Raccoon Vigilance and Activity Patterns When Sympatric with Coyotes
Abstract
1. Introduction
2. Materials and Methods
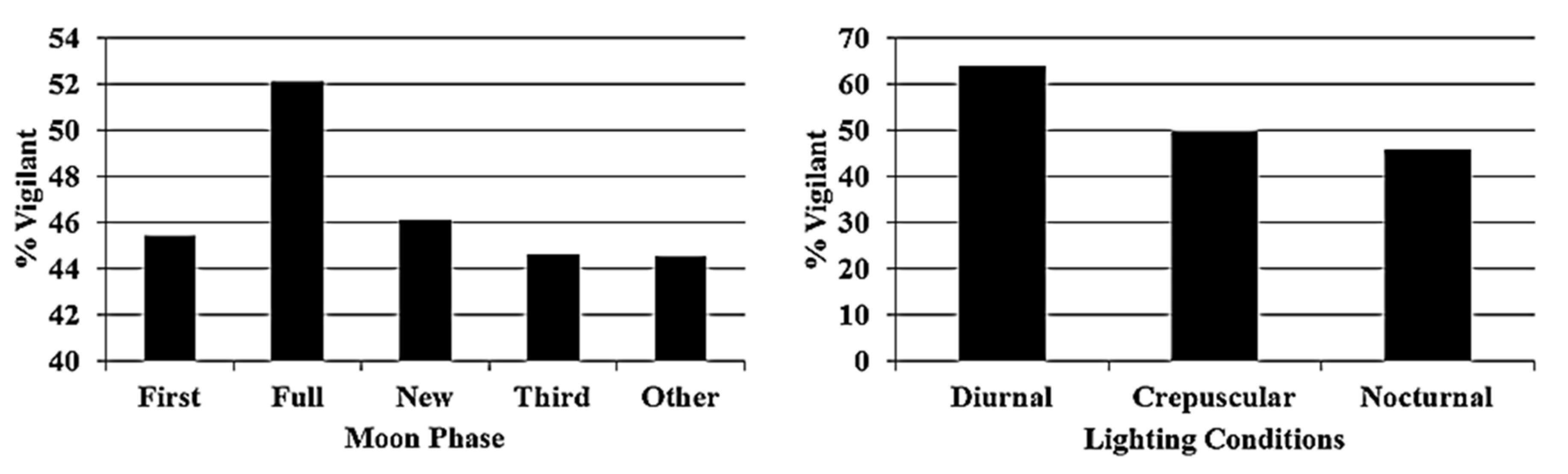
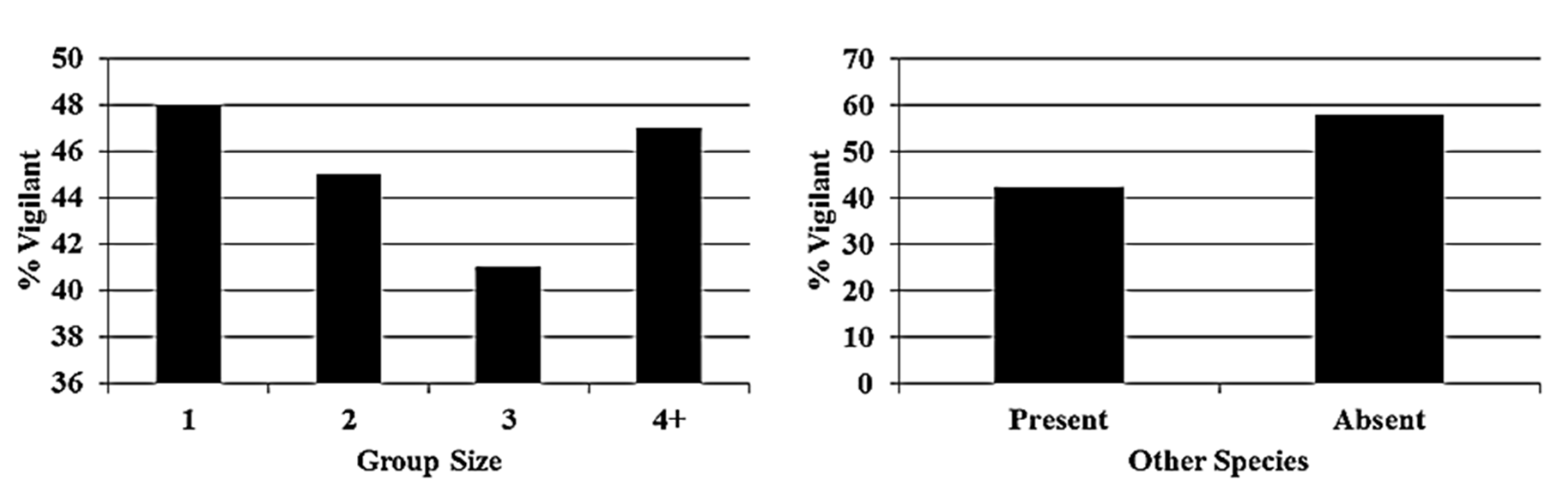
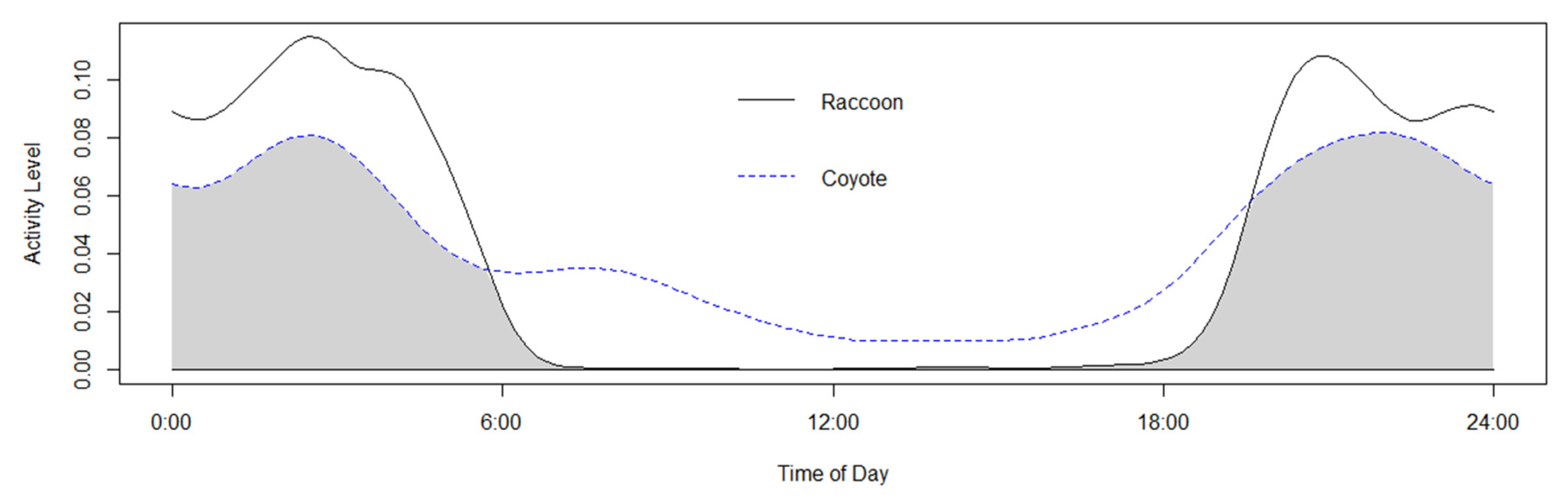
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werner, E.E.; Peacor, S.D. A review of trait-mediated indirect interactions in ecological communities. Ecology 2003, 84, 1083–1100. [Google Scholar] [CrossRef]
- Winnie, J., Jr.; Christianson, D.; Creel, S.; Maxwell, B. Elk decision-making rules are simplified in the presence of wolves. Behav. Ecol. Sociobiol. 2006, 61, 277–289. [Google Scholar] [CrossRef]
- Nelson, E.H.; Matthews, C.E.; Rosenheim, J.A. Predators reduce prey population growth by inducing changes in prey behavior. Ecology 2004, 85, 1853–1858. [Google Scholar] [CrossRef]
- Sih, A.; McCarthy, T.M. Prey responses to pulses of risk and safety: Testing the risk allocation hypothesis. Anim. Behav. 2002, 63, 437–443. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Creel, S.; Winnie, J.A. Responses of elk herd size to fine-scale spatial and temporal variation in the risk of predation by wolves. Anim. Behav. 2005, 69, 1181–1189. [Google Scholar] [CrossRef]
- Bergerud, A.T.; Wyett, W.; Snider, B. The role of wolf predation in limiting a moose population. J. Wildl. Manag. 1983, 47, 977–988. [Google Scholar] [CrossRef]
- Formanowicz, D.R., Jr.; Bobka, M.S. Predation risk and microhabitat preference: An experimental study of the behavioral responses of prey and predator. Am. Midl. Nat. 1989, 121, 379–386. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Daniel, J.C. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. Lond. B Biol. Sci. 2005, 272, 1663–1668. [Google Scholar] [CrossRef]
- Heithaus, M.R.; Dill, L.M. Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology 2002, 83, 480–491. [Google Scholar] [CrossRef]
- Hughes, J.J.; Ward, D. Predation risk and distance to cover affect foraging behaviour in Namib Desert gerbils. Anim. Behav. 1993, 46, 1243–1245. [Google Scholar] [CrossRef]
- Abramsky, Z.; Rosenzweig, M.L.; Subach, A. The costs of apprehensive foraging. Ecology 2002, 83, 1330–1340. [Google Scholar] [CrossRef]
- Elgar, M.A. Predator vigilance and group size in mammals and birds: A critical review of the empirical evidence. Biol. Rev. 1989, 64, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Houston, A.I.; McNamara, J.M.; Hutchinson, J.M.C. General Results concerning the trade-off between gaining energy and avoiding predation. Philos. Trans. R. Soc. B Biol. Sci. 1993, 341, 375–397. [Google Scholar]
