, 547–556. [Google Scholar] [CrossRef]
- Hylleberg, J.; Nateewathana, A. Temporal and Spatial Distribution of Subtidal Magelonid Polychaetes at Phuket Island, Thailand, Andaman Sea. Ophelia 1991, 5, 573–578. [Google Scholar]
- Hannan, C.A. The Life Histories and Population Dynamics of the Polychaetes, Nothria elegans (Johnson) (Onuphidae) and Magelona sacculata Hartman (Magelonidae) in Monterey Bay, California. Master’s Thesis, San Jose State University, San Jose, CA, USA, 1980. [Google Scholar]
- Fajardo, M.; Andrade, D.; Bonicelli, J.; Bon, M.; Gómez, G.; Riascos, J.M.; Pacheco, A.S. Macrobenthic Communities in a Shallow Normoxia to Hypoxia Gradient in the Humboldt Upwelling Ecosystem. PLoS ONE 2018, 13, e0200349. [Google Scholar] [CrossRef] [PubMed]
- Gaston, G. Effects of Hypoxia on Macrobenthos of the Inner Shelf off Cameron, Louisiana. Estuar. Coast. Shelf Sci. 1985, 20, 603–613. [Google Scholar] [CrossRef]
- Sivadas, S.K.; Carvalho, R. Marine Annelida of India: Taxonomy and Status Evaluation and an Updated Checklist. J. Threat. Taxa 2020, 12, 16647–16714. [Google Scholar] [CrossRef]
- Meißner, K.; Darr, A. Distribution of Magelona Species (Polychaeta: Magelonidae) in the German Bight (North Sea): A Modelling Approach. Zoosymposia 2009, 2, 567–586. [Google Scholar] [CrossRef]
- Fauchald, K.; Jumars, P. The Diet of Worms: A Study of Polychaete Feedings Guilds. Oceanogr. Mar. Biol. Ann. Rev. 1979, 17, 193–284. [Google Scholar]
- Wolff, W.J. The Estuary as a Habitat: An Analysis of Data on the Soft-Bottom Macrofauna of the Estuarine Area of the Rivers Rhine, Meuse, and Scheldt. Zool. Verh. 1973, 126, 1–242. [Google Scholar]
- Hartmann-Schroder, G. Annelida, Borstenwurmer, Polychaeta. Tierwelt Dtschl. 1971, 58, 1–594. [Google Scholar]
- McMahon, R.F.; Jones, M.L. Observations on Feeding in Magelona sp. Biol. Bull. 1967, 133, 476. [Google Scholar]
- Hunt, O.D. The Food of the Bottom Fauna of the Plymouth Fishing Grounds. J. Mar. Biol. Ass. UK 1925, 13, 560–599. [Google Scholar] [CrossRef]
- Mare, M.F. A Study of a Marine Benthic Community with Special Reference to the Micro-Organisms. J. Mar. Biol. Ass. UK 1942, 25, 517–554. [Google Scholar] [CrossRef]
- Kühl, H. Über Vorkommen Und Nahrung der Larven von Magelona papillicornis O.F. Müller (Polychaeta Sedentaria) Im Mündungsgebiet von Elbe, Weser Und Ems. Ber. Dtsch. Wiss. Komm. Meeresfirsch. 1974, 23, 296–301. [Google Scholar]
- Rouse, G.W. Polychaete Sperm: Phylogenetic and Functional Considerations. Hydrobiologia 1999, 402, 215–224. [Google Scholar] [CrossRef]
- Rouse, G.W. Chapter 3. In Annelid Sperm and Spermiogenesis; Rouse, G.W., Pleijel, F., Eds.; Science Publishers Inc: Enfield, NH, USA, 2006; pp. 45–76. [Google Scholar]
- Johnson, K.B.; Brink, L.A. Predation on Bivalve Veligers by Polychaete Larvae. Biol. Bull. 1998, 194, 297–303. [Google Scholar] [CrossRef]
- Plate, S.; Husemann, E. Identification Guide to the Planktonic Larvae around the Island of Helgoland (German Bight). Helgol. Meeresunters. 1994, 48, 1–58. [Google Scholar] [CrossRef]
