Exotic Prey Facilitate Coexistence between Pumas and Culpeo Foxes in the Andes of Central Chile
Abstract
1. Introduction
2. Materials and Methods
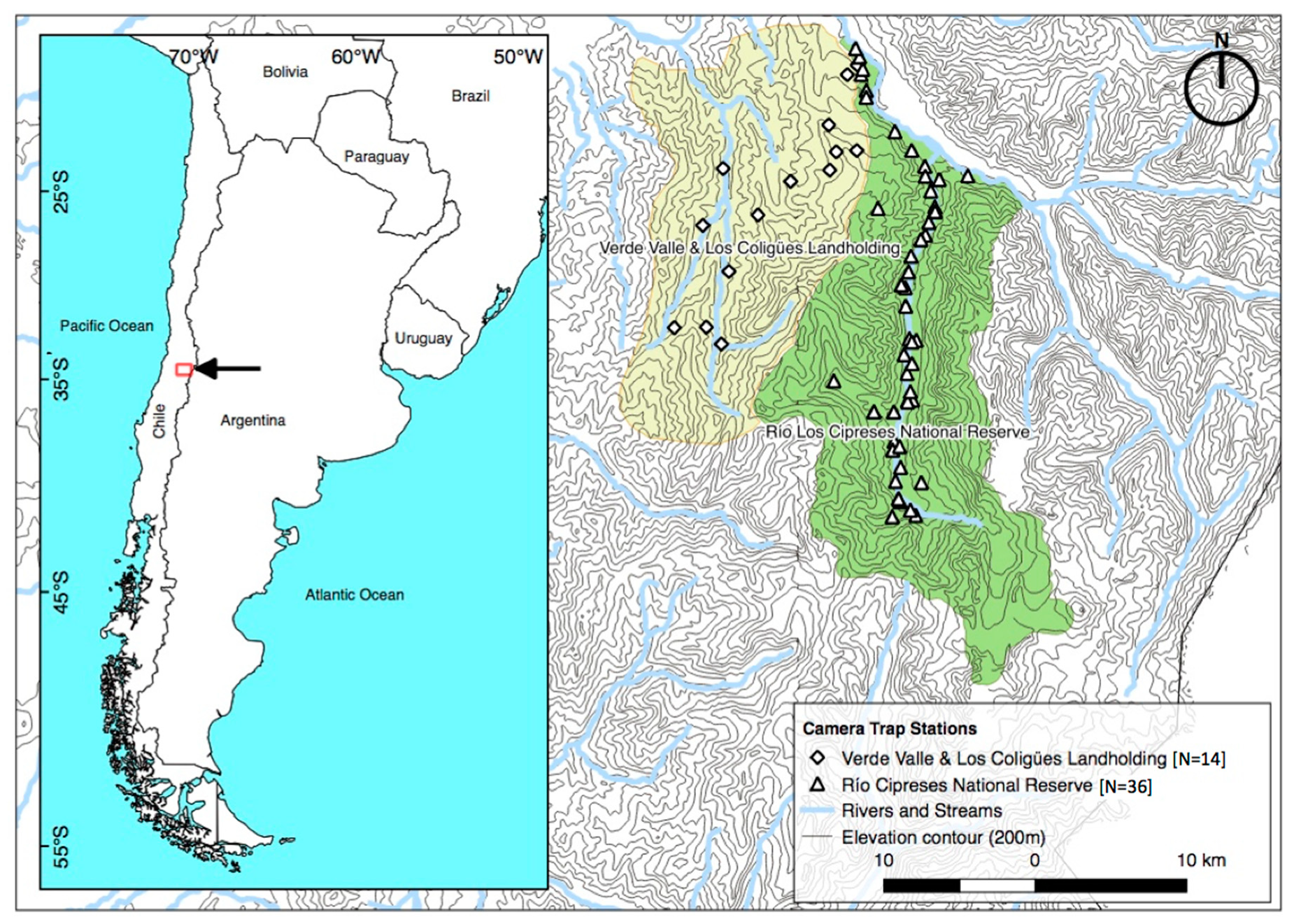
2.1. Study Site
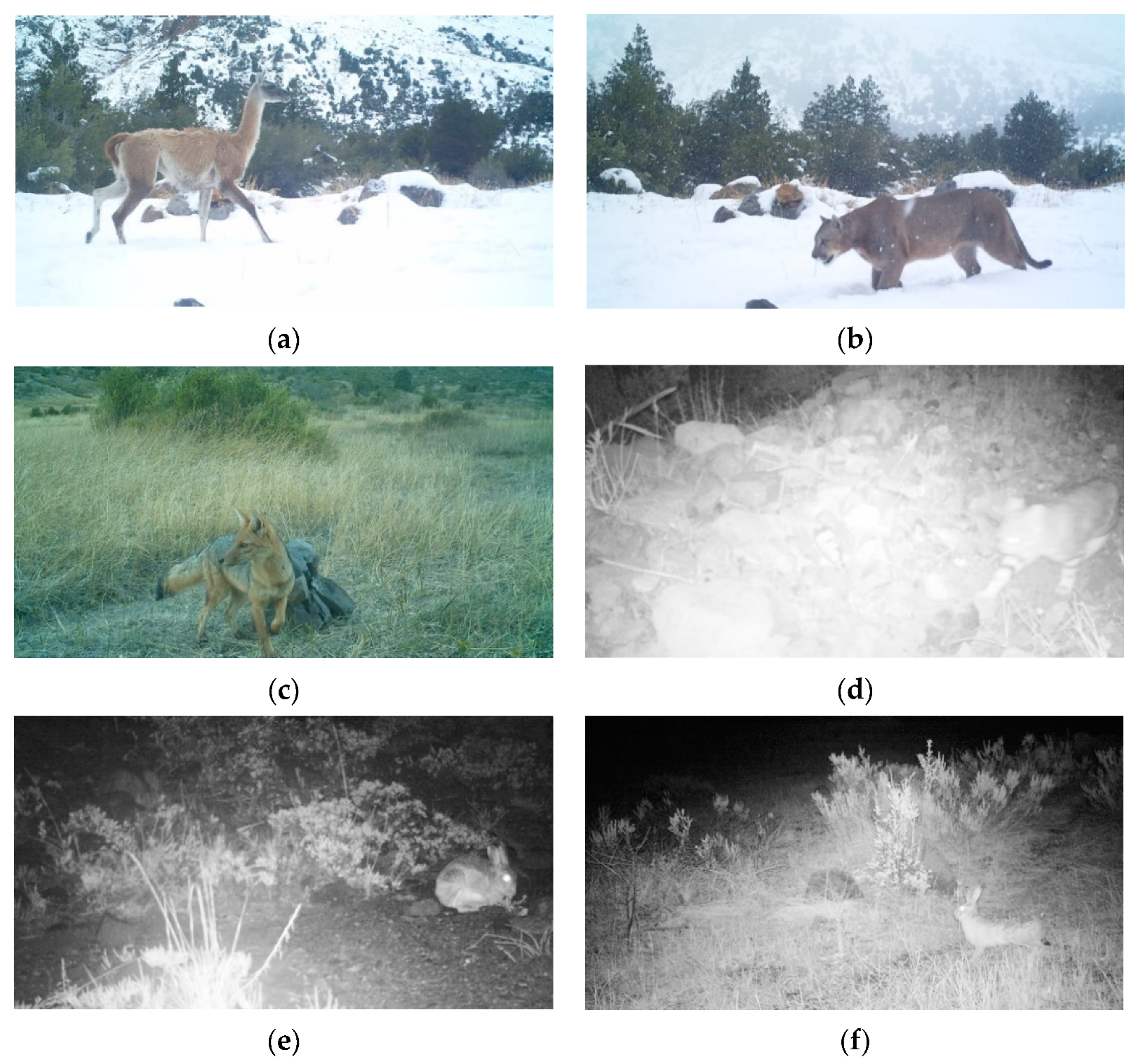
2.2. Camera-Trap Surveys and Trap Rates
2.3. Single-Species, Single-Season Models, and Co-Occurrence Modeling
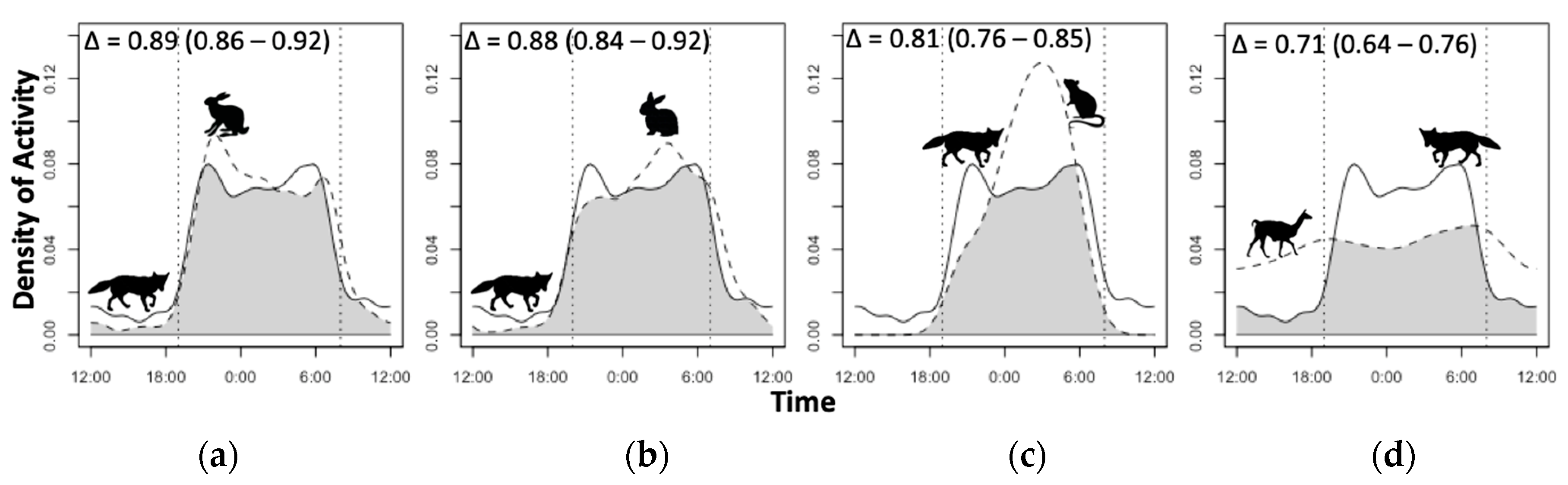
2.4. Temporal Activity Overlap Estimation
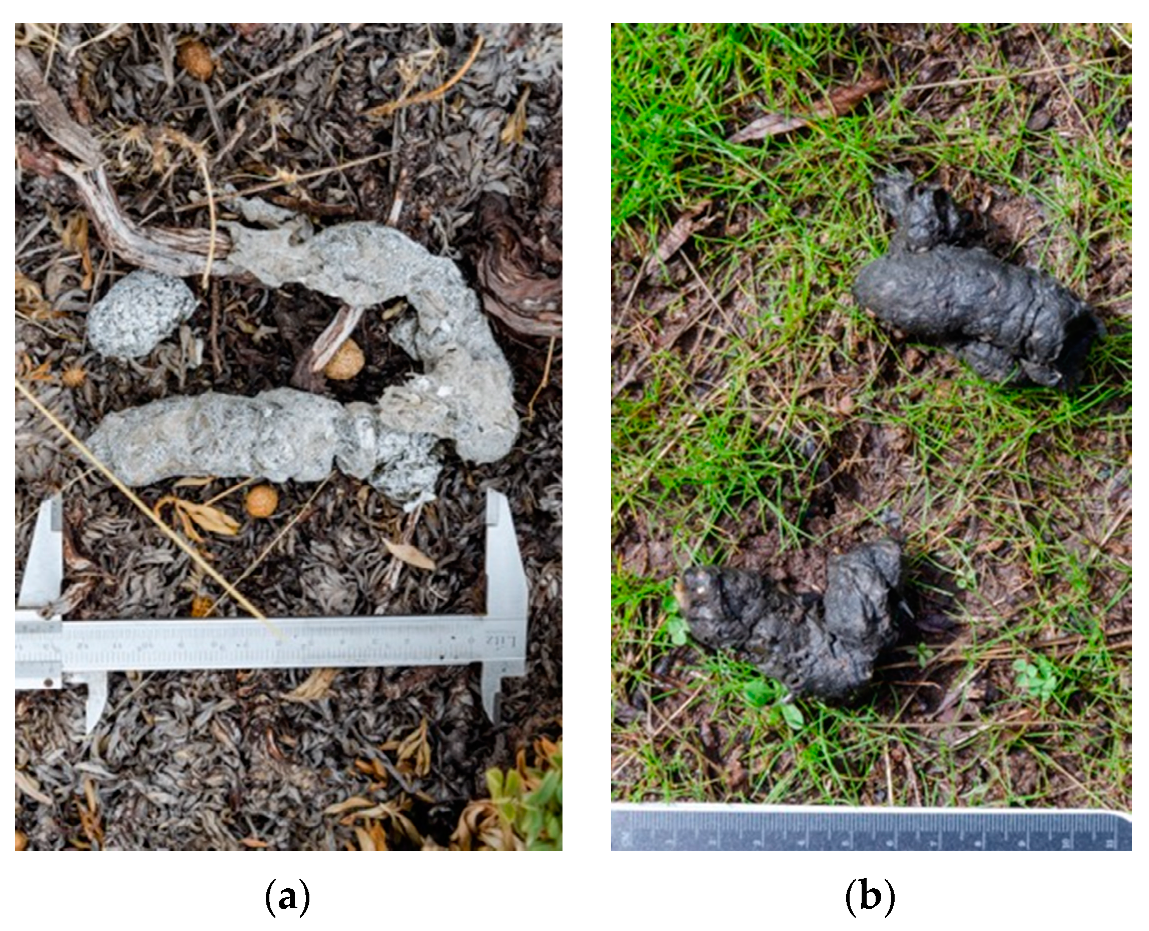
2.5. Scat Collection and Diet Analysis
3. Results
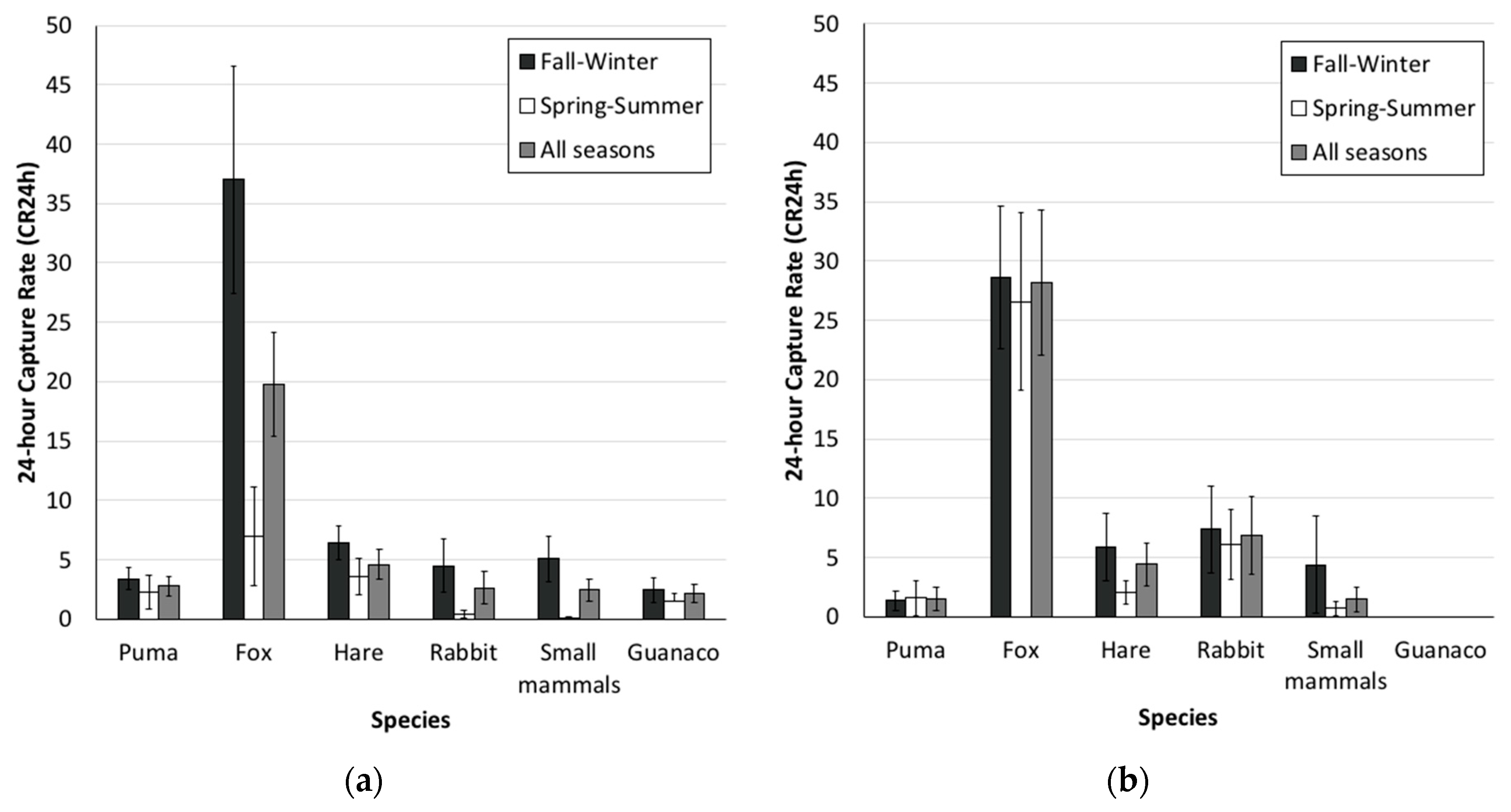
3.1. Camera-Trap Records and Trap Rates
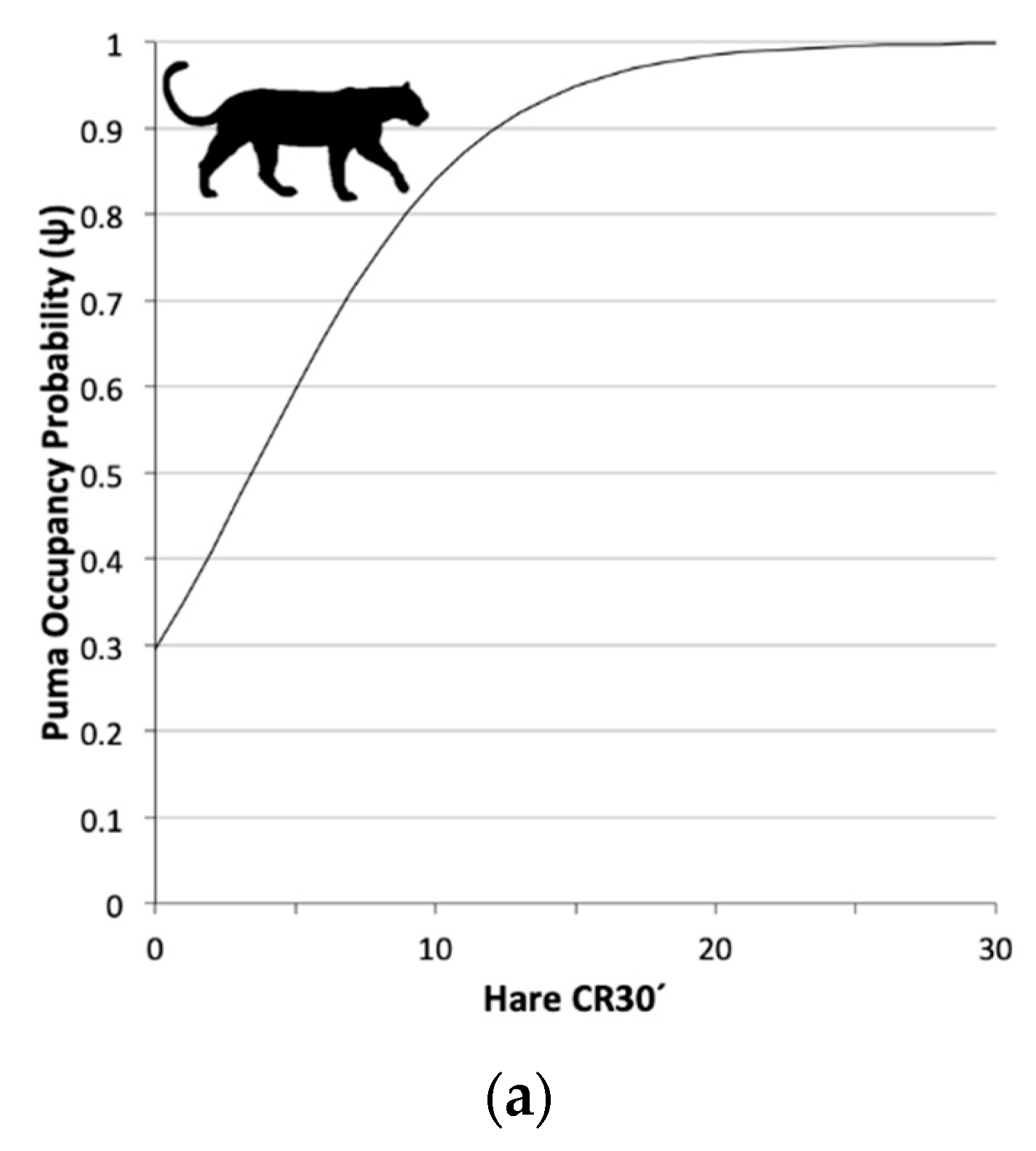
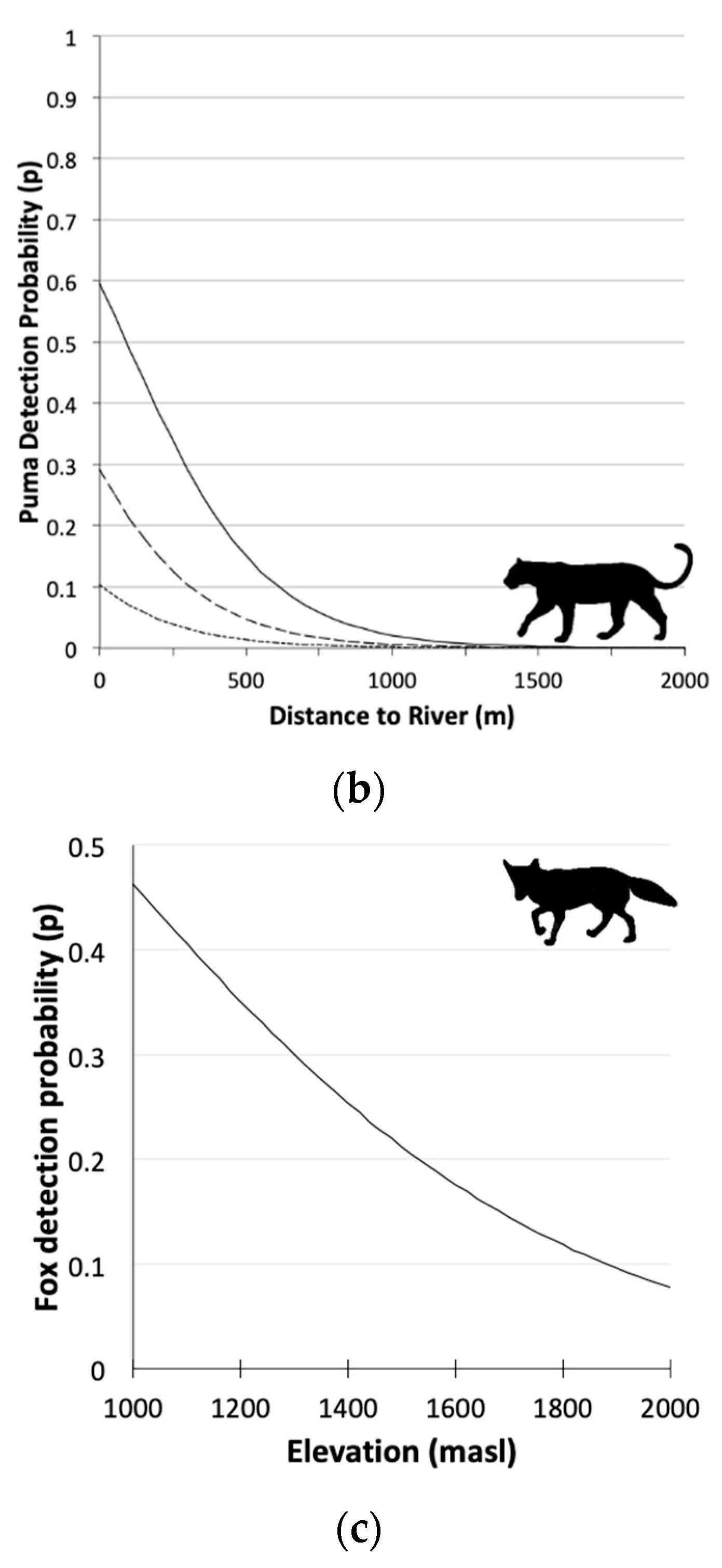
3.2. Single Season Occupancy Models
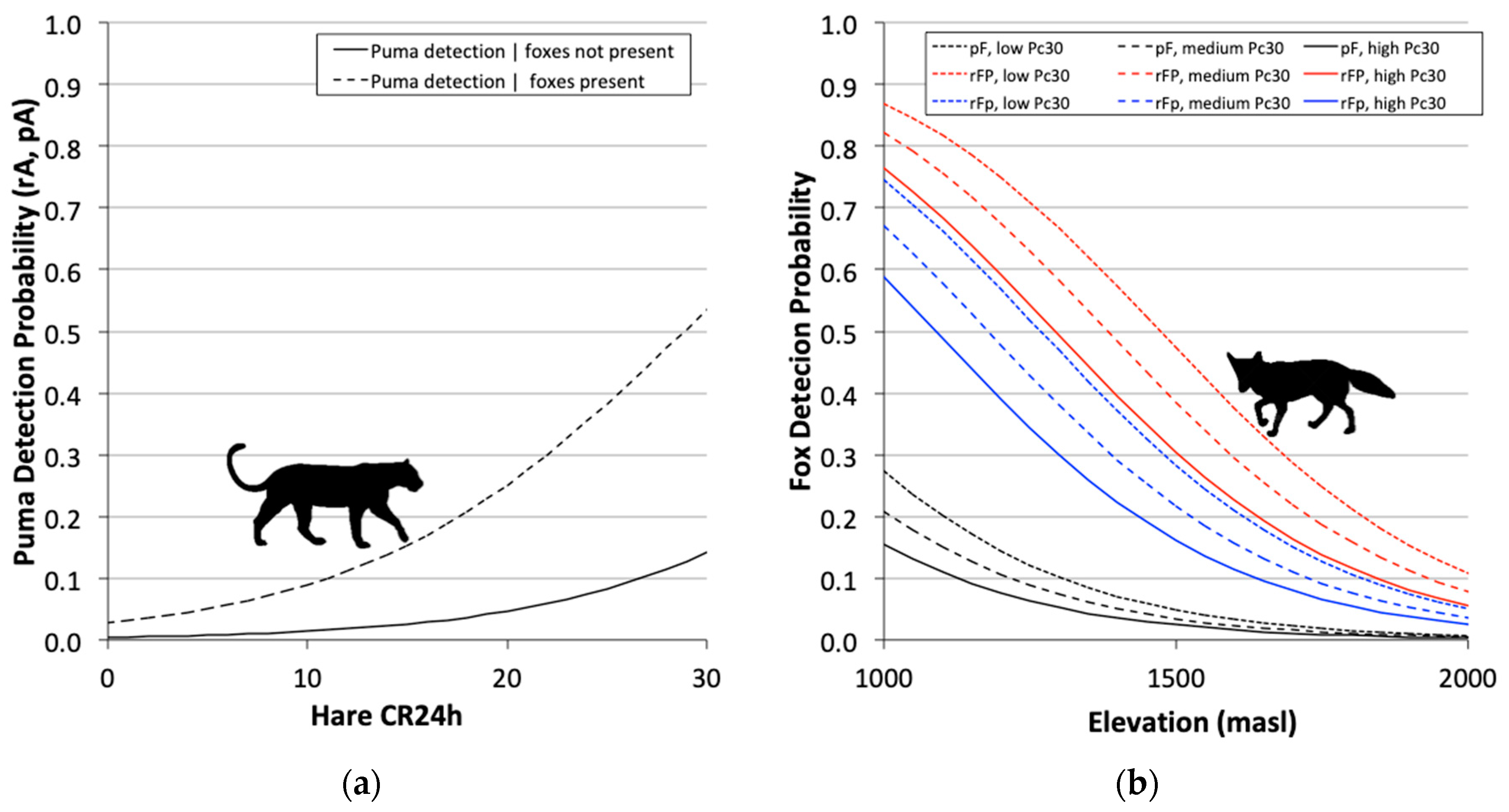
3.3. Two-Species Co-Occurrence Models
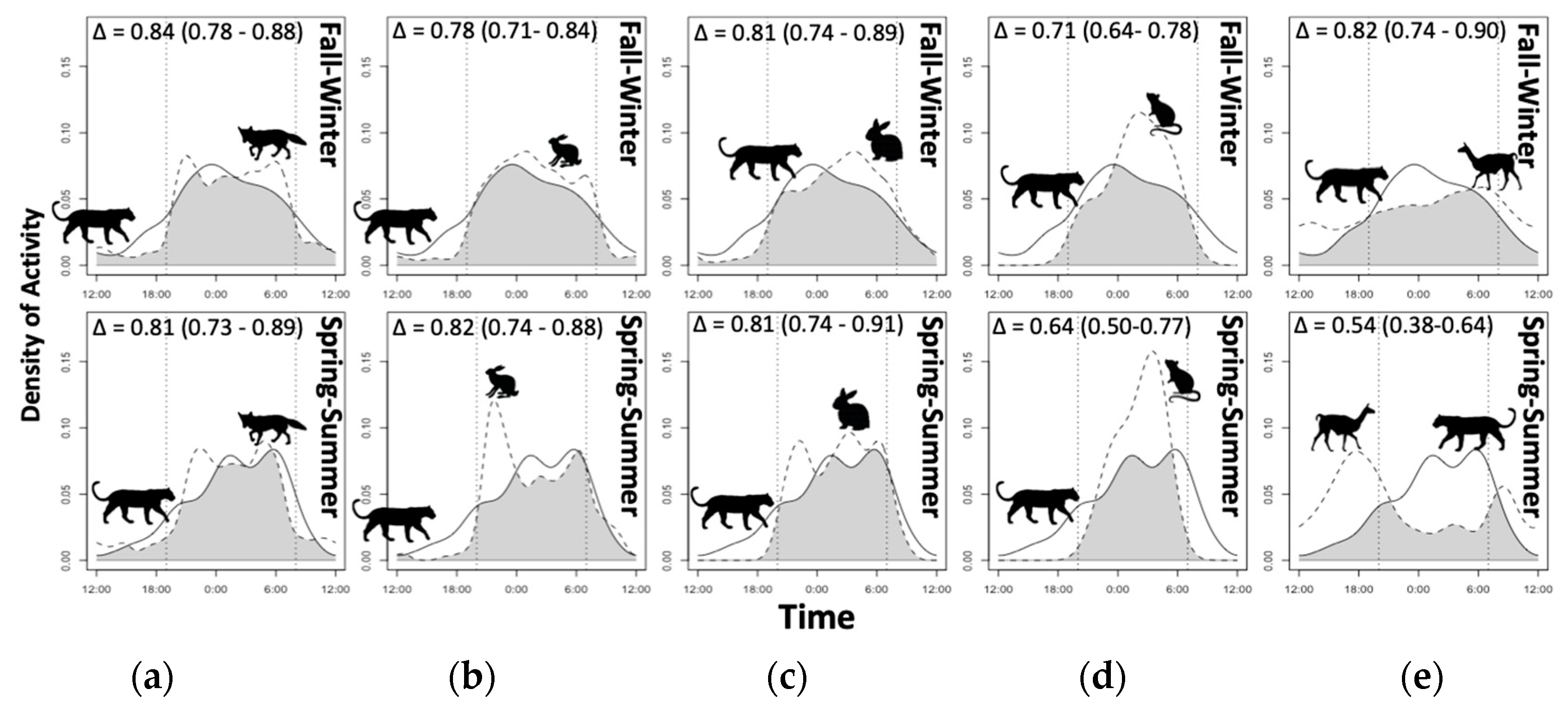
3.4. Diel Selectivity and Activity Pattern Overlap
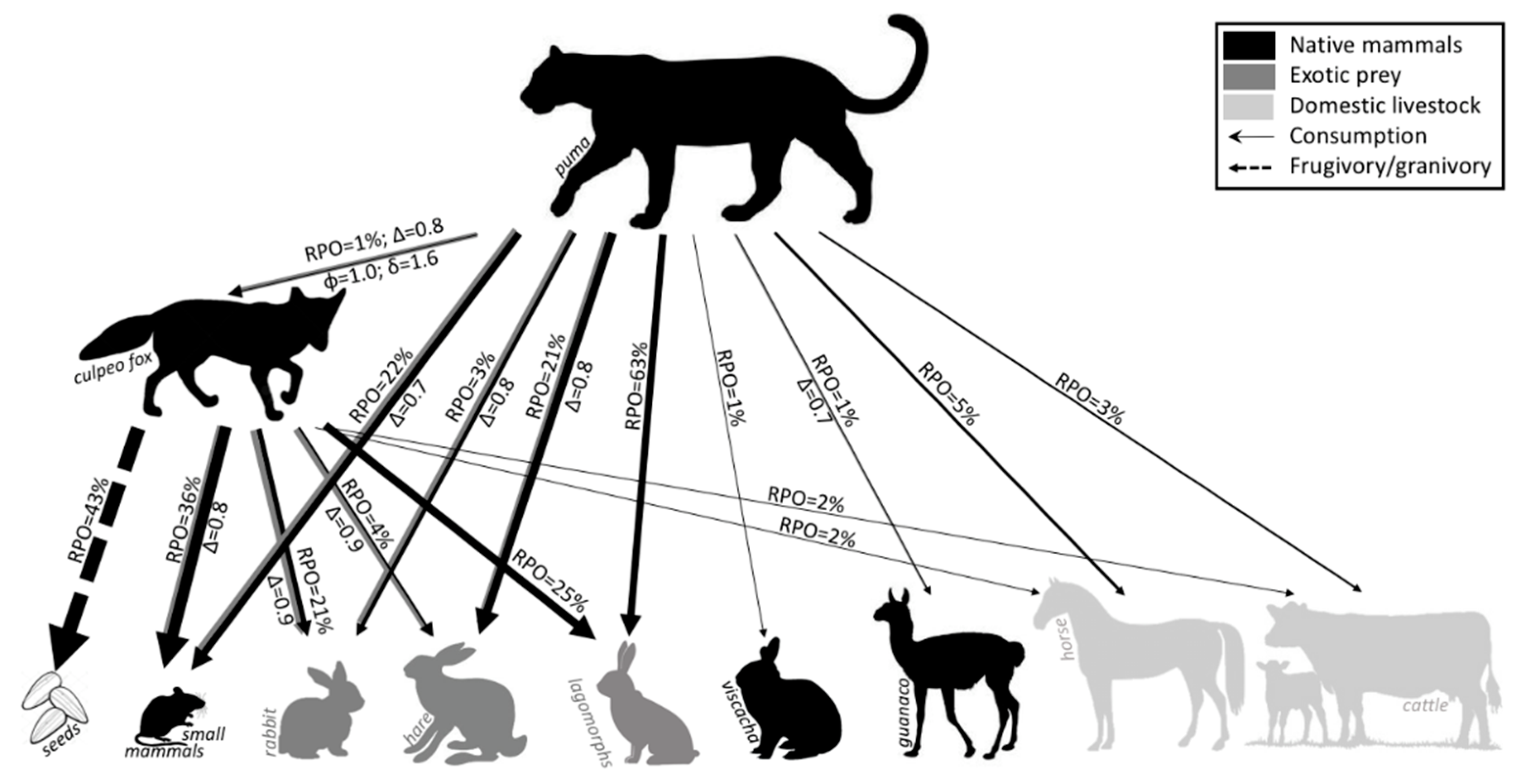
3.5. Diet Analysis
3.6. Carnivores Trophic Niche Breadth and Overlap
4. Discussion
4.1. Camera-Trap Records and Trap Rates
4.2. Space Use, Detection, and Overlap
4.3. Temporal Activity and Overlap
4.4. Trophic, Dietary Overlap
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Model Type | Fox Site Use | Puma Detection | Fox Detection |
|---|---|---|---|
| ΨFP≠ ΨFp, pP ≠ rp, rFP ≠ rFp | Non-independent | Non-independent | Non-independent |
| ΨFP ≠ ΨFp, pP ≠ rP, rFP = rFp | Non-independent | Non-independent | Independent |
| ΨFP ≠ ΨFp, pP = rP, rFP ≠ rFp | Non-independent | Independent | Non-independent |
| ΨFP ≠ ΨFp, pP = rP, rFP = rFp | Non-independent | Independent | Independent |
| ΨFP = ΨFp, pP ≠ rP, rFP ≠ rFp | Independent | Non-independent | Non-independent |
| ΨFP = ΨFp, pP ≠ rP, rFP = rFp | Independent | Non-independent | Independent |
| ΨFP = ΨFp, pP = rP, rFP ≠ rFp | Independent | Independent | Non-independent |
| ΨFP = ΨFp, pP = rP, rFP = rFp | Independent | Independent | Independent |
References