- Brown, J.S. Vigilance, patch use and habitat selection: Foraging under predation risk. Evol. Ecol. Res. 1999, 1, 49–71. [Google Scholar]
- Fortin, D.; Boyce, M.S.; Merrill, E.H.; Fryxell, J.M. Foraging costs of vigilance in large mammalian herbivores. Oikos 2004, 107, 172–180. [Google Scholar] [CrossRef]
- Sönnichsen, L.; Bokje, M.; Marchal, J.; Hofer, H.; Jędrzejewska, B.; Kramer-Schadt, S.; Ortmann, S. Behavioural responses of European roe deer to temporal variation in predation risk. Ethology 2013, 119, 233–243. [Google Scholar] [CrossRef]
- Kotler, B.P.; Brown, J.; Mukherjee, S.; Berger-Tal, O.; Bouskila, A. Moonlight avoidance in gerbils reveals a sophisticated interplay among time allocation, vigilance and state-dependent foraging. Proc. R. Soc. B Biol. Sci. 2010, 277, 1469–1474. [Google Scholar] [CrossRef]
- Parker, G.R. Eastern Coyote: The Story of Its Success; Nimbus Publishing: Halifax, NS, Canada, 1995. [Google Scholar]
- Gompper, M.E. Top carnivores in the suburbs? Ecological and conservation issues raised by colonization of north-eastern North America by coyotes. BioScience 2002, 52, 185–190. [Google Scholar] [CrossRef]
- Hill, E.P.; Sumner, P.W.; Wooding, J.B. Human influences on range expansion of coyotes in the southeast. Wildl. Soc. Bull. 1987, 15, 521–524. [Google Scholar]
- Jones, B.M.; Cove, M.V.; Lashley, M.A.; Jackson, V.L. Do coyotes Canis latrans influence occupancy of prey in suburban forest fragments? Curr. Zool. 2016, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, M.C.; Lashley, M.A.; Kilgo, J.C.; Moorman, C.E.; DePerno, C.S. White-Tailed deer population dynamics and adult female survival in the presence of a novel predator. J. Wildl. Manag. 2015, 79, 211–219. [Google Scholar] [CrossRef]
- Cherry, M.J.; Conner, L.M.; Warren, R.J. Effects of predation risk and group dynamics on white-tailed deer foraging behavior in a longleaf pine savanna. Behav. Ecol. 2015, 26, 1091–1099. [Google Scholar] [CrossRef]
- Cherry, M.J.; Morgan, K.E.; Rutledge, B.T.; Conner, L.M.; Warren, R.J. Can coyote predation risk induce reproduction suppression in white-tailed deer? Ecosphere 2016, 7, e01481. [Google Scholar] [CrossRef]
- Phillips, M.K.; Parker, W.T. Red wolf recovery: A progress report. Conserv. Biol. 1988, 2, 139–141. [Google Scholar] [CrossRef]