- Carson, H.S.; Hentschel, B.T. Estimating the Dispersal Potential of Polychaete Species in the Southern California Bight: Implications for Designing Marine Reserves. Mar. Ecol. Prog. Ser. 2006, 316, 105–113. [Google Scholar] [CrossRef][Green Version]
- Criales-Hernández, M.I.; Schwamborn, R.; Graco, M.; Ayón, P.; Hirche, H.-J.; Wolff, M. Zooplankton Vertical Distribution and Migration off Central Peru in Relation to the Oxygen Minimum Layer. Helgol. Mar. Res. 2008, 62, 85–100. [Google Scholar] [CrossRef]
- Dean, H.K. The Use of Polychaetes (Annelida) as Indicator Species of Marine Pollution: A Review. Rev. Biol. Trop. 2008, 56, 29. [Google Scholar]
- Pagliosa, P.R. Another Diet of Worms: The Applicability of Polychaete Feeding Guilds as a Useful Conceptual Framework and Biological Variable. Mar. Ecol. 2005, 26, 246–254. [Google Scholar] [CrossRef]
- Liwanow, N.A.; Porfirjewa, N.A. Die Organisation Der Pogonophoren Und Deren Beziehungen Zu Den Polychäten. Biol. Zbl. 1967, 86, 177–204. [Google Scholar]
- Gardiner, S.L. Fine Structure of the Ciliated Epidermis on the Tentacles of Owenia fusiformis (Polychaeta, Oweniidae). Zoomorphologie 1978, 91, 37–48. [Google Scholar] [CrossRef]
- Smith, P.R.; Ruppert, E.E.; Gardiner, S.L. A Deuterostome-like Nephridium in the Mitraria Larva of Owenia fusiformis (Polychaeta, Annelida). Biol. Bull. 1987, 172, 315–323. [Google Scholar] [CrossRef]
- Westheide, W. The Direction of Evolution within the Polychaeta. J. Nat. Hist. 1997, 31, 1–15. [Google Scholar] [CrossRef]
- Hausen, H. Chaetae and chaetogenesis in polychaetes (Annelida). Hydrobiologia 2005, 535, 37–52. [Google Scholar] [CrossRef]
- Rimskaya-Korsakova, N.N.; Kristof, A.; Malakhov, V.V.; Wanninger, A. Neural Architecture of Galathowenia oculata Zach, 1923 (Oweniidae, Annelida). Front. Zool. 2016, 13, 1–19. [Google Scholar] [CrossRef]
- Rimskaya-Korsakova, N.; Dyachuk, V.; Temereva, E. Parapodial Glandular Organs in Owenia borealis (Annelida: Oweniidae) and Their Possible Relationship with Nephridia. J. Exp. Zool. Part B 2020, 334, 88–99. [Google Scholar] [CrossRef]
- Kirkegaard, J.B. The Polychaeta of West Africa Part I. Sedentary Species. Atlantide Rep. 1959, 5, 7–117. [Google Scholar]
- Kirkegaard, J.B. Bathyal Benthic Polychaetes from the N.E. Atlantic Ocean, S.W. of the British Isles. J. Mar. Biol. Ass. UK 1983, 63, 593–608. [Google Scholar] [CrossRef]
- Fauvel, P. Polychètes Sédentaires. Addenda aux Errantes, Archiannélides, Myzostomaires. Faune France 1927, 16, 1–494. [Google Scholar]
- Uschakov, P. Polychaeta of the Far Eastern Seas of the U.S.S.R.; Israel Program for Scientific Translations: Jerusalem, Israel, 1965. [Google Scholar]
- Hartman, O. Polychaeta Myzostomidae and Sedentaria of Antarctica. Antarctic Res. Ser. 1966, 7, 1–158. [Google Scholar]
- Day, J.H. A Monograph on the Polychaeta of Southern Africa 2. Sedentaria. Brit. Mus. (Nat. Hist.) Lond. 1967, 459–878. [Google Scholar]
- Hartmann-Schröder, G. Annelida, Borstenwürmer, Polychaeta. Tierwelt Dtschl. 1996, 58, 1–648. [Google Scholar]
- Gil, J. The European Fauna of Annelida Polychaeta. Ph.D. Thesis, University of Lisboa, Lisbon, Portugal, 2011. [Google Scholar]
- Rouse, G.W. Oweniidae. In Polychaetes; Rouse, G.W., Pleijel, F., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 185–188. [Google Scholar] [CrossRef]
