Taxonomic and Functional Diversity and Composition of Bats in a Regenerating Neotropical Dry Forest
Abstract
1. Introduction
2. Materials and Methods
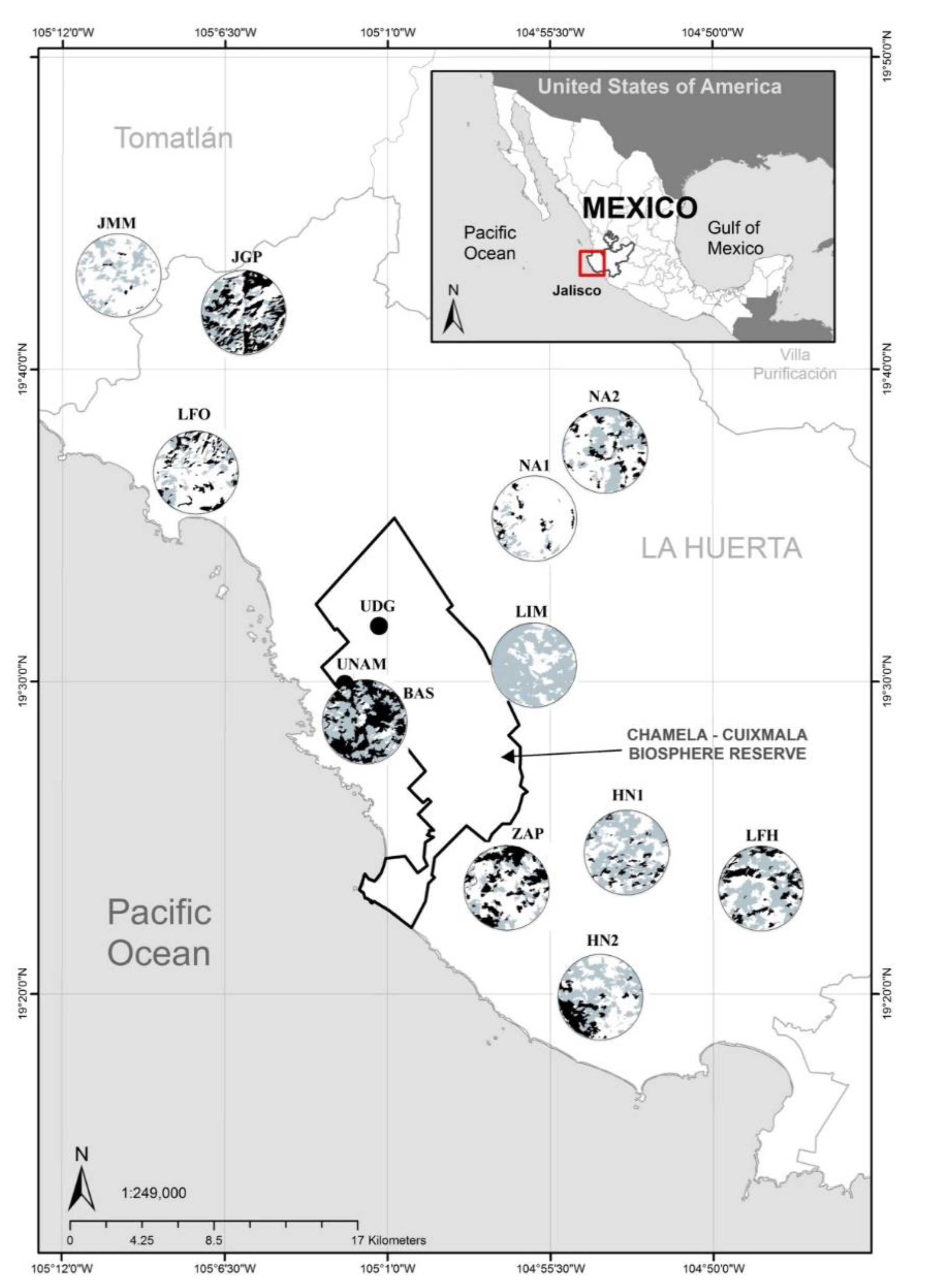
2.1. Study Site
2.2. Sampling and Taxonomic and Functional Characterization of Bat Communities
2.3. Sampling and Characterization of the Structure and Composition of the Vegetation
2.4. Landscape Characterization
2.5. Data Analysis
2.5.1. Sample Representability
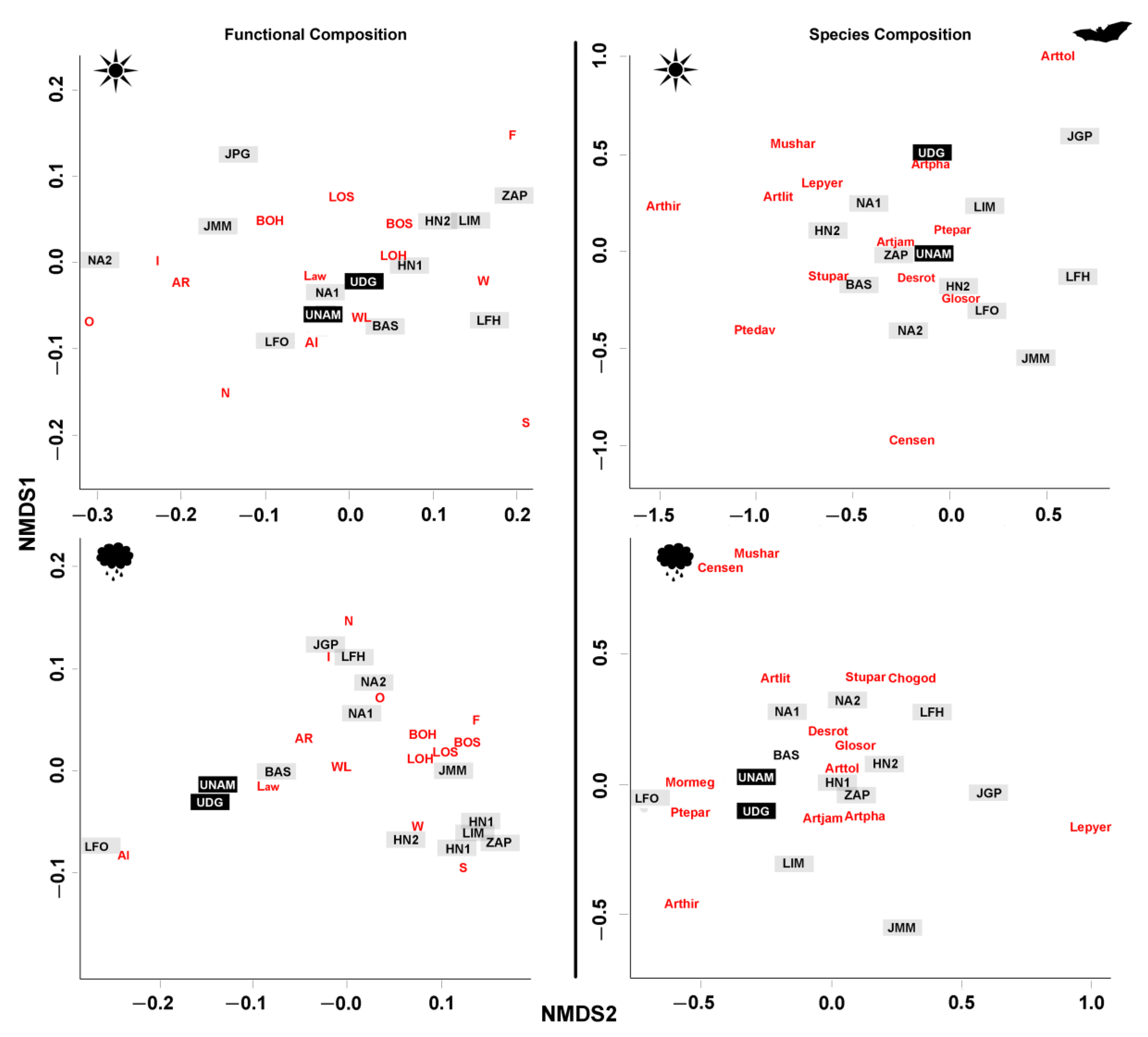
2.5.2. Comparison among Bat Communities
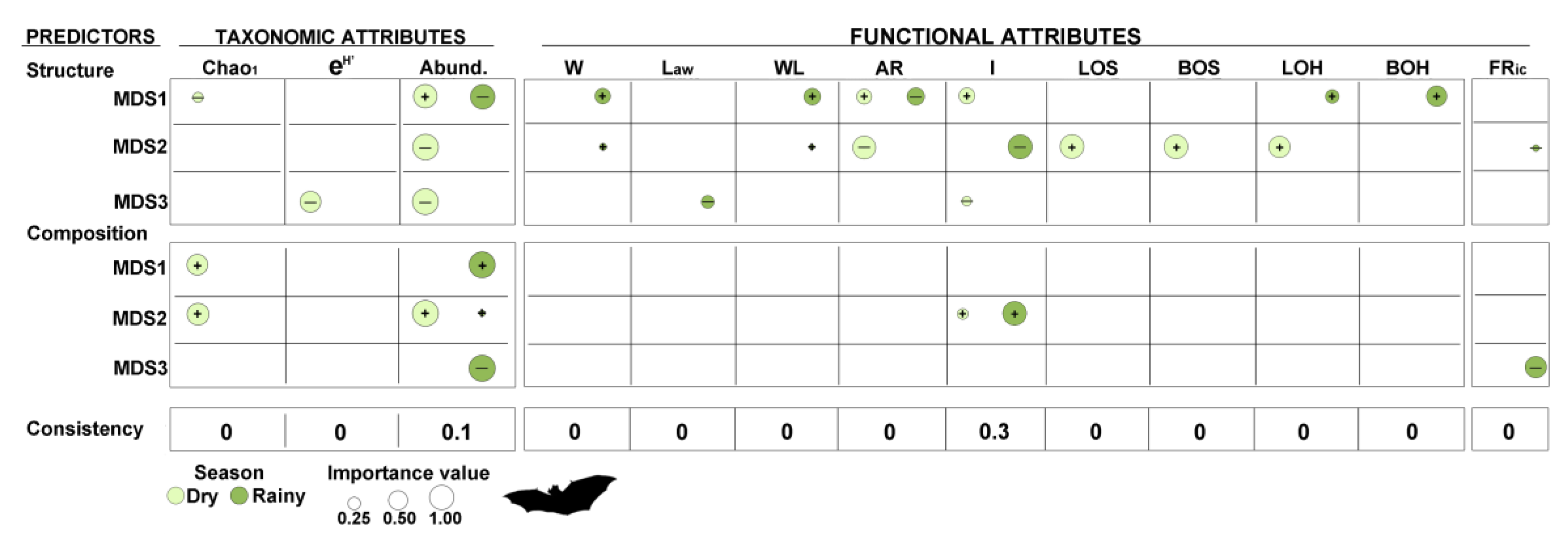
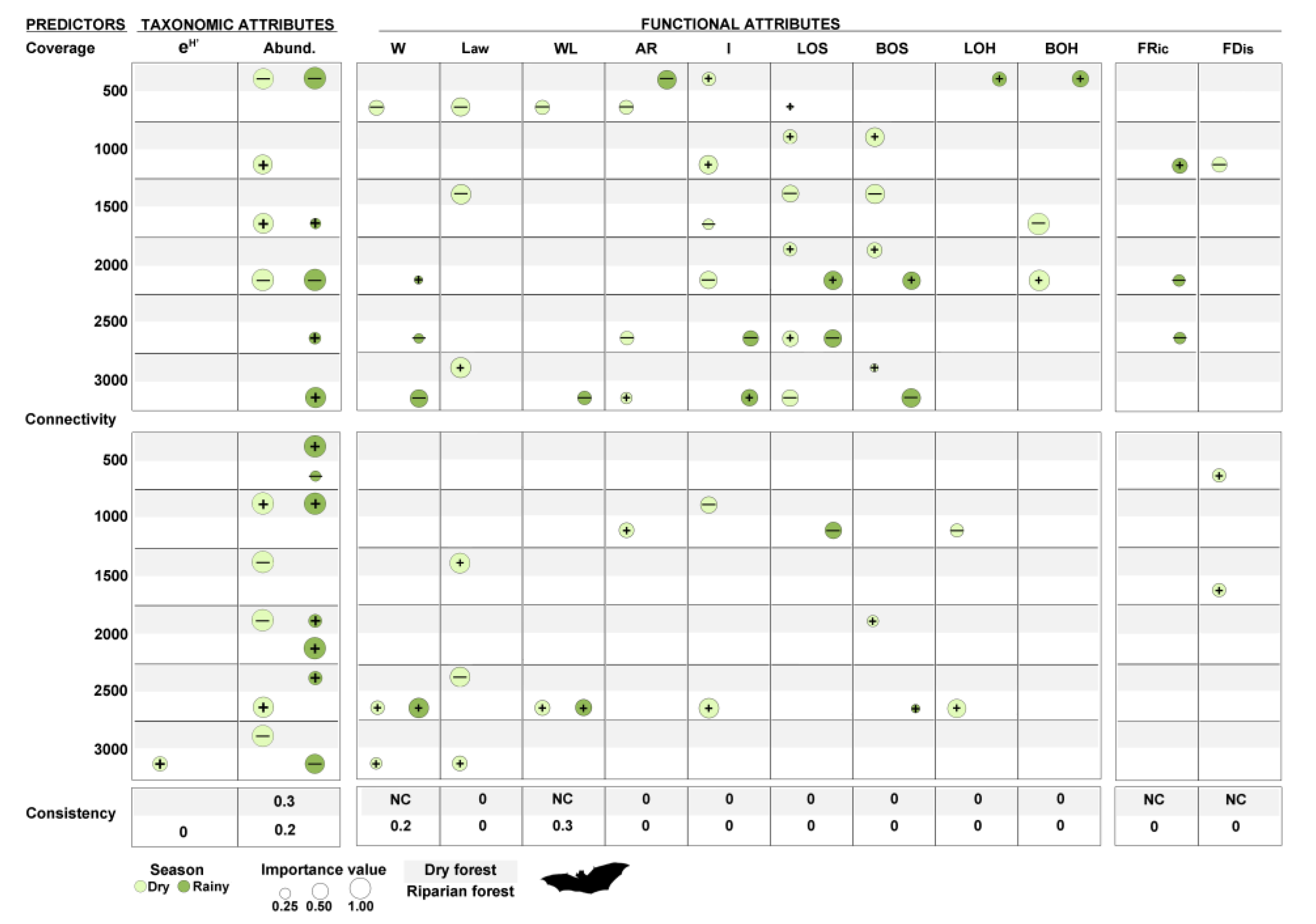
2.5.3. Response of Bats to Variation in Vegetation and Landscape Attributes
3. Results
3.1. Characterization of Early Successional Bat Communities
3.2. Early Successional Bat Community Response to Vegetation and Landscape Attributes
4. Discussion
4.1. Characterization of Early Successional Bat Communities
4.2. Early Successional Bat Community Response to Vegetation and Landscape Attributes
4.3. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Details on Methodology
Appendix A.1. Land-Use History and General Characteristic of Vegetation in Study Sites
Appendix A.2. Body Mass and Morphometric Measurements
Appendix A.3. Spatial Structure Evaluation on Response Variables
References
- Muscarella, R.; Fleming, T.H. The Role of Frugivorous Bats in Tropical Forest Succession. Biol. Rev. 2007, 82, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; DellaSala, D.A.; Hutto, R.L.; Lindenmayer, D.B.; Swanson, F.J. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 2011, 9, 117–125. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Peres, C.A.; Dent, D.; Sheil, D.; Lugo, A.E.; Lamb, D.; Stork, N.E.; Miller, S.E. The Potential for Species Conservation in Tropical Secondary Forests. Conserv. Biol. 2009, 23, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M. Conceptual Synthesis in Community Ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef]