- Lucherini, M.; Reppucci, J.I.; Walker, R.S.; Villalba, M.L.; Wurstten, A.; Gallardo, G.; Iriarte, A.; Villalobos, R.; Perovic, P. Activity pattern segregation of carnivores in the high Andes. J. Mammal. 2009, 90, 1404–1409. [Google Scholar] [CrossRef]
- Romero-Munoz, A.; Maffei, L.; Cuéllar, E.; Noss, A.J. Temporal separation between jaguar and puma in the dry forests of southern Bolivia. J. Trop. Ecol. 2010, 26, 303–311. [Google Scholar] [CrossRef]
- Oliveira-Santos, L.G.R.; Graipel, M.E.; Tortato, M.A.; Zucco, C.A.; Cáceres, N.C.; Goulart, F.V. Abundance changes and activity flexibility of the oncilla, Leopardus tigrinus (Carnivora Felidae), appear to reflect avoidance of conflict. Zool. Curitiba 2012, 29, 115–120. [Google Scholar] [CrossRef]
- Monterroso, P.; Alves, P.C.; Ferreras, P. Catch me if you can: Diel activity patterns of mammalian prey and predators. Ethology 2013, 119, 1044–1056. [Google Scholar] [CrossRef]
- MacArthur, R.; Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Palomares, F.; Caro, T.M. Interspecific killing among mammalian carnivores. Am. Nat. 1999, 153, 492–508. [Google Scholar] [CrossRef]
- Davis, M.; Kelly, M.; Stauffer, D. Carnivore co-existence and habitat use in the Mountain Pine Ridge Forest Reserve, Belize. Anim. Conserv. 2011, 14, 56–65. [Google Scholar] [CrossRef]
- Hernández-SaintMartín, A.D.; Rosas-Rosas, O.C.; Palacio-Núñez, J.; Tarango-Arámbula, L.A.; Clemente-Sánchez, F.; Hoogesteijn, A.L. Activity patterns of jaguar, puma and their potential prey in San Luis Potosi, Mexico. Acta Zool. Mex. Nueva Ser. 2013, 29, 520–533. [Google Scholar]
- Monterroso, P.; Alves, P.C.; Ferreras, P. Plasticity in circadian activity patterns of mesocarnivores in Southwestern Europe: Implications for species coexistence. Behav. Ecol. Sociobiol. 2014, 68, 1403–1417. [Google Scholar] [CrossRef]