- Watson, A.T. On the structure and habits of the Polychaeta of the family Ammocharidae. J. Linn. Soc. 1901, 28, 230–260. [Google Scholar] [CrossRef]
- Wilson, D.P. On the Mitraria Larva of Owenia fusiformis Delle Chiaje. Philos. T. R. Soc. Lon. B. 1932, 22, 231–334. [Google Scholar]
- Dales, R.P. The Feeding Mechanism and Structure of the Gut of Owenia fusiformis Delle Chiaje. J. Mar. Biol. Ass. UK 1957, 36, 81–89. [Google Scholar] [CrossRef]
- Beckers, P.; Helm, C.; Purschke, G.; Worsaae, K.; Hutchings, P.; Bartolomaeus, T. The central nervous system of Oweniidae (Annelida) and its implications for the structure of the ancestral annelid brain. Front. Zool. 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, P.A. Family Oweniidae. In Polychaetes & Allies: The Southern Synthesis; Fauna of Australia. Vol. 4A Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula; Beesley, P.L., Ross, G.J.B., Glasby, C.J., Eds.; CSIRO Publishing: Melbourne, Australia, 2000; pp. 173–176. [Google Scholar]
- Koh, B.-S.; Bhaud, M. Identification of New Criteria for Differentiating between Populations of Owenia fusiformis (Annelida Polychaeta) from Different Origins: Rehabilitation of Old Species and Erection of New Species. Vie Milieu 2003, 53, 65–95. [Google Scholar]
- Silva, L.; Lana, P. Strategies for tube construction in Owenia caissara (Oweniidae, Annelida) from southern Brazil. Zoology 2018, 129, 9–16. [Google Scholar] [CrossRef]
- Caullery, M. Polychètes Sédentaire de l’Expédition Du Siboga: Ariciidae, Spionidae, Chaetopteridae, Chlorhaemidae, Opheliidae, Oweniidae, Sabellariidae, Sternaspidae, Amphictenidae, Ampharetidae, Terebellidae. In Siboga-Expeditie Uitkomsten op Zoologisch, Bonatisch, Oceanographisch en Geologisch gebied Verzameld in Nederlandsch Oost-Indië 1899-1900; World Register of Marine Species: Ostend, Belgium, 1944; Volume XXIV 2 bis, pp. 1–204. [Google Scholar]
- Verrill, A.E. Notice of Recent Additions to the Marine Invertebrata of the Northeastern Coast of America, with Descriptions of New Genera and Species and Critical Remarks on Others. Part V. Annelida, Echinodermata, Hydroida, Tunicata. Proc. US Nat. Mus. 1885, 8, 424–448. [Google Scholar] [CrossRef]
- Rioja, E. Estudio de Los Poliquetos de La Península Ibérica. Mem. Real Acad. Ci. Exact. Madrid Ser. Cienc. Nat. 1931, 2, 1–471. [Google Scholar]
- Díaz-Díaz, O.F.; Parapar, J.; Moreira, J. Validation of Owenia vieitezi Díaz-Díaz, Parapar & Moreira, a polychaete from the coast of Venezuela (Annelida: Oweniidae). Zootaxa 2020, 4729, 145–146. [Google Scholar]
- Malmgren, A.J. Annulata Polychaeta Spetsbergiae, Grönlandiae et Scandinaviae Hactenus Cognita. Öfvers. K. Vet. Akad. Förh. 1867, 24, 127–235. [Google Scholar]
- Delle Chiaje, S. Descrizione e Notomia degli Animali Invertebrati della Sicilia Citeriore osservati vivi negli anni 1822–1830. Tomo Ottavo. Appendice, Osservazioni Critiche, Indice Generale; Stabilimento Tipografico di C. Batelli e Comp.: Naples, Italy, 1844. [Google Scholar]
- Berkeley, E. Morphological characters of Myriochele heeri Malmgren. Nature 1949, 164, 239. [Google Scholar] [CrossRef]