- Avila-Cabadilla, L.D.; Stoner, K.E.; Henry, M.; Añorve, M.Y.A. Composition, structure and diversity of phyllostomid bat assemblages in different successional stages of a tropical dry forest. For. Ecol. Manag. 2009, 258, 986–996. [Google Scholar] [CrossRef]
- Herrera, J.P.; Duncan, N.; Clare, E.; Fenton, M.B.; Simmons, N. Disassembly of Fragmented Bat Communities in Orange Walk District, Belize. Acta Chiropterologica 2018, 20, 147. [Google Scholar] [CrossRef]
- Morales-Díaz, S.P.; Alvarez-Añorve, M.Y.; Zamora-Espinoza, M.E.; Dirzo, R.; Oyama, K.; Avila-Cabadilla, L.D. Rodent community responses to vegetation and landscape changes in early successional stages of tropical dry forest. For. Ecol. Manag. 2019, 433, 633–644. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.A.; Kembel, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Macarthur, R.; Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography (Mpb-32); Princeton University Press: Princeton, NJ, USA, 2001; ISBN 9780691021287. [Google Scholar]
- Givnish, T.J. On the causes of gradients in tropical tree diversity. J. Ecol. 1999, 87, 193–210. [Google Scholar] [CrossRef]
- Miller, P.M.; Kauffman, J.B. Effects of slash and burn agriculture on species abundance and composition of a tropical deciduous forest. For. Ecol. Manag. 1998, 103, 191–201. [Google Scholar] [CrossRef]
- Alvarez-Añorve, M.Y.; Quesada, M.; Arturo Sánchez-Azofeifa, G.; Avila-Cabadilla, L.D.; Gamon, J.A. Functional regeneration and spectral reflectance of trees during succession in a highly diverse tropical dry forest ecosystem. Am. J. Bot. 2012, 99, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Avila-Cabadilla, L.D.; Stoner, K.E.; Nassar, J.M.; Espírito-Santo, M.M.; Alvarez-Añorve, M.Y.; Aranguren, C.I.; Henry, M.; González-Carcacía, J.A.; Falcão, L.A.D.; Sanchez-Azofeifa, G.A. Phyllostomid Bat Occurrence in Successional Stages of Neotropical Dry Forests. PLoS ONE 2014, 9, e84572. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Hannon, S.J.; Schmiegelow, F.K.A. Fragments are not islands: Patch vs landscape perspectives on songbird presence and abundance in a harvested boreal forest. Ecography 2000, 23, 209–223. [Google Scholar] [CrossRef]