- Phillips, M.K.; Henry, V.G.; Kelly, B.T. Restoration of the red wolf. In Wolves: Behavior, Ecology, and Conservation; Mech, L.D., Boitani, L., Eds.; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Suraci, J.P.; Clinchy, M.; Dill, L.M.; Roberts, D.; Zanette, L.Y. Fear of large carnivores causes a trophic cascade. Nat. Commun. 2016, 7, 10698. [Google Scholar] [CrossRef]
- Downes, S. Trading heat and food for safety: Costs of predator avoidance in a lizard. Ecology 2001, 82, 2870–2881. [Google Scholar] [CrossRef]
- Harmsen, B.J.; Foster, R.J.; Silver, S.C.; Ostro, L.E.; Doncaster, C.P. Jaguar and puma activity patterns in relation to their main prey. Mamm. Biol. 2011, 76, 320–324. [Google Scholar] [CrossRef]
- Foster, V.C.; Sarmento, P.; Sollmann, R.; Tôrres, N.; Jácomo, A.T.A.; Negrões, N.; Fonseca, C.; Silveira, L. Jaguar and puma activity patterns and predator-prey interactions in four Brazilian biomes. Biotropica 2013, 45, 373–379. [Google Scholar] [CrossRef]
- Ross, J.; Hearn, A.J.; Johnson, P.J.; Macdonald, D.W. Activity patterns and temporal avoidance by prey in response to Sunda clouded leopard predation risk. J. Zool. 2013, 290, 96–106. [Google Scholar] [CrossRef]
- Delibes-Mateos, M.; Díaz-Ruiz, F.; Caro, J.; Ferreras, P. Activity patterns of the vulnerable guiña (Leopardus guigna) and its main prey in the Valdivian rainforest of southern Chile. Mamm. Biol. 2014, 79, 393–397. [Google Scholar] [CrossRef]
- Gehrt, S.D.; Clark, W.R. Raccoons, coyotes, and reflections on the mesopredator release hypothesis. Wildl. Soc. Bull. 2003, 31, 836–842. [Google Scholar]
- Gehrt, S.D.; Prange, S. Interference competition between coyotes and raccoons: A test of the mesopredator release hypothesis. Behav. Ecol. 2007, 18, 204–214. [Google Scholar] [CrossRef]
- Lesmeister, D.B.; Nielsen, C.K.; Schauber, E.M.; Hellgren, E.C. Spatial and temporal structure of a mesocarnivore guild in midwestern North America. Wildl. Monogr. 2015, 191, 1–61. [Google Scholar] [CrossRef]
- Lashley, M.A.; Chitwood, M.C.; Prince, A.; Elfelt, M.B.; Kilburg, E.L.; DePerno, C.S.; Moorman, C.E. Subtle effects of a managed fire regime: A case study in the longleaf pine ecosystem. Ecol. Indic. 2014, 38, 212–217. [Google Scholar] [CrossRef]
- Chitwood, M.C.; Lashley, M.A.; Moorman, C.E.; DePerno, C.S. Confirmation of coyote predation on adult female white-tailed deer in the southeastern United States. Southeast. Nat. 2014, 13, N30–N32. [Google Scholar] [CrossRef]