- Rullier, F. Étude Morphologique, Histologique et Physiologique de l’organe Nucal Chez Les Annélides Polychètes Sédentaires. Ann. Inst. Ocean. 1951, 25, 207–341. [Google Scholar]
- Gardiner, S.L.; Rieger, R.M. Rudimentary Cilia in Muscle Cells of Annelids and Echinoderms. Cell Tissue Res. 1980, 213, 247–252. [Google Scholar] [CrossRef]
- Tzetlin, A.B.; Filippova, A.V. Muscular system in polychaetes (Annelida). Hydrobiologia 2005, 535, 113–126. [Google Scholar] [CrossRef]
- Fransen, M.E. Coelomic and Vascular Systems. Chapter XII. In The Ultrastructure of Polychaeta. Microfauna Marina; Westheide, W., Hermans, C.O., Eds.; Fischer Gustav Verlag GmbH & Co. KG.: Sttutgart, Germany, 1988; Volume 4, pp. 199–213. [Google Scholar]
- Parapar, J.; Candás, M.; Cunha-Veira, X.; Moreira, J. Exploring annelid anatomy using micro-computed tomography: A taxonomic approach. Zool. Anz. 2017, 270, 19–42. [Google Scholar] [CrossRef]
- Tzetlin, A.B.; Purschke, G. Pharynx and intestine. Hydrobiologia 2005, 535, 199–205. [Google Scholar] [CrossRef]
- Purschke, G.; Tzetlin, A.B. Dorsolateral Ciliary Folds in the Polychaete Foregut: Structure, Prevalence and Phylogenetic Significance. Acta Zool. 1996, 77, 33–49. [Google Scholar] [CrossRef]
- Bubko, O.V.; Minichev, Y.S. The Nervous System of Oweniidae (Polychaeta). Zool. Zhurnal 1972, 51, 1288–1299. [Google Scholar]
- Coulon, J.; Bessone, R. Autoradiographic Detection of Indolamine and Catecholamine Neurons in the Nervous System of Owenia fusiformis (Polychaeta, Annelida). Cell Tissue Res. 1979, 198, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Purschke, G. Sense Organs in Polychaetes (Annelida). Hydrobiologia 2005, 535, 53–78. [Google Scholar] [CrossRef]
- Purschke, G.; Arendt, D.; Hausen, H.; Müller, M.C.M. Photoreceptor Cells and Eyes in Annelida. Arthropod Struct. Dev. 2006, 35, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Helm, C.; Vöcking, O.; Kourtesis, I.; Hausen, H. Owenia fusiformis—A Basally Branching Annelid Suitable for Studying Ancestral Features of Annelid Neural Development. BMC Evol. Biol. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Rioja, E. Datos para el conocimiento de la fauna de anélidos poliquetos del Cantábrico. Trab. Mus. Nac. Cienc. Nat. Ser. Zool. 1917, 29, 1–111. [Google Scholar]
- Day, J.H. New Polychaeta from Beaufort, with a Key to All Species Recorded from North Carolina; NOAA tech. Rep. NMFS, circ. # 375; U.S. Dept. of Commerce, NOAA, Marine Fisheries Service: Seattle, WA, USA, 1973; pp. 1–140. [Google Scholar]
- Fauchald, K. The Polychaete Worms. Definitions and Keys to the Orders, Families and Genera. Nat. Hist. Mus. Los Angel. Cty. Sci. Ser. 1977, 28, 1–188. [Google Scholar]
- Pettibone, M.H. Annelida. In Synopsis and Classification of Living Organisms; Parker, S.P., Ed.; McGraw-Hill Book Co: New York, NY, USA, 1982; Volume 2, pp. 1–43. [Google Scholar]
- Hartman, O. Catalogue of the polychaetous annelids of the world. Allan Hancock Found. Publ. Occas. Pap. 1959, 23, 1–628. [Google Scholar]
- Hutchings, P.A.; Rainer, S. The Polychaete Fauna of Careel Bay, Pittwater, New South Wales, Australia. J. Nat. Hist. 1979, 13, 745–796. [Google Scholar] [CrossRef]
