Abstract
Usually, biospeleological studies focus on cave-specialist taxa showing strong adaptation to the subterranean environment, as their unusual morphological and ecological features represent intriguing case studies. On the other hand, species occurring in subterranean environments but without marked adaptations have been generally overlooked, probably because they are thought to be accidental and not very important for the ecosystem. Particularly exemplificative is the case of Tipuloidea crane flies (Diptera), which although abundant, are rarely considered in biospeleological studies. Here, by analyzing the crane fly occupancy, we observed that individuals occur within the shallowest areas of subterranean environments throughout the year, with a peak of presence during hot season. Crane flies occupy dark and warm areas close to the connection with surface and with smoother walls. Furthermore, we observed that the presence of crane flies is positively related to the abundance and the richness of cave predators, highlighting their importance for the sustainment of the local community. With this study we aim to stimulate future researches on these important, but still neglected cave species.
1. Introduction
Studies on cave adapted species have gained large interest during the last decades, a trend demonstrated by the growing available literature [1,2,3,4,5]. The high interest in subterranean environments and its biota is often related to the peculiar study-cases offered to taxonomists, ecologists, and evolutionary biologists [6]. Indeed, subterranean environments have represented (and still represent) a source of unknown and bizarre organisms that help understanding adaptation and evolution in extreme environments [7,8,9]. The appearance of troglomorphic characters (e.g., reduction in pigmentation, anophthalmia, and elongation of appendages) is related to the peculiar ecological conditions of subterranean environments, which are very different from those found in any surface ones [1]. The subterranean environments show high stability and have a natural microclimatic gradient making them an excellent natural study-system [10,11,12]. The most obvious difference is the lack of light, a condition which not only contributes in reducing the fluctuation of the subterranean microclimatic conditions, but also impedes the colonization of plants, therefore strongly limiting the availability of food resources [1]. The lack of light also means that the sense of sight is useless and subterranean species often rely on alternative senses to perform their natural activities [13,14,15]. A missing day–night cycle in subterranean environments promotes a prolonged stability of the inner microclimate, which is more evident in the deepest areas not reached by incoming light [10,16].
A large number of species with different degree of adaptations can be found within subterranean environments. The most specialised species are called troglobionts; these animals are usually found in the deepest areas laying in complete darkness and are unable to reproduce in surface environments [17]. Troglobionts often show the well-known morphological, physiological (e.g., resistance to starvation), and behavioral (e.g., loss of fear) adaptations occurring in cave animals [18,19,20]. Troglophiles can maintain stable populations in subterranean environments, but are still able to exit and reproduce in both surface and subterranean environment [6]. These animals can be found throughout the subterranean environment (but generally not too far from the connection with surface) and might show specific traits suitable for subterranean life [21,22,23]. Trogloxenes are species usually found within the first meters from the cave entrance, as these animals usually do not show evident adaptations to the cave environment, and thus are unable to persist in the deepest parts [24]; these species can successfully reproduce only outside caves or in proximity of the entrance [6,17]. The species showing higher degree of adaptation to subterranean life (troglobionts and troglophiles) have been intensively studied and used as model species in a large number of researches, from macro-ecology to evolutionary studies [25,26,27,28]. On the other hand, the interest on trogloxenes has often lagged behind, probably because these species have been thought to have little importance for the subterranean environment [29].
This misleading idea has been proven wrong by recent research, which highlighted that some species traditionally considered to be “occasional” actually show strong ecological relationships with the underground environment, and play a major role in subterranean ecosystems [30,31,32]. Some of such neglected species are the Tipuloidea crane flies (Diptera). Crane flies represent a large taxonomic group (>3300 described species for Palaearctic; [33]) with at least two genera, Limonia Meigen, 1803, and Neolimonia Alexander, 1964, widespread in Italy [34]. Generally, crane flies show similar size and habitus: the typical features are an elongated yellow-brownish abdomen and wings with particular venation and dark pigmentation [35] (Figure 1A). Crane flies inhabit forested areas [36,37], but are also found in subterranean environments, particularly from spring to autumn, where they shelter from external unsuitable climatic conditions (i.e., when it is too hot and dry) [38,39,40,41]. Considering the lack of specific adaptation to cave life, crane flies are usually observed in areas not too far from the connection with the surface, where they can form dense aggregations (Figure 1B) [39,42]. This condition probably makes tipuloids one of the most abundant taxonomic groups in the cave-entrance; thus, representing the most abundant prey for several cave-dwelling predators [43,44,45,46].