- Fleming, T.H.; Muchhala, N. Nectar-feeding bird and bat niches in two worlds: Pantropical comparisons of vertebrate pollination systems. J. Biogeogr. 2008, 35, 764–780. [Google Scholar] [CrossRef]
- Kalka, M.B.; Smith, A.R.; Kalko, E.K.V. Bats Limit Arthropods and Herbivory in a Tropical Forest. Science 2008, 320, 71. [Google Scholar] [CrossRef] [PubMed]
- Lobova, T.A.; Geiselman, C.K.; Mori, S.A. Seed Dispersal by Bats in the Neotropics; New York Botanical Garden: New York, NY, USA, 2009. [Google Scholar]
- Kuzmin, I.V.; Bozick, B.; Guagliardo, S.A.; Kunkel, R.; Shak, J.R.; Tong, S.; Rupprecht, C.E. Bats, emerging infectious diseases, and the rabies paradigm revisited. Emerg. Health Threats J. 2011, 4, 7159. [Google Scholar] [CrossRef]
- Jones, G.; Jacobs, D.; Kunz, T.; Willig, M.; Racey, P. Carpe noctem: The importance of bats as bioindicators. Endanger. Species Res. 2009, 8, 93–115. [Google Scholar] [CrossRef]
- Meyer, C.F.J.; Struebig, M.J.; Willig, M.R. Responses of tropical bats to habitat fragmentation, logging, and deforestation. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer International Publishing: Cham, Switzerland, 2016; pp. 63–103. [Google Scholar]
- Rocha, R.; López-Baucells, A.; Farneda, F.Z.; Groenenberg, M.; Bobrowiec, P.E.D.; Cabeza, M.; Palmeirim, J.M.; Meyer, C.F.J. Consequences of a large-scale fragmentation experiment for Neotropical bats: Disentangling the relative importance of local and landscape-scale effects. Landsc. Ecol. 2017, 32, 31–45. [Google Scholar] [CrossRef]
- Ferreira, D.F.; Rocha, R.; López-Baucells, A.; Farneda, F.Z.; Carreiras, J.M.B.; Palmeirim, J.M.; Meyer, C.F.J. Season-modulated responses of Neotropical bats to forest fragmentation. Ecol. Evol. 2017, 7, 4059–4071. [Google Scholar] [CrossRef]