- Kiszka, J.; Simon-Bouhet, B.; Martinez, L.; Pusineri, C.; Richard, P.; Ridoux, V. Ecological niche segregation within a community of sympatric dolphins around a tropical island. Mar. Ecol. Prog. Ser. 2011, 433, 273–288. [Google Scholar] [CrossRef]
- Karanth, K.U.; Srivathsa, A.; Vasudev, D.; Puri, M.; Parameshwaran, R.; Kumar, N.S. Spatio-temporal interactions facilitate large carnivore sympatry across a resource gradient. Proc. R. Soc. B Biol. Sci. 2017, 284, 20161860. [Google Scholar] [CrossRef] [PubMed]
- Fedriani, J.M.; Palomares, F.; Delibes, M. Niche relations among three sympatric Mediterranean carnivores. Oecologia 1999, 121, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Šálek, M.; Červinka, J.; Padyšáková, E.; Kreisinger, J. Does spatial co-occurrence of carnivores in a Central European agricultural landscape follow the null model? Eur. J. Wildl. Res. 2014, 60, 99–107. [Google Scholar] [CrossRef]
- Davis, C.L.; Rich, L.N.; Farris, Z.J.; Kelly, M.J.; Di Bitetti, M.S.; Blanco, Y.D.; Albanesi, S.; Farhadinia, M.S.; Gholikhani, N.; Hamel, S.; et al. Ecological correlates of the spatial co-occurrence of sympatric mammalian carnivores worldwide. Ecol. Lett. 2018, 21, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Palomares, F. Coexistence of sympatric carnivores in relatively homogeneous Mediterranean landscapes: Functional importance of habitat segregation at the fine-scale level. Oecologia 2015, 179, 223–235. [Google Scholar] [CrossRef]
- Gerber, B.D.; Karpanty, S.M.; Randrianantenaina, J. Activity patterns of carnivores in the rain forests of Madagascar: Implications for species coexistence. J. Mammal. 2012, 93, 667–676. [Google Scholar] [CrossRef]
- Harmsen, B.J.; Foster, R.J.; Silver, S.C.; Ostro, L.E.; Doncaster, C. Jaguar and puma activity patterns in relation to their main prey. Mamm. Biol. 2011, 76, 320–324. [Google Scholar] [CrossRef]
- Foster, V.C.; Sarmento, P.; Sollmann, R.; Tôrres, N.; Jácomo, A.T.; Negrões, N.; Fonseca, C.; Silveira, L. Jaguar and puma activity patterns and predator-prey interactions in four Brazilian biomes. Biotropica 2013, 45, 373–379. [Google Scholar] [CrossRef]