Figure 1.
Two photos showing crane flies in subterranean environments: (A) two individuals mating and (B) high density aggregation. Photo credit: Enrico Lunghi.
Given the scarce quantitative ecological information on crane flies, our aim is to study the use of subterranean environments by these species and evaluate its potential relationships with cave-dwelling predators, as crane flies may represent an important food resource in an ecosystem characterized by constant scarcity [1]. Although, in the literature, only the presence of Limonia nubeculosa Meigen, 1804 is generally reported within Italian subterranean environments [30,39,47], the broad distribution of multiple species with similar habitus makes challenging their identification without manipulation [34,35]. Therefore, to avoid potential misidentification, we refer here to the whole superfamily Tipuloidea. We focused in assessing the major environmental features related to the occupancy of tipuloids within subterranean environments. Despite no specific adaptation toward the cave life, crane flies regularly frequent these environments [30,39], and previous studies suggested that ecological relationships with the cave environment can be extremely strong even for some species traditionally considered to be “accidental” [31,48]. Furthermore, we evaluated relationships between the distribution of crane flies and cave predators, as crane-flies can represent a major food resource in prey-deprived environments [30,43].
2. Methods
2.1. Study Sites and Surveys
Fifteen subterranean environments located in the north of Tuscany (Italy) have been monthly surveyed throughout a year (2013). Three of them are semi-natural environments, one is a subterranean drainage tunnel, while 11 are natural caves (Table 1). These sites show a specific microclimatic gradient, going from the entrance to the deepest sectors of caves [10]. The microclimate of subterranean environments is affected by the influence of external climatic conditions especially through the opening connecting them with the surface [1]. Consequently, the areas close to the entrance have microclimatic conditions similar to those found outside. On the other hand, the external influences become weaker with depth and the microclimate of the deepest areas do not experience the same variability, but rather high stability [11,12,16]. We surveyed the 15 subterranean environments following a standardized procedure [49]. The environments were subdivided in sections of three linear meters (hereafter, sector) from the entrance (i.e., the main connection with the surface) to the deepest point reachable without the use of speleological equipment. At the end of each sector, multiple environmental data on both cave morphology (height, width, and wall heterogeneity) and microclimate (temperature, humidity, and incident light) were recorded [10]. Sector height and width were recorded using a laser meter (Anself RZE-70, accuracy 2 mm), while the wall heterogeneity was estimated by unrolling a one-meter length string following the vertical wall shape, then with a tape meter, we measured the linear distance between the two string extremities [50]. A Lafayette TDP92 thermo-hygrometer (accuracy: 0.1 °C and 0.1%) was used to record sector temperature and humidity, while a Velleman DVM1300 light meter (minimum recordable light: 0.1 lx) was used to record the minimum incident light. For further details on data recording refer to Lunghi, Corti, Mulargia, Zhao, Manenti, Ficetola and Veith [49].

Table 1.
List of the surveyed subterranean environments. For each Site we provide: Latitude (N), Longitude (E), Elevation (m a.s.l.), Origin (N = natural; S = semi-natural; and A = artificial), and the number of surveyed Sectors. Latitude and longitude are shown with reduced precision to increase species protection [51].
We surveyed each sector to assess the presence of crane flies through Visual Encounter Survey (VES) [52]; sectors were surveyed with similar effort to limit bias due to imperfect species detection [53]. Adopting the same methodology, we also recorded the presence and the abundance of five predators (traditionally considered to be troglophiles or trogloxenes) usually occurring on cave walls: the cave salamander Hydromantes italicus (Dunn, 1923), and four spider species, Meta menardi (Latreille, 1804), Metellina merianae (Scopoli, 1763), spiders of the family Agelenidae C. L. Koch, 1837, and Amaurobius ferox (Walckenaer, 1830) [10,48,54].