- Cisneros, L.M.; Fagan, M.E.; Willig, M.R. Effects of human-modified landscapes on taxonomic, functional and phylogenetic dimensions of bat biodiversity. Divers. Distrib. 2015, 21, 523–533. [Google Scholar] [CrossRef]
- Klingbeil, B.T.; Willig, M.R. Guild-specific responses of bats to landscape composition and configuration in fragmented Amazonian rainforest. J. Appl. Ecol. 2009, 46, 203–213. [Google Scholar] [CrossRef]
- Gonçalves, F.; Fischer, E.; Dirzo, R. Forest conversion to cattle ranching differentially affects taxonomic and functional groups of Neotropical bats. Biol. Conserv. 2017, 210, 343–348. [Google Scholar] [CrossRef]
- Norberg, U.M.; Rayner, J.M.V. Ecological morphology and flight in bats (Mammalia; Chiroptera): Wing adaptations, flight performance, foraging strategy and echolocation. Philos. Trans. R. Soc. B Biol. Sci. 1987, 316, 335–427. [Google Scholar] [CrossRef]
- Wordley, C.F.R.; Sankaran, M.; Mudappa, D.; Altringham, J.D. Bats in the Ghats: Agricultural intensification reduces functional diversity and increases trait filtering in a biodiversity hotspot in India. Biol. Conserv. 2017, 210, 48–55. [Google Scholar] [CrossRef]
- Klingbeil, B.T.; Willig, M.R. Seasonal differences in population-, ensemble- and community-level responses of bats to landscape structure in Amazonia. Oikos 2010, 119, 1654–1664. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.A.; Quesada, M.; Cuevas-Reyes, P.; Castillo, A.; Sánchez-Montoya, G. Land cover and conservation in the area of influence of the Chamela-Cuixmala Biosphere Reserve, Mexico. For. Ecol. Manag. 2009, 258, 907–912. [Google Scholar] [CrossRef]
- Avila-Cabadilla, L.D.; Sanchez-Azofeifa, G.A.; Stoner, K.E.; Alvarez-Añorve, M.Y.; Quesada, M.; Portillo-Quintero, C.A. Local and landscape factors determining occurrence of phyllostomid bats in tropical secondary forests. PLoS ONE 2012, 7, e35228. [Google Scholar] [CrossRef]