- Day, J.H.; Hutchings, P.A. Descriptive Notes on the Fauna and Flora of Merimbula, Pambula and Black Lakes, New South Wales. Aust. Zool. 1984, 21, 269–289. [Google Scholar]
- Hutchings, P.; Murray, A. Taxonomy of Polychaetes from the Hawkesbury River and the Southern Estuaries of New South Wales, Australia. Rec. Aust. Mus. Suppl. 1984, 3, 1–118. [Google Scholar] [CrossRef]
- Nygren, A. Cryptic Polychaete Diversity: A Review. Zool. Scr. 2014, 43, 172–183. [Google Scholar] [CrossRef]
- Hutchings, P.; Kupriyanova, E. Cosmopolitan Polychaetes—Fact or Fiction? Personal and Historical Perspectives. Invert. Syst. 2018, 32, 1. [Google Scholar] [CrossRef]
- Jolly, M.T.; Viard, F.; Gentil, F.; Thiébaut, E.; Jollivet, D. Comparative Phylogeography of Two Coastal Polychaete Tubeworms in the Northeast Atlantic Supports Shared History and Vicariant Events: Comparative Phylogeography Of Coastal Tubeworms. Mol. Ecol. 2006, 15, 1841–1855. [Google Scholar] [CrossRef] [PubMed]
- Gentil, F.; Dauvin, J.-C.; Ménard, F. Reproductive Biology of the Polychaete Owenia fusiformis Delle Chiaje in the Bay of Seine (Eastern English Channel). J. Exp. Mar Biol. Ecol. 1990, 142, 13–23. [Google Scholar] [CrossRef]
- McNulty, J.K.; López, N.N. Year-Round Production of Ripe Gametes by Benthic Polychaetes in Biscayne Bay, Florida. Bull. Mar. Sci. 1969, 19, 945–954. [Google Scholar]
- Thiébaut, E.; Dauvin, J.C. Modelling of population dynamics of Owenia fusiformis (Annelida, Polychaeta) in eastern Bay of Seine. In Report of the Workshop Modelling the Benthos Yerseke, The Netherlands; Herman, P.M.J., Ed.; Delta Institute Communication, Delta Institute for Hydrobiological Research: Yerseke, The Netherlands, 1991; Volume 538, pp. 189–190. [Google Scholar]
- Okada, K.A. Ch. 7. Annelida. In Invertebrate Embryology; Kume, M., Dan, K., Eds.; Bai Fu Kan Press: Tokyo, Japan, 1970; pp. 192–241. [Google Scholar]
- Oliver, J.S. Selection for Asexual Reproduction in an Antarctic Polychaete Worm. Mar. Ecol. Prog. Ser. 1984, 19, 3–38. [Google Scholar] [CrossRef]
- Aguirrezabalaga, F.; Gil, J.; Viéitez, J.M. Presence of Myriochele danielsseni Hansen, 1879 (Polychaeta, Oweniidae) en las costas de la Península Ibérica. Bol. R. Soc. Esp. Hist. Nat. 2000, 96, 57–68. [Google Scholar]
- Signor, P.W.; McMenamin, M.A.S. The early Cambrian worm tube Onuphionella from California and Nevada. J. Paleontol. 1988, 62, 233–240. [Google Scholar] [CrossRef]
- Gambi, M.C. Osservazioni su morfologia funzionale e comportamento trofico di Owenia fusiformis delle Chiaje (Polychaeta, Oweniidae) in rapporto ai fattori ambientali. Oebalia 1989, 15, 145–155. [Google Scholar]
- Dales, R.P. The Nature of the Pigments in the Crown of Sabellid and Serpulid Polychaetes. J. Mar. Biol. Assoc. UK 1962, 42, 259–274. [Google Scholar] [CrossRef]
- Ziegelmeier, E. Neue Untersuchungen über die Wohnröhren-Bauweise von Lanice conchilega (Polychaeta, Sedentaria). Helgol. Wiss. Meeresunters. 1969, 19, 216–229. [Google Scholar] [CrossRef]
- Künitzer, A. Zur Verbreitung und Populationsdynamik der Bodenfauna der Zentralen Nordsee. PhD. Thesis, Universität Bremen, Bremen, Germany, 1990. [Google Scholar]