- Farris, Z.J.; Golden, C.D.; Karpanty, S.; Murphy, A.; Stauffer, D.; Ratelolahy, F.; Andrianjakarivelo, V.; Holmes, C.M.; Kelly, M.J. Hunting, exotic carnivores, and habitat loss: Anthropogenic effects on a native carnivore community, Madagascar. PLoS ONE 2015, 10, e0136456. [Google Scholar] [CrossRef]
- Sunarto, S.; Kelly, M.J.; Parakkasi, K.; Hutajulu, M.B. Cat coexistence in central Sumatra: Ecological characteristics, spatial and temporal overlap, and implications for management. J. Zool. 2015, 296, 104–115. [Google Scholar] [CrossRef]
- Carothers, J.H.; Jaksić, F.M. Time as a niche difference: The role of interference competition. Oikos 1984, 403–406. [Google Scholar] [CrossRef]
- Harmsen, B.; Foster, R.; Silver, S.; Ostro, L.; Doncaster, C. Spatial and temporal interactions of two sympatric cats in a neotropical forest: The jaguar (Panthera onca) and the puma (Puma concolor). J. Mammal. 2009, 90, 224–236. [Google Scholar] [CrossRef]
- Gómez-Ortiz, Y.; Monroy-Vilchis, O.; Mendoza-Martínez, G.D. Feeding interactions in an assemblage of terrestrial carnivores in central Mexico. Zool. Stud. 2015, 54, 16. [Google Scholar] [CrossRef] [PubMed]
- Lanszki, Z.; Horváth, G.F.; Bende, Z.; Lanszki, J. Differences in the diet and trophic niche of three sympatric carnivores in a marshland. Mamm. Res. 2020, 65, 93–104. [Google Scholar] [CrossRef]
- Santos, M.J.; Pinto, B.M.; Santos-Reis, M. Trophic niche partitioning between two native and two exotic carnivores in SW Portugal. Web Ecol. 2007, 7, 53–62. [Google Scholar] [CrossRef]
- Murphy, A.; Kelly, M.J.; Karpanty, S.M.; Andrianjakarivelo, V.; Farris, Z.J. Using camera traps to investigate spatial co-occurrence between exotic predators and native prey species: A case study from northeastern Madagascar. J. Zool. 2019, 307, 264–273. [Google Scholar] [CrossRef]