- Fraga-Ramírez, Y.; Suazo-Ortuño, I.; Avila-Cabadilla, L.D.; Alvarez-Añorve, M.; Alvarado-Díaz, J. Multiscale analysis of factors influencing herpetofaunal assemblages in early successional stages of a tropical dry forest in western Mexico. Biol. Conserv. 2017, 209, 196–210. [Google Scholar] [CrossRef]
- Jones, K.E.; Purvis, A.; Maclarnon, A.; Bininda-Emonds, O.R.P.; Simmons, N.B. A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biol. Rev. Camb. Philos. Soc. 2002, 77, S1464793101005899. [Google Scholar] [CrossRef]
- Ceballos, G.; Miranda, A. Guía de Campo de Los Mamíferos de la Costa de Jalisco, México; No. QL722; Fundación Ecológica de Cuixmala A.C.: Jalisco, Mexico, 2000. [Google Scholar]
- Fenton, M.B.; Kunz, T. Movements and behavior. In Biology of Bats of the New World Family Phyllosto-Matidae; Baker, R.J., Jones, J.K., Jr., Carter, D.C., Eds.; University Press: Lubbock, TX, USA, 1977; pp. 351–364. [Google Scholar]
- Saldaña-Vázquez, R.A.; Munguía-Rosas, M.A. Lunar phobia in bats and its ecological correlates: A meta-analysis. Mamm. Biol. 2013, 78, 216–219. [Google Scholar] [CrossRef]
- Marques, J.T.; Pereira, M.J.R.; Marques, T.A.; Santos, C.D.; Santana, J.; Beja, P.; Palmeirim, J.M. Optimizing sampling design to deal with mist-net avoidance in Amazonian birds and bats. PLoS ONE 2013, 8, e74505. [Google Scholar] [CrossRef] [PubMed]
- Medellín, R.A.; Arita, H.T.; Sanchez, O. Identificación de los Murciélagos de México, 2nd ed.; Instituto de Ecología de la UNAM: Mexico City, Mexico, 2007. [Google Scholar]
- Brunet-Rossinni, A.K.; Wilkinson, G.S. Methods for age estimation and the study of senescence in bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; pp. 315–325. [Google Scholar]
- Racey, P.A. Reproductive assessment of bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; pp. 249–264. [Google Scholar]
- Arita, H.T. Noseleaf morphology and ecological correlates in phyllostomid bats. J. Mammal. 1990, 71, 36–47. [Google Scholar] [CrossRef]