2.2. Statistical Analyses
We first estimated the detection probability of crane flies; the detectability of small cave species usually is <1 [53,55]. We calculated the species detection probability on the base of 35 pairs of survey performed within 14 days; this ensures to meet the assumption of population closure [55]. We built a model including the linear distance from the entrance (hereafter, depth) as potential variable affecting the species occupancy suitability. Starting from this, we built a second model adding the wall heterogeneity as potential variable affecting species detection. Models were compared following the Akaike Information Criterion (AIC) [56] and the best one (with lowest AIC value) used to estimate the detection probability of crane flies.
We then used a binomial generalized linear mixed model to assess which environmental factors influence the occupancy of crane flies in subterranean environments [57]. Their presence/absence was used as dependent variable, while the morphological and microclimatic features of cave sectors, along with depth, as independent variables; site and sector identity were used as random variables. We included as further independent variables the interaction between month of survey and microclimatic features. Considering the imperfect detection of tipuloids (see Results), we weighted their absence with the estimated detection probability [58]. The values of AIC corrected for small samples (AICc) were used to estimate a set of positive Akaike weights wi summing to 1, and used to rank the models; the one with the highest weight was the best [59]. The significance of variables included in the best AICc model was tested using the likelihood ratio test [60].
Moreover, to assess the relationships between the occurrence of crane flies and cave predators we also built two linear mixed models [61]. We used predators’ abundance (total N of individuals) and richness (total N of species), respectively, as dependent variables, the presence/absence of crane flies as independent variable, cave, and sector identity as random factors.
3. Results
We performed a total of 1417 cave sector surveys (Table 1) and we detected crane flies in 709 of them; both the number of occupied sectors and their average depth increased during the hot season (Figure 2). The microclimatic features at which Diptera occurred were: air temperature, average = 12.18 ± 3.08 (SD), max = 25.7, min = 2.5; humidity, average = 89.16 ± 4.95, max = 96.5, min = 47.2; and minimum illuminance, average = 0.53 ± 3.22, max = 58.2, min = 0.
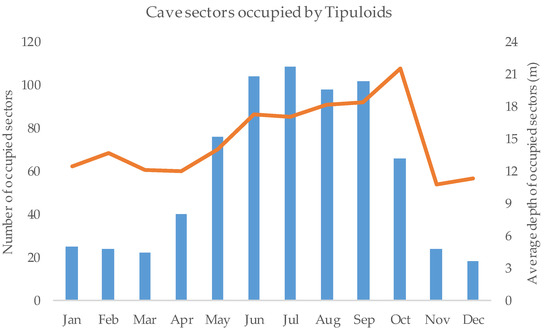
Figure 2.
Number and distribution of occupied cave sectors. The plot shows the monthly number of cave sectors occupied by crane flies and their average depth (linear meters from the connection with surface).
The best model estimating detection probability of crane flies did not include the covariate wall heterogeneity (AIC = 625.49 vs. 626.63); the model suggested that crane flies have a detection probability of 0.58 (SE = 0.05).
The best-AIC model suggested that the occurrence of crane flies within caves was significantly related to temperature, illuminance, sector depth, wall heterogeneity, to the month of survey and its interaction with temperature (Table 2 and Table 3). Crane flies occupied sectors close to the cave entrance, showing low illuminance, relatively high temperature and smooth walls; during the hot season, the species occupied colder sectors.

Table 2.
Five best corrected Akaike Information Criterion (AICc) models relating crane flies presence within cave sectors. We considered as dependent variable the presence of the species. We used as independent variables: sector Width, Height, Depth, wall heterogeneity (Het), sector humidity (Humid), illuminance (Lux) and temperature (Temp), Month of survey and its interaction between microclimatic features (×). For each continuous variable, the regression coefficient is reported if the variable is included into a given model. For categorical variables and interactions, + indicates that the variable or the interaction is included into the model. If not shown in table, the variables were not included in the first five best AICc models. The best model is highlighted in bold.

Table 3.
Parameters related to the presence of crane flies within cave sectors. The significance of variables included in the best AICc model are shown; significant variables are in bold.
Finally, we found a strong, positive relationship between the presence of crane flies and both predator species’ abundance (F1,1295 = 4.71, P = 0.03) and richness (F1,1295 = 12.95, P < 0.001).