- Roemer, G.W.; Gompper, M.E.; Van Valkenburgh, B. The ecological role of the mammalian mesocarnivore. BioScience 2009, 59, 165–173. [Google Scholar] [CrossRef]
- Paviolo, A.; Di Blanco, Y.E.; De Angelo, C.D.; Di Bitetti, M.S. Protection affects the abundance and activity patterns of pumas in the Atlantic Forest. J. Mammal. 2009, 90, 926–934. [Google Scholar] [CrossRef]
- Guarda, N.; Gálvez, N.; Leichtle, J.; Osorio, C.; Bonacic, C. Puma concolor density estimation in the Mediterranean Andes of Chile. Oryx 2017, 51, 263–267. [Google Scholar] [CrossRef]
- Pia, M.V. Trophic interactions between puma and endemic culpeo fox after livestock removal in the high mountains of central Argentina. Mammalia 2013, 77, 273–283. [Google Scholar] [CrossRef]
- Palacios, R.; Walker, R.S.; Novaro, A.J. Differences in diet and trophic interactions of Patagonian carnivores between areas with mostly native or exotic prey. Mamm. Biol. 2012, 77, 183–189. [Google Scholar] [CrossRef]
- Bonacic, C.; Ibarra, J.T. Fauna Andina: Historia Natural y Conservación; Ibarra, J.T., Ed.; Editorial Académica Española: Guadalajara, Mexico, 2010. [Google Scholar]
- Guarda, N. Abundance and Trophic Ecology of Puma Concolor in the Andean Environments of Mediterranean Chile. Ph.D. Thesis, Pontificia Universidad Católica de Chile, Santiago, Chile, 2015. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853. [Google Scholar] [CrossRef] [PubMed]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile; Editorial Universitaria: Santiago, Chile, 2006. [Google Scholar]
- Di Castri, F.; Hajek, E. Bioclimatología de Chile; Editorial Universidad Católica de Chile: Santiago, Chile, 1976. [Google Scholar]
- Gajardo, R. La Vegetación Natural de Chile: Clasificación y Distribución Geográfica; Editorial Universitaria: Santiago, Chile, 1995. [Google Scholar]
- Rau, J.R.; Jiménez, J.E. Diet of puma (Puma concolor, Carnivora Felidae) in coastal and Andean ranges of southern Chile. Stud. Neotrop. Fauna Environ. 2002, 37, 201–205. [Google Scholar] [CrossRef]