- Arita, H.T.; Fenton, M.B. Flight and echlocation in the ecology and evolution of bats. Trends Ecol. Evol. 1997, 12, 53–58. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing [Computer Software Manual]; R Core Team: Viena, Austria, 2016; ISBN 3-900051-07-0. [Google Scholar]
- Colwell, R.K. EstimateS (Statistical Estimation of Species Richness and Shared Species from Samples). V 9. Available online: http://purl.oclc.org/estimates (accessed on 9 May 2019).
- Marsden, S.J.; Fielding, A.H.; Mead, C.; Hussin, M.Z. A technique for measuring the density and complexity of understorey vegetation in tropical forests. For. Ecol. Manag. 2002, 165, 117–123. [Google Scholar] [CrossRef]
- Fournier, R.A.; Mailly, D.; Walter, J.M.N.; Soudani, K. Indirect measurements of forest canopy structure from in situ optical sensors. In Remote Sensing of Forest Environments; Wulder, M.A., Franklin, S.E., Eds.; Springer: Boston, MA, USA, 2003; pp. 78–114. ISBN 978-1-4613-5014-9. [Google Scholar]
- Mc Cune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; Mjm Software Design: Gleneden Beach, OR, USA, 2002; Volume 28, ISBN 0972129006. [Google Scholar]
- Jaeger, J.A.G. Landscape division, splitting index, and effective mesh size: New measures of landscape fragmentation. Landsc. Ecol. 2000, 15, 115–130. [Google Scholar] [CrossRef]
- Jung, M. LecoS—A python plugin for automated landscape ecology analysis. Ecol. Inform. 2016, 31, 18–21. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Payton, M.E. Contrasting Diversity Values: Statistical Inferences Based on Overlapping Confidence Intervals. PLoS ONE 2013, 8, e56794. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95364-9. [Google Scholar]
- Bonthoux, S.; Barnagaud, J.-Y.; Goulard, M.; Balent, G. Contrasting spatial and temporal responses of bird communities to landscape changes. Oecologia 2013, 172, 563–574. [Google Scholar] [CrossRef]
- Calcagno, V. Glmulti: Model Selection and Multimodel Inference Made Easy. Available online: https://cran.r-project.org/web/packages/glmulti/glmulti.pdf (accessed on 26 May 2020).