4. Discussion
Crane flies occurred within the subterranean environments throughout the year, although there was a clear peak during the hot season (Figure 2). These species, as many other taxa without specific adaptations, probably seek refuge in subterranean environments when outdoor climatic conditions become too dry and hot [10,30,39]. Indeed, when species are strongly dependent on fine scale environmental conditions [62], they need to track suitable microclimate when the local conditions become too harsh [63]. Occupancy was the highest in areas not far from the connection with the surface, where microclimatic conditions are more influenced by external climate [10]. Dipterans, as many other trogloxenes, usually actively seek habitats closely connected to cave entrances, likely because they are phototactic and lack specific adaptations to cope with the peculiar environmental conditions found in the deepest areas (e.g., darkness and food scarcity) [64]. Considering the wide range of microclimatic variables at which Diptera occurred, it is likely that multiple species of Diptera occurred within the studied subterranean environments, but detailed studies are required to ascertain this hypothesis. Furthermore, the buffered microclimatic conditions found in the shallowest areas are probably suitable enough for crane flies; indeed, individuals mate and occur in large numbers (Figure 1). Tipuloids seems to be less linked to the subterranean environments if compared with Trichoceridea, as these Diptera have been usually found in areas distant from the connection with surface [65,66]. Cave walls having less irregularities seem to promote the occupancy of crane flies. A smooth wall may facilitate the attachment of individuals, so they probably choose this feature to facilitate their resting. On the other hand, a wall with pronounced irregularities may shield their presence from surveyor view, and reduce the overall species detection [53]. However, this hypothesis was not confirmed by the analysis of detection probability, as we did not find relationships between crane-fly detectability and wall heterogeneity.
Both richness and abundance of cave predators were positively related to the occurrence of crane flies. Subterranean environments in temperate areas are often food deprived [1], and cave predators have to develop specific adaptations to cope with that. Indeed, some species are able to reduce their metabolism to save energies and withstand prolonged starvation periods [67], others develop specific morphologies to store energy [68], while some others exit and forage on the surface [63]. Crane flies in subterranean environments can represent a large proportion of local biomass [69], thus being an important food resource for the local community; sure enough, dipterans represent one of the main food resources for multiple species [44,45] (Figure 3), as we often saw live and dead ones on different spiders’ webs.

Figure 3.
Oxychilus draparnaudi (Beck, 1837), a facultative cave-dwelling snail, feeding on a dead crane fly. In caves, crane flies are not only important for species directly preying on them, but also for the many scavengers. Photo credit: Simone Giachello.
Crane flies can be highly suitable prey for many cave predators. First, individuals show a particular aggregative behavior, and during the harsher months, they can form dense “clouds” on the cave walls (Figure 1B). The reason of such behavior is still unknown, but it provides to predators an easy way to catch multiple prey with minimum effort. For example the cave salamanders of the genus Hydromantes Gistel, 1848, catch their prey darting their protrusible tongue [70,71]; therefore, if they target a dense aggregation of dipterans they could even catch multiple individuals with one “shot”. In fact, crane flies are the most abundant prey of European cave salamanders [72]. A second advantage is given by location. Compared to the surface, subterranean environments represent a safer place for many species, not only because the suitable microclimatic conditions, but also because the lower presence of potential predators [73,74,75]. Some predators mostly living in subterranean environments can exit caves for foraging, but this exposes them to several risks (e.g., predation or unsuitable climatic conditions), while hunting inside caves is likely safer [43,63].
This was the first study only focusing on the ecology of crane flies within subterranean environments of Italy. Even though many open questions remain, our study highlighted the importance of expanding ecological analyses toward often neglected cave-dwellers. Indeed, there are still so many gaps in the knowledge of the ecology and distribution of crane flies for both their surface and subterranean phase. For examples, we still have no information about species richness of crane flies within subterranean environments, their phenology and whether spatial segregation occurs. Indeed, in this study we attributed all Diptera observed within subterranean environments to the superfamily Tipuloidea, while similar species of the superfamily Trichoceridea can also occur in caves [66]; thus, further refinements are required to better comprehend the occurrence of the different Diptera in subterranean environments and their habitat preference. Nonetheless, we only analysed the main determinants of their occurrence, leaving unexplored all the causes affecting their abundance and aggregation. It would be also interesting to evaluate the energetic contribution provided by crane flies assessing their role in the food web, and evaluating how the flow of individuals between outdoor and subterranean environments affects the functioning of these ecosystems [76].