- Rau, J.; Tillería, M.S.; Martínez, D.R.; Muñoz, A. Dieta de Felis Concolor (Carnivora: Felidae) en áreas silvestres protegidas del sur de Chile. Rev. Chil. Hist. Nat. 1991, 64, 139–144. [Google Scholar]
- Walker, R.S.; Novaro, A.J.; Perovic, P.; Palacios, R.; Donadio, E.; Lucherini, M.; Pia, M.; López, M.S. Diets of three species of Andean carnivores in high-altitude deserts of Argentina. J. Mammal. 2007, 88, 519–525. [Google Scholar] [CrossRef]
- Jaksic, F.M. Vertebrate invaders and their ecological impacts in Chile. Biodivers. Conserv. 1998, 7, 1427–1445. [Google Scholar] [CrossRef]
- Jaksic, F.M.; Iriarte, J.A.; Jiménez, J.E.; Martínez, D.R. Invaders without frontiers: Cross-border invasions of exotic mammals. Biol. Invasions 2002, 4, 157–173. [Google Scholar] [CrossRef]
- Tislerics, A. Oryctolagus cuniculus. Available online: http://animaldiversity.org/accounts/Oryctolagus_cuniculus/ (accessed on 11 July 2016).
- Vu, A. Lepus europaeus. Available online: http://animaldiversity.org/accounts/Lepus_europaeus/ (accessed on 14 May 2016).
- Macdonald, D.W. The Encyclopedia of Mammals; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Novaro, A.J.; Funes, M.C.; Walker, R.S. Ecological extinction of native prey of a carnivore assemblage in Argentine Patagonia. Biol. Conserv. 2000, 92, 25–33. [Google Scholar] [CrossRef]
- Pia, M.V.; López, M.S.; Novaro, A.J. Effects of livestock on the feeding ecology of endemic culpeo foxes (Pseudalopex culpaeus smithersi) in central Argentina. Rev. Chil. Hist. Nat. 2003, 76, 313–321. [Google Scholar] [CrossRef]
- Jaksic, F.M.; Schlatter, R.P.; Yáñez, J.L. Feeding ecology of central Chilean foxes, Dusicyon culpaeus and Dusicyon griseus. J. Mammal. 1980, 61, 254–260. [Google Scholar] [CrossRef]
- Correa, P.; Roa, A. Relaciones tróficas entre Oncifelis guigna, Lycalopex culpaeus, Lycalopex griseus y Tyto alba en un ambiente fragmentado de la zona central de Chile. Mastozool. Neotrop. 2005, 12, 57–60. [Google Scholar]