- Chitwood, M.C.; Lashley, M.A.; Kilgo, J.C.; Pollock, K.; Moorman, C.E.; DePerno, C.S. Do biological and bedsite characteristics influence survival of neonatal white-tailed deer? PLoS ONE 2015, 10, e0119070. [Google Scholar] [CrossRef]
- Elfelt, M.B. Coyote Movement Ecology and Food Habits at Fort Bragg Military Installation. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2014. [Google Scholar]
- Stevenson, E.R.; Chitwood, M.C.; Lashley, M.A.; Pollock, K.H.; Swingen, M.B.; Moorman, C.E.; DePerno, C.S. Survival and cause-specific mortality of coyotes on a large military installation. Southeast. Nat. 2016, 15, 459–466. [Google Scholar] [CrossRef]
- Stevenson, E.R.; Lashley, M.A.; Chitwood, M.C.; Garabedian, J.E.; Swingen, M.B.; DePerno, C.S.; Moorman, C.E. Resource selection by coyotes (Canis latrans) in a longleaf pine (Pinus palustris) ecosystem: Effects of anthropogenic fires and landscape features. Can. J. Zool. 2019, 97, 165–171. [Google Scholar] [CrossRef]
- Jacobson, H.A.; Kroll, J.C.; Browning, R.W.; Koerth, B.H.; Conway, M.H. Infrared-Triggered cameras for censusing white-tailed deer. Wildl. Soc. Bull. 1997, 25, 547–556. [Google Scholar]
- Lashley, M.A.; Chitwood, M.C.; Biggerstaff, M.T.; Morina, D.L.; Moorman, C.E.; DePerno, C.S. White-tailed deer vigilance: The influence of social and environmental factors. PLoS ONE 2014, 9, e90652. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.S.; Noss, A.J. Behavior and activity patterns. In Camera-Traps in Animal Ecology; O’Connell, A.F., Nichols, J.D., Karanth, K.U., Eds.; Springer: New York, NY, USA, 2011; pp. 57–70. [Google Scholar]
- Rowcliffe, J.M.; Kays, R.; Kranstauber, B.; Carbone, C.; Jansen, P.A. Quantifying levels of animal activity using camera trap data. Methods Ecol. Evol. 2014, 5, 1170–1179. [Google Scholar] [CrossRef]
- Lashley, M.A.; Cove, M.V.; Chitwood, M.C.; Penido, G.; Gardner, B.; DePerno, C.S.; Moorman, C.E. Estimating wildlife activity curves: Comparison of methods and sample size. Sci. Rep. 2018, 8, 4173. [Google Scholar] [CrossRef] [PubMed]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overlap: Estimates of Animal Activity. R Package Version 0.2.3. Available online: https://cran.r-project.org/web/packages/overlap/ (accessed on 14 July 2015).
- Lund, U.; Agostinelli, C. CircStats: Circular Statistics. R Package Version 0.2-4. Available online: https://cran.r-project.org/web/packages/CircStats/ (accessed on 14 July 2015).