Author Contributions
Conceptualization, E.L.; Data curation, E.L.; Formal analysis, E.L. and G.F.F.; Investigation, E.L. Methodology, E.L.; Validation, E.L., G.F.F., Y.Z. and R.M.; Visualization, E.L.; Writing—original draft, E.L.; Writing—review & editing, E.L., G.F.F., Y.Z. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the National Natural Science Foundation of China (NSFC-31972868).
Acknowledgments
E. Lunghi is supported by the Chinese Academy of Sciences President’s International Fellowship Initiative for postdoctoral researchers (2019PB0143).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Culver, D.C.; Pipan, T. (Eds.) The Biology of Caves and Other Subterranean Habitats, 3rd ed.; Oxford University Press: New York, NY, USA, 2019; p. 336. [Google Scholar]
- Moldovan, O.T.; Kovác, L.; Halse, S. Cave Ecology; Springer Nature Switzerland: Cham, Switzerland, 2018. [Google Scholar]
- Romero, A. Cave Biology; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Christiansen, K. Proposition pour la classification des animaux cavernicoles. Spelunca 1962, 2, 76–78. [Google Scholar]
- Mammola, S.; Isaia, M. Spiders in cave. Proc. R. Soc. B 2017, 284, 20170193. [Google Scholar] [CrossRef]
- Mammola, S. Finding answers in the dark: Caves as models in ecology fifty years after Poulson and White. Ecography 2019, 42, 1331–1351. [Google Scholar] [CrossRef]
- Barr, T.C.J. Cave ecology and the evolution of troglobites. Evol. Biol. 1968, 2, 35–102. [Google Scholar]
- Culver, D.C.; Holsinger, J.R. How many species of troglobites are there? Bull. Natl. Speleol. Soc. 1992, 54, 79–80. [Google Scholar]
- Ficetola, G.F.; Canedoli, C.; Stock, F. The Racovitzan impediment and the hidden biodiversity of unexplored environments. Conserv. Biol. 2019, 33, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Seasonal variation in microhabitat of salamanders: Environmental variation or shift of habitat selection? PeerJ 2015, 3, e1122. [Google Scholar] [CrossRef]
- Sánchez-Fernández, D.; Rizzo, V.; Bourdeau, C.; Cieslak, A.; Comas, J.; Faille, A.; Fresneda, J.; Lleopart, E.; Millán, A.; Montes, A.; et al. The deep subterranean environment as a potential model system in ecological, biogeographical and evolutionary research. Subterr. Biol. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Lunghi, E.; Canedoli, C.; Padoa-Schioppa, E.; Pennati, R.; Manenti, R. Differences between microhabitat and broad-scale patterns of niche evolution in terrestrial salamanders. Sci. Rep. 2018, 8, 10575. [Google Scholar] [CrossRef]
- Sharma, S.; Coombs, S.; Patton, P.; Burt de Perera, T. The function of wall-following behaviors in the Mexican blind cavefish and a sighted relative, the Mexican tetra (Astyanax). J. Comp. Physiol. A Neuroethol. Sens. NeuralBehav. Physiol. 2009, 195, 225–240. [Google Scholar] [CrossRef]
- Varatharasan, N.; Croll, R.P.; Franz-Odendaal, T. Taste bud development and patterning in sighted and blind morphs of Astyanax mexicanus. Dev. Dyn. 2009, 238, 3056–3064. [Google Scholar] [CrossRef] [PubMed]
- Plath, M.; Parzefall, J.; Körner, K.E.; Schlupp, I. Sexual selection in darkness? Female mating preferences in surface- and cave-dwelling Atlantic mollies, Poecilia mexicana (Poeciliidae, Teleostei). Behav. Ecol. Sociobiol. 2004, 55, 596–601. [Google Scholar] [CrossRef]
- Biswas, J. Kotumsar cave ecosystem: An interaction between geophysical, chemical, and biological characteristics. NSS Bull. 1992, 54, 7–10. [Google Scholar]
- Howarth, F.G.; Moldovan, O.T. The ecological classification of cave animals and their adaptations. In Cave Ecology; Moldovan, O.T., Kováč, L., Halse, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 41–67. [Google Scholar]