- Rubio, A.V.; Alvarado, R.; Bonacic, C. Introduced European rabbit as main prey of the native carnivore culpeo fox (Lycalopex culpaeus) in disturbed ecosystems of central Chile. Stud. Neotrop. Fauna Environ. 2013, 48, 89–94. [Google Scholar] [CrossRef]
- Iriarte, J.A.; Johnson, W.E.; Franklin, W.L. Feeding ecology of the Patagonia puma in southernmost Chile. Rev. Chil. Hist. Nat. 1991, 64, 145–156. [Google Scholar]
- Skewes, O.; Moraga, C.A.; Arriagada, P.; Rau, J.R. The european wild boar (Sus scrofa): A biological invader as a recent prey of the American puma (Puma concolor) in southern Chile. Rev. Chil. Hist. Nat. 2012, 85, 227–232. [Google Scholar] [CrossRef]
- Jackson, R.M.; Roe, J.D.; Wangchuk, R.; Hunter, D.O. Estimating snow leopard population abundance using photography and capture-recapture techniques. Wildl. Soc. Bull. 2006, 34, 772–781. [Google Scholar] [CrossRef]
- Delibes-Mateos, M.; Díaz-Ruiz, F.; Caro, J.; Ferreras, P. Activity patterns of the vulnerable guiña (Leopardus guigna) and its main prey in the Valdivian rainforest of southern Chile. Mamm. Biol. 2014, 79, 393–397. [Google Scholar] [CrossRef]
- Gelin, M.L.; Branch, L.C.; Thornton, D.H.; Novaro, A.J.; Gould, M.J.; Caragiulo, A. Response of pumas (Puma concolor) to migration of their primary prey in Patagonia. PLoS ONE 2017, 12, e0188877. [Google Scholar] [CrossRef]
- Kelly, M.J.; Holub, E.L. Camera trapping of carnivores: Trap success among camera types and across species, and habitat selection by species, on Salt Pond Mountain, Giles County, Virginia. Northeast. Nat. 2008, 15, 249–262. [Google Scholar] [CrossRef]
- Srbek-Araujo, A.; Chiarello, A. Domestic dogs in Atlantic forest preserves of south-eastern Brazil: A camera-trapping study on patterns of entrance and site occupancy rates. Braz. J. Biol. 2008, 68, 771–779. [Google Scholar] [CrossRef]
- Hines, J.E. Presence; USGS: Reston, VA, USA, 2006.