- Etheredge, C. Ecology and Impacts of Coyotes (Canis latrans) in the Southeastern United States. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2013. [Google Scholar]
- Brown, J.S.; Kotler, B.P.; Smith, R.J.; Wirtz, W.O. The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia 1988, 76, 408–415. [Google Scholar] [CrossRef]
- Kotler, B.P.; Brown, J.S.; Smith, R.J.; Wirtz, W.O. The effects of morphology and body size on rates of owl predation on desert rodents. Oikos 1988, 53, 145–152. [Google Scholar] [CrossRef]
- Kotler, B.P.; Brown, J.S.; Hasson, O. Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 1991, 72, 2249–2260. [Google Scholar] [CrossRef]
- Biebouw, K.; Blumstein, D.T. Tammar wallabies (Macropus eugenii) associate safety with higher levels of nocturnal illumination. Ethol. Ecol. Evol. 2003, 15, 159–172. [Google Scholar] [CrossRef]
- Roberts, G. Why individual vigilance declines as group size increases. Anim. Behav. 1996, 51, 1077–1086. [Google Scholar] [CrossRef]
- Kays, R.; Costello, R.; Forrester, T.; Baker, M.C.; Parsons, A.W.; Kalies, E.L.; Hess, G.; Millspaugh, J.J.; McShea, W. Cats are rare where coyotes roam. J. Mammal. 2015, 96, 981–987. [Google Scholar] [CrossRef]
- Crooks, K.R.; Soulé, M.E. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 1999, 400, 563–566. [Google Scholar] [CrossRef]
- Sergio, F.; Marchesi, L.; Pedrini, P.; Penteriani, V. Coexistence of a generalist owl with its intraguild predator: Distance-Sensitive or habitat-mediated avoidance? Anim. Behav. 2007, 74, 1607–1616. [Google Scholar] [CrossRef]
- Prugh, L.R.; Stoner, C.J.; Epps, C.W.; Bean, W.T.; Ripple, W.J.; Laliberte, A.S.; Brashares, J.S. The rise of the mesopredator. BioScience 2009, 59, 779–791. [Google Scholar] [CrossRef]
- Ritchie, E.G.; Johnson, C.N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 2009, 12, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Cove, M.V.; Jones, B.M.; Bossert, A.J.; Clever, D.R., Jr.; Dunwoody, R.K.; White, B.C.; Jackson, V.L. Use of camera traps to examine the mesopredator release hypothesis in a fragmented midwestern landscape. Am. Midl. Nat. 2012, 168, 456–465. [Google Scholar] [CrossRef]
- Prange, S.; Gehrt, S.D. Response of skunks to a simulated increase in coyote activity. J. Mammal. 2007, 88, 1040–1049. [Google Scholar] [CrossRef]
- Swingen, M.B.; DePerno, C.S.; Moorman, C.E. Seasonal coyote diet composition at a low-productivity site. Southeast. Nat. 2015, 14, 397–404. [Google Scholar] [CrossRef]