- Hervant, F.; Mathieu, J.; Durand, J. Behavioural, physiological and metabolic responses to long-term starvation and refeeding in a blind cave-dwelling (Proteus anguinus) and a surface-dwelling (Euproctus asper) salamander. J. Exp. Biol. 2001, 204, 269–281. [Google Scholar] [PubMed]
- Christiansen, K.A. Morphological adaptations. In Encyclopedia of Caves; White, W., Culver, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 386–397. [Google Scholar]
- Parzefall, J.; Trajano, E. Behavioral patterns in subterranean fishes. In Biology of Subterranean Fishes; Trajano, E., Bichuette, M.E., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2010; pp. 81–114. [Google Scholar]
- Lipovšek, S.; Leitinger, G.; Janžekovič, F.; Kozel, P.; Dariš, B.; Perc, M.; Devetak, D.; Weiland, N.; Novak, T. Towards understanding partial adaptation to the subterranean habitat in the European cave spider, Meta menardi: An ecocytological approach. Sci. Rep. 2019, 9, 9121. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Kiefer, A. Bats of Britain and Europe; Bloomsbury: London, UK, 2016. [Google Scholar]
- Hesselberg, T.; Simonsen, D. A comparison of morphology and web geometry between hypogean and epigean species of Metellina orb spiders (family Tetragnathidae). Subterr. Biol. 2019, 32, 1–13. [Google Scholar] [CrossRef]
- Manenti, R.; Siesa, M.E.; Ficetola, G.F. Odonata occurrence in caves: Active or accidentals? A new case study. J. Cave Karst. Stud. 2013, 75, 205–209. [Google Scholar] [CrossRef]
- Aljančič, G. History of research on Proteus anguinus Laurenti 1768 in Slovenia. Folia Biol. Geol. 2019, 60, 39–69. [Google Scholar] [CrossRef]
- Poulson, T.L. Cave adaptation in Amblyopsid fishes. Am. Midl. Nat. 1963, 70, 257–290. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Lunghi, E.; Manenti, R. Microhabitat analyses support relationships between niche breadth and range size when spatial autocorrelation is strong. Ecography 2020, 43, 1–11. [Google Scholar] [CrossRef]
- Culver, D.C.; Kane, T.C.; Fong, D.W. (Eds.) Adaptation and Natural Selection in Caves. The Evolution of Gammarus Minus; Harvard University Press: Cambridge, UK, 1995; p. 223. [Google Scholar]
- Trajano, E.; de Carvalho, M.R. Towards a biologically meaningful classification of subterranean organisms: A critical analysis of the Schiner-Racovitza system from a historical perspective, difficulties of its application and implications for conservation. Subterr. Biol. 2017, 22, 1–26. [Google Scholar] [CrossRef]
- Lunghi, E.; Bruni, G.; Ficetola, G.F.; Manenti, R. Is the Italian stream frog (Rana italica Dubois, 1987) an opportunistic exploiter of cave twilight zone? Subterr. Biol. 2018, 25, 49–60. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Do cave features affect underground habitat exploitation by non-troglobite species? Acta Oecol. 2014, 55, 29–35. [Google Scholar] [CrossRef]
- Pape, R.B. The importance of ants in cave ecology, with new records and behavioral observations of ants in Arizona caves. Int. J. Speleol. 2016, 45, 185–205. [Google Scholar] [CrossRef]
- Oosterbroek, P. Catalogue of the Craneflies of the World. Available online: https://ccw.naturalis.nl/ (accessed on 13 August 2020).
- de Jong, Y.; Verbeek, M.; Michelsen, V.; de Place Bjørn, P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E.; et al. Fauna Europaea—All European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef] [PubMed]
- Fetzner, J.W.J. The Crane Flies (Diptera: Tipulidae) of Pennsylvania. Available online: https://www.invertebratezoology.org/cranefly/idkeys.htm (accessed on 7 August 2020).