- Stoner, K.E.; Timm, R.M. Seasonally dry tropical forest mammals: Adaptations and seasonal patterns. In Seasonally Dry Tropical Forests; Dirzo, R., Young, H.S., Mooney, H.A., Ceballos, G., Eds.; Island Press: Washington, DC, USA, 2011; pp. 85–106. [Google Scholar]
- Medellín, R.A.; Equihua, M.; Amin, M.A. Bat diversity and abundance as indicators of disturbance in neotropical rainforests. Conserv. Biol. 2000, 14, 1666–1675. [Google Scholar] [CrossRef]
- Denzinger, A.; Schnitzler, H.-U. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front. Physiol. 2013, 4, 164. [Google Scholar] [CrossRef]
- Hopkins, H.L.; Sánchez-Hernández, C.; Romero-Almaraz, M.D.L.; Gilley, L.M.; Schnell, G.D.; Kennedy, M.L. Flight speeds of four species of neotropical bats. Southwest. Nat. 2003, 48, 711–714. [Google Scholar] [CrossRef]
- Sánchez-Hernández, C.; Romero-Almaraz, M.D.L.; Wooten, M.C.; Schnell, G.D.; Kennedy, M.L. Speed in flight of common vampire bats (Desmodus rotundus). Southwest. Nat. 2006, 51, 422–426. [Google Scholar] [CrossRef]
- Wilson, D.E. Bats in Question: The Smithsonian Answer Book; Smithsonian Books: Whasinhgton, DC, USA, 1997; ISBN 978-1560987390. [Google Scholar]
- Haber, W.A.; Frankie, G.W. A Tropical Hawkmoth Community: Costa Rican Dry Forest Sphingidae. Biotropica 1989, 21, 155. [Google Scholar] [CrossRef]
- Rezsutek, M.; Cameron, G.N. Mormoops megalophylla. Mamm. Species 1993, 1–5. [Google Scholar] [CrossRef]
- Medina, A.; Harvey, C.A.; Merlo, D.S.; Vílchez, S.; Hernández, B. Bat diversity and movement in an agricultural landscape in Matiguás, Nicaragua. Biotropica 2007, 39, 120–128. [Google Scholar] [CrossRef]
- Jakobsen, L.; Brinkløv, S.; Surlykke, A. Intensity and directionality of bat echolocation signals. Front. Physiol. 2013, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Vanderelst, D.; De Mey, F.; Peremans, H.; Geipel, I.; Kalko, E.; Firzlaff, U. What noseleaves do for fm bats depends on their degree of sensorial specialization. PLoS ONE 2010, 5, e11893. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.; Hallam, J.; Moss, C.F.; Hedenström, A. Directionality of nose-emitted echolocation calls from bats without a nose leaf (Plecotus auritus). J. Exp. Biol. 2018, 221, jeb171926. [Google Scholar] [CrossRef] [PubMed]
- Brinkløv, S.; Kalko, E.K.V.; Surlykke, A. Dynamic adjustment of biosonar intensity to habitat clutter in the bat Macrophyllum macrophyllum (Phyllostomidae). Behav. Ecol. Sociobiol. 2010, 64, 1867–1874. [Google Scholar] [CrossRef]
- Feng, L.; Gao, L.; Lu, H.; Müller, R. Noseleaf Dynamics during Pulse Emission in Horseshoe Bats. PLoS ONE 2012, 7, e34685. [Google Scholar] [CrossRef]
- Voss, R.S.; Fleck, D.W.; Strauss, R.E.; Velazco, P.M.; Simmons, N.B. Roosting ecology of Amazonian bats: Evidence for guild structure in hyperdiverse mammalian communities. Am. Mus. Novit. 2016, 3870, 1–43. [Google Scholar] [CrossRef]
- Zarazúa-Carbajal, M.; Avila-Cabadilla, L.D.; Alvarez-Añorve, M.Y.; Benítez-Malvido, J.; Stoner, K.E. Importance of riparian habitat for frugivorous bats in a tropical dry forest in western Mexico. J. Trop. Ecol. 2017, 33, 74–82. [Google Scholar] [CrossRef]
- García-Morales, R.; Moreno, C.E.; Badano, E.I.; Zuria, I.; Galindo-González, J.; Rojas-Martínez, A.E.; Ávila-Gómez, E.S. Deforestation impacts on bat functional diversity in tropical landscapes. PLoS ONE 2016, 11, e0166765. [Google Scholar] [CrossRef]
- Farneda, F.Z.; Rocha, R.; López-Baucells, A.; Groenenberg, M.; Silva, I.; Palmeirim, J.M.; Bobrowiec, P.E.D.; Meyer, C.F.J. Trait-related responses to habitat fragmentation in Amazonian bats. J. Appl. Ecol. 2015, 52, 1381–1391. [Google Scholar] [CrossRef]
- Marinello, M.M.; Bernard, E. Wing morphology of Neotropical bats: A quantitative and qualitative analysis with implications for habitat use. Can. J. Zool. 2014, 92, 141–147. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. Linear and Nonlinear Mixed Effects Models. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 24 May 2020).