- Schrecengost, J.D.; Kilgo, J.C.; Mallard, D.; Ray, H.S.; Miller, K.V. Seasonal food habits of the coyote in the South Carolina coastal plain. Southeast. Nat. 2008, 7, 135–144. [Google Scholar] [CrossRef]
- McVey, J.M.; Cobb, D.T.; Powell, R.A.; Stoskopf, M.K.; Bohling, J.H.; Waits, L.P.; Moorman, C.E. Diets of sympatric red wolves and coyotes in northeastern North Carolina. J. Mammal. 2013, 94, 1141–1148. [Google Scholar] [CrossRef]
- Cherry, M.J.; Turner, K.L.; Howze, M.B.; Cohen, B.S.; Conner, L.M.; Warren, R.J. Coyote diets in a longleaf pine ecosystem. Wildl. Biol. 2016, 22, 64–70. [Google Scholar] [CrossRef]
- Morin, D.J.; Higdon, S.D.; Holub, J.L.; Montague, D.M.; Fies, M.L.; Waits, L.P.; Kelly, M.J. Bias in carnivore diet analysis resulting from misclassification of predator scats based on field identification. Wildl. Soc. Bull. 2016, 40, 669–677. [Google Scholar] [CrossRef]
- McDonald, R.A.; O’Hara, K.; Morrish, D.J. Decline of invasive alien mink (Mustela vison) is concurrent with recovery of native otters (Lutra lutra). Divers. Distrib. 2007, 13, 92–98. [Google Scholar] [CrossRef]
- Caut, S.; Casanovas, J.G.; Virgós, E.; Lozano, J.; Witmer, G.W.; Courchamp, F. Rats dying for mice: Modelling the competitor release effect. Austral Ecol. 2007, 32, 858–868. [Google Scholar] [CrossRef]
- Casanovas, J.G.; Barrull, J.; Mate, I.; Zorrilla, J.M.; Ruiz-Olmo, J.; Gosalbez, J.; Salicrú, M. Shaping carnivore communities by predator control: Competitor release revisited. Ecol. Res. 2012, 27, 603–614. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitwood, M.C.; Lashley, M.A.; Higdon, S.D.; DePerno, C.S.; Moorman, C.E. Raccoon Vigilance and Activity Patterns When Sympatric with Coyotes. Diversity 2020, 12, 341. https://doi.org/10.3390/d12090341
Chitwood MC, Lashley MA, Higdon SD, DePerno CS, Moorman CE. Raccoon Vigilance and Activity Patterns When Sympatric with Coyotes. Diversity. 2020; 12(9):341. https://doi.org/10.3390/d12090341
Chicago/Turabian StyleChitwood, M. Colter, Marcus A. Lashley, Summer D. Higdon, Christopher S. DePerno, and Christopher E. Moorman. 2020. "Raccoon Vigilance and Activity Patterns When Sympatric with Coyotes" Diversity 12, no. 9: 341. https://doi.org/10.3390/d12090341
APA StyleChitwood, M. C., Lashley, M. A., Higdon, S. D., DePerno, C. S., & Moorman, C. E. (2020). Raccoon Vigilance and Activity Patterns When Sympatric with Coyotes. Diversity, 12(9), 341. https://doi.org/10.3390/d12090341