- Ebejer, M.J. The craneflies (Diptera, Tipulidae and Limoniidae) and winter gnats (Diptera, Trichoceridae) of Malta. Bull. Entomol. Soc. Malta 2015, 7, 51–55. [Google Scholar] [CrossRef]
- Freeman, B.E. Studies on the ecology of adult Tipulidae (Diptera) in Southern England. J. Anim. Ecol. 1968, 37, 339–362. [Google Scholar] [CrossRef]
- Service, M.W. Spatial and temporal distributions of aerial populations of woodland tipulids (Diptera). J. Anim. Ecol. 1973, 42, 295–303. [Google Scholar] [CrossRef]
- Di Russo, C.; Carchini, G.; Rampini, M.; Lucarelli, M.; Sbordoni, V. Long term stability of a terrestrial cave community. Int. J. Speleol. 1999, 26, 75–88. [Google Scholar] [CrossRef]
- Østbye, E.; Lauritzen, S.-E. A checklist of invertebrates from Norwegian caves and mines. Fauna Nor. 2013, 33, 35–51. [Google Scholar] [CrossRef]
- Kjærandsen, J. Diptera in mines and other cave systems in southern Norway. Entomol. Fenn. 1993, 4, 151–160. [Google Scholar] [CrossRef]
- Novak, T.; Sambol, J.; Janžekovič, F. Faunal dynamics in the Železna jama cave. Acta Carsologica 2004, 33, 249–267. [Google Scholar] [CrossRef]
- Manenti, R.; Lunghi, E.; Ficetola, G.F. Distribution of spiders in cave twilight zone depends on microclimatic features and trophic supply. Invertebr. Biol. 2015, 134, 242–251. [Google Scholar] [CrossRef]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Mulargia, M.; Cogoni, R.; Barzaghi, B.; Cornago, L.; Avitabile, D.; Veith, M.; Manenti, R.; et al. Field-recorded data on the diet of six species of European Hydromantes cave salamanders. Sci. Data 2018, 5, 180083. [Google Scholar] [CrossRef]
- Novak, T.; Tkavc, T.; Kuntner, M.; Arnett, A.E.; Lipovšek Delakorda, S.; Perc, M.; Janžekovič, F. Niche partitioning in orbweaving spiders Meta menardi and Metellina merianae (Tetragnathidae). Acta Oecol. 2010, 36, 522–529. [Google Scholar] [CrossRef]
- Salvidio, S. Diet and food utilization in the European plethodontid Speleomantes ambrosii. Vie et Milieu 1992, 42, 35–39. [Google Scholar]
- Mazza, G.; Cianferoni, F.; Bottacci, A.; Zoccola, A. Primo contributo alla conoscenza della biospeleologia all’interno delle riserve naturali biogenetiche casentinesi (Parco Nazionale Foreste Casentinesi, Monte Falterona e Campigna) e zone limitrofe. Quad. Di Studi E Not. Di Stor. Nat. Della Romagna 2008, 27, 1–72. [Google Scholar]
- Lunghi, E. Occurrence of the Black lace-weaver spider, Amaurobius ferox, in caves. Acta Carsologica 2020, in press. [Google Scholar]
- Lunghi, E.; Corti, C.; Mulargia, M.; Zhao, Y.; Manenti, R.; Ficetola, G.F.; Veith, M. Cave morphology, microclimate and abundance of five cave predators from the Monte Albo (Sardinia, Italy). Biodivers. Data J. 2020, 8, e48623. [Google Scholar] [CrossRef]
- Camp, C.D.; Jensen, J.B. Use of twilight zones of caves by plethodontid salamanders. Copeia 2007, 2007, 594–604. [Google Scholar] [CrossRef]
- Lunghi, E.; Corti, C.; Manenti, R.; Ficetola, G.F. Consider species specialism when publishing datasets. Nat. Ecol. Evol. 2019, 3, 319. [Google Scholar] [CrossRef] [PubMed]
- Crump, M.L.; Scott, N.J. Visual Encounter Surveys. In Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians; Heyer, W.R., Donnelly, M.A., McDiarmid, R.W., Hayek, L.C., Foster, M.S., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1994; pp. 84–92. [Google Scholar]
- Lunghi, E. Ecology and life history of Meta bourneti (Araneae: Tetragnathidae) from Monte Albo (Sardinia, Italy). PeerJ 2018, 6, e6049. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Cave features, seasonality and subterranean distribution of non-obligate cave dwellers. PeerJ 2017, 5, e3169. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling. Inferring Patterns and Dynamics of Species Occurrence; Academic Press: San Diego, CA, USA, 2006. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Gorosito, I.L.; Bermúdez, M.M.; Douglass, R.J.; Busch, M. Evaluation of statistical methods and sampling designs for the assessment of microhabitat selection based on point data. Methods in Ecol. Evol. 2016, 1316–1324. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, C.; Bustamante, J.; Díaz-Paniagua, C.; Guisan, A. Integrating detection probabilities in species distribution models of amphibians breeding in Mediterranean temporary ponds. Divers. Distrib. 2012, 18, 260–272. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multi-Model inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2008, 24, 127–135. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-128. 2017. Available online: http://CRAN.R-project.org/package=nlme (accessed on 7 July 2020).