- Dauvin, J.-C.; Gillet, P. Spatio-Temporal Variability in Population Structure of Owenia fusiformis Delle Chiaje (Annelida: Polychaeta) from the Bay of Seine (Eastern English Channel). J. Exp. Mar. Biol. Ecol. 1991, 152, 105–122. [Google Scholar] [CrossRef]
- Pinedo, S.; Sardá, R.; Rey, C.; Bhaud, M. Effect of Sediment Particle Size on Recruitment of Owenia fusiformis in the Bay of Blanes (NW Mediterranean Sea): An Experimental Approach to Explain Field Distribution. Mar. Ecol. Prog. Ser. 2000, 203, 205–213. [Google Scholar] [CrossRef][Green Version]
- Deart, Y.V.; Britayev, T.A. “New” Benthic Community Dominated by Oweniidae (Polychaeta, Oweniidae) at the Murman Coast: Structure and Causes of Appearance. Dokl. Biol. Sci. 2014, 454, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Bailey-Brock, J.H.; McGurr, M.M. Temporal Changes in the Polychaete Infaunal Community Surrounding a Hawaiian Mariculture Operation. Mar. Ecol. Prog. Ser. 2006, 307, 175–185. [Google Scholar] [CrossRef]
- Kopp, D.; Le Bris, H.; Grimaud, L.; Nérot, C.; Brind’Amour, A. Spatial Analysis of the Trophic Interactions between Two Juvenile Fish Species and Their Preys along a Coastal–Estuarine Gradient. J. Sea Res. 2013, 81, 40–48. [Google Scholar] [CrossRef][Green Version]
- Fager, E.W. Marine Sediments: Effects of Tube-Dwelling Polychaete. Science 1964, 143, 356–359. [Google Scholar] [CrossRef]
- Eckman, J.E.; Nowell, A.R.M.; Jumars, P.A. Sediment destabilization by animal tubes. J. Mar. Res. 1981, 39, 361–374. [Google Scholar]
- Somaschini, A. A Mediterranean Fine-Sand Polychaete Community and the Effect of the Tube-Dwelling Owenia fusiformis Delle Chiaje on Community Structure. Int. Rev. Gesamten Hydrobiol. 1993, 78, 219–233. [Google Scholar] [CrossRef]
- Ambrogi, R.; Fontana, P.; Gambi, M.C. Population dynamics and estimate of secondary production of Owenia fusiformis Delle Chiaje (Polychaeta, Oweniidae) in the coastal area of the Po river Delta (Italy). In Biology and Ecology of Shallow Coastal Waters; Eleftheriou, A., Smith, C.J., Ansell, A.D., Eds.; Olsen & Olsen: Fredensborg, Denmark, 1995; pp. 207–214. [Google Scholar]
- Oyarzún, C.; Fernández, C.; Landaeta, M.; Cortés, N. Ontogenetic and Temporal Fluctuations in Feeding Habits of the Chilean Croaker Cilus gilberti (Perciformes, Sciaenidae) in Southern Chile. Cah. Biol. Mar. 2001, 42, 295–302. [Google Scholar]
- Hartman, O. Systematic account of some marine invertebrate animals from the deep basins of southern California. In The Benthic Fauna of the Deep Basins off Southern California. Part II; Hartman, O., Barnard, J.L., Eds.; Allan Hancock Pacific Expeditions, University of Southern California Press: Los Angeles, CA, USA, 1960; Volume 22, pp. 69–215. [Google Scholar]
- Annenkova, N.P. The Polychaete Fauna of the Northern Part of the Japan Sea. Issled. Rus. Mor. 1937, 23, 139–216. [Google Scholar]
- McIntosh, W.C. Report on the Annelida Polychaeta Collected by H.M.S. Challenger during the Years 1873-1876. Chall. Rep. (Zool.) 1885, 12, 1–554. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).