| Trait Complex | Abbreviation/Trait | Measure/Calculus | Interpretation |
|---|---|---|---|
| Size | W/weight | W = M × 9.81 m/s2 (gravity) | Larger species tend to have smaller populations, slower life histories, and larger home ranges owing to greater energy requirements. |
| Law/arm-wing length | Directly measured | Larger species tend to have smaller populations, slower life histories, and larger home ranges owing to greater energy requirements. | |
| Wing | WL/wing loading | WL = W/S | It is a measure of the required pressure to sustain the flight. Flight speed is proportional to the square root of wing loading. |
| AR/aspect-ratio | AR = Lws2/S | It is a measure of the general bat’s body shape. Lower values correspond to lower aerodynamic efficiency and higher energy losses in flight (short and wide wings). | |
| I/wing tip index | I = Ts/Tl-Ts | It provides a measure of the wing tip angle, and hence of wingtip shape. Lower values correspond to more acute wingtips. | |
| Leaf-nose | LOS/length of the spear | Directly measured | Larger dimensions can be potentially associated with an increased ability to direct sound. |
| BOS/breadth of the spear | Directly measured | Larger dimensions can be potentially associated with an increased ability to direct sound. | |
| LOH/length of the horseshoe | Directly measured | Larger dimensions can be potentially associated with an increased ability to direct sound and also to the frequency of emission. | |
| BOH/breadth of the horseshoe | Directly measured | Larger dimensions can be potentially associated with an increased ability to direct sound as well as with the frequency of emission. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ferreira, S.R.; Alvarez-Añorve, M.Y.; Bravo-Monzón, A.E.; Montiel-González, C.; Flores-Puerto, J.I.; Morales-Díaz, S.P.; Chiappa-Carrara, X.; Oyama, K.; Avila-Cabadilla, L.D. Taxonomic and Functional Diversity and Composition of Bats in a Regenerating Neotropical Dry Forest. Diversity 2020, 12, 332. https://doi.org/10.3390/d12090332
Martínez-Ferreira SR, Alvarez-Añorve MY, Bravo-Monzón AE, Montiel-González C, Flores-Puerto JI, Morales-Díaz SP, Chiappa-Carrara X, Oyama K, Avila-Cabadilla LD. Taxonomic and Functional Diversity and Composition of Bats in a Regenerating Neotropical Dry Forest. Diversity. 2020; 12(9):332. https://doi.org/10.3390/d12090332
Chicago/Turabian StyleMartínez-Ferreira, Sergio Ramón, Mariana Yolotl Alvarez-Añorve, Angel E. Bravo-Monzón, Cristina Montiel-González, Jose Israel Flores-Puerto, Sharon Patricia Morales-Díaz, Xavier Chiappa-Carrara, Ken Oyama, and Luis Daniel Avila-Cabadilla. 2020. "Taxonomic and Functional Diversity and Composition of Bats in a Regenerating Neotropical Dry Forest" Diversity 12, no. 9: 332. https://doi.org/10.3390/d12090332
APA StyleMartínez-Ferreira, S. R., Alvarez-Añorve, M. Y., Bravo-Monzón, A. E., Montiel-González, C., Flores-Puerto, J. I., Morales-Díaz, S. P., Chiappa-Carrara, X., Oyama, K., & Avila-Cabadilla, L. D. (2020). Taxonomic and Functional Diversity and Composition of Bats in a Regenerating Neotropical Dry Forest. Diversity, 12(9), 332. https://doi.org/10.3390/d12090332