- Lunghi, E.; Manenti, R.; Canciani, G.; Scarì, G.; Pennati, R.; Ficetola, G.F. Thermal equilibrium and temperature differences among body regions in European plethodontid salamanders. J. Therm. Biol. 2016, 60, 79–85. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Mulargia, M.; Veith, M.; Corti, C.; Ficetola, G.F. Environmental suitability models predict population density, performance and body condition for microendemic salamanders. Sci. Rep. 2018, 8, 7527. [Google Scholar] [CrossRef]
- White, W.; Culver, D.C.; Pipan, T. (Eds.) Encyclopedia of Caves; Academic Press: Waltham, UK, 2019; p. 1250. [Google Scholar]
- Plachter, P. Cave-dwelling flies in Central Europe: Adaptation to environment, especially to low temperatures (Diptera, Nematocera: Trichoceridae et Sciaridae). Oecologia 1983, 58, 367–372. [Google Scholar] [CrossRef]
- Petrašiūnas, A.; Weber, D. Winter crane flies (Insecta, Diptera, Trichoceridae) from caves of the Grand Duchy of Luxembourg. Ferrantia 2013, 69, 276–283. [Google Scholar]
- Hervant, F. Starvation in subterranean species versus surface-dwelling species: Crustaceans, fish, and salamanders. In Comparative Physiology of Fasting, Starvation, and Food Limitation; McCue, M.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 91–102. [Google Scholar]
- Lunghi, E.; Zhao, Y. Do Chinese cavefish show intraspecific variability in morphological traits? Ecol. Evol. 2020, 10, 7723–7730. [Google Scholar] [CrossRef]
- Barnes, J.K.; Slay, M.E.; Taylor, S.J. Adult Diptera from Ozark Caves. Proc. Entomol. Soc. Wash. 2009, 111, 335–353. [Google Scholar] [CrossRef]
- Deban, S.M.; Dicke, U. Motor control of tongue movement during prey capture in Plethodontid salamanders. J. Exp. Biol. 1999, 202, 3699–3714. [Google Scholar] [PubMed]
- Deban, S.M.; Dicke, U. Activation patterns of the tongue-projector muscle during feeding in the imperial cave salamander Hydromantes imperialis. J. Exp. Biol. 2004, 207, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Veith, M.; Manenti, R.; Mancinelli, G.; Corti, C.; Ficetola, G.F. What shapes the trophic niche of European plethodontid salamanders? PLoS ONE 2018, 13, e0205672. [Google Scholar] [CrossRef]
- Manenti, R.; Lunghi, E.; Barzaghi, B.; Melotto, A.; Falaschi, M.; Ficetola, G.F. Do salamanders limit the abundance of groundwater invertebrates in subterranean habitats? Diversity 2020, 12, 161. [Google Scholar] [CrossRef]
- Biswas, J.; Pradhan, R.K.; Pati, A.K. Studies on burying behaviour in epigean and hypogean fish, Oreonectus evezardi: An example of behavioural divergence. Mem. De Biospéleologie 1990, 17, 33–41. [Google Scholar]
- Bradley, J.G.; Eason, P.K. Predation risk and microhabitat selection by cave salamanders, Eurycea lucifuga (Rafinesque, 1822). Behaviour 2019, 155, 841–859. [Google Scholar] [CrossRef]
- Barzaghi, B.; Ficetola, G.F.; Pennati, R.; Manenti, R. Biphasic predators provide biomass subsidies in small freshwater habitats: A case study of spring and cave pools. Freshw. Biol. 2017, 62, 1637–1644. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).