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Andrew Royle, J.; Langtimm, C.A. Estimating site occupancy rates when detection probabilities are less than one. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Farris, Z.; Kelly, M.; Karpanty, S.; Ratelolahy, F. Patterns of spatial co-occurrence among native and exotic carnivores in north-eastern Madagascar. Anim. Conserv. 2016, 19, 189–198. [Google Scholar] [CrossRef]
- Thomas, J.P.; Reid, M.L.; Barclay, R.M.; Jung, T.S. Salvage logging after an insect outbreak reduces occupancy by snowshoe hares (Lepus americanus) and their primary predators. Glob. Ecol. Conserv. 2019, 17, e00562. [Google Scholar] [CrossRef]
- Spencer, W.; Rustigian-Romsos, H.; Strittholt, J.; Scheller, R.; Zielinski, W.; Truex, R. Using occupancy and population models to assess habitat conservation opportunities for an isolated carnivore population. Biol. Conserv. 2011, 144, 788–803. [Google Scholar] [CrossRef]
- Webb, N.F.; Merrill, E.H. Simulating carnivore movements: An occupancy–abundance relationship for surveying wolves. Wildl. Soc. Bull. 2012, 36, 240–247. [Google Scholar] [CrossRef]
- Long, R.A.; Donovan, T.M.; MacKay, P.; Zielinski, W.J.; Buzas, J.S. Predicting carnivore occurrence with noninvasive surveys and occupancy modeling. Landsc. Ecol. 2011, 26, 327–340. [Google Scholar] [CrossRef]
- Ackerman, B.B.; Lindzey, F.G.; Hemker, T.P. Cougar food habits in southern Utah. J. Wildl. Manag. 1984, 48, 147–155. [Google Scholar] [CrossRef]
- US Geological Survey; National Aeronautical and Space Agency USGS. Shuttle Radar Topographic Mission, SRTM 2000; USGS: Reston, VA, USA, 2000.
- Burnham, K.P.; Anderson, D.R. A Practical Information-Theoretic Approach. Model Selection Multimodel Inference, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Richmond, O.M.; Hines, J.E.; Beissinger, S.R. Two-species occupancy models: A new parameterization applied to co-occurrence of secretive rails. Ecol. Appl. 2010, 20, 2036–2046. [Google Scholar] [CrossRef]
- Steen, D.A.; McClure, C.J.; Brock, J.C.; Craig Rudolph, D.; Pierce, J.B.; Lee, J.R.; Jeffrey Humphries, W.; Gregory, B.B.; Sutton, W.B.; Smith, L.L.; et al. Snake co-occurrence patterns are best explained by habitat and hypothesized effects of interspecific interactions. J. Anim. Ecol. 2014, 83, 286–295. [Google Scholar] [CrossRef]
- Powell, L.A. Approximating variance of demographic parameters using the delta method: A reference for avian biologists. Condor 2007, 109, 949–954. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Bailey, L.L.; Reid, J.A.; Forsman, E.D.; Nichols, J.D. Modeling co-occurrence of northern spotted and barred owls: Accounting for detection probability differences. Biol. Conserv. 2009, 142, 2983–2989. [Google Scholar] [CrossRef]
- Ivlev, V.S. Experimental Ecology of the Feeding of Fishes; Yale University Press: New Haven, CT, USA, 1961. [Google Scholar]
- Jacobs, J. Quantitative measurement of food selection. Oecologia 1974, 14, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Garrido, F.; Prenda, J.; Narvaez, M. Eurasian otter (Lutra lutra) diet and prey selection in Mediterranean streams invaded by centrarchid fishes. Biol. Invasions 2008, 10, 641–648. [Google Scholar] [CrossRef]
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and Their Applications; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Canty, A.; Ripley, B.D. Boot: Bootstrap R (S-Plus) Functions; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Harrison, D.; Kanji, G.K. The development of analysis of variance for circular data. J. Appl. Stat. 1988, 15, 197–223. [Google Scholar] [CrossRef]
- Harrison, D.; Kanji, G.K.; Gadsden, R.J. Analysis of variance for circular data. J. Appl. Stat. 1986, 13, 123–138. [Google Scholar] [CrossRef]
- Berens, P. CircStat: A MATLAB toolbox for circular statistics. J. Stat. Softw. 2009, 31, 1–21. [Google Scholar] [CrossRef]
- Linkie, M.; Ridout, M.S. Assessing tiger–prey interactions in Sumatran rainforests. J. Zool. 2011, 284, 224–229. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overlap: Estimates of Coefficient of Overlapping for Animal Activity Patterns; R Package Version 02; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Chehébar, C.; Martín, S. Guía para el reconocimiento microscópico de los pelos de los mamíferos de la Patagonia. Doñana Acta Vertebr. 1989, 16, 247–291. [Google Scholar]
- Reise, D. Clave para la Determinación de los Cráneos de Marsupiales y Roedores Chilenos; Instituto de Biología, Universidad de Concepción: Conception, Chile, 1973. [Google Scholar]
- Gladfelter, W.B.; Johnson, W.S. Feeding niche separation in a guild of tropical reef fishes (Holocentridae). Ecology 1983, 64, 552–563. [Google Scholar] [CrossRef]
- Cardona, L. Measurement of trophic niche breadth using occurrence frequencies. J. Fish Biol. 1991, 39, 901–903. [Google Scholar] [CrossRef]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Shrestha, A.; Thapa, K.; Subba, S.A.; Dhakal, M.; Devkota, B.P.; Thapa, G.J.; Shrestha, S.; Malla, S.; Thapa, K. Cats, canines, and coexistence: Dietary differentiation between the sympatric snow leopard and grey wolf in the western landscape of Nepal Himalaya. J. Threat. Taxa 2019, 11, 13815–13821. [Google Scholar] [CrossRef]
- Morin, D.J.; Higdon, S.D.; Holub, J.L.; Montague, D.M.; Fies, M.L.; Waits, L.P.; Kelly, M.J. Bias in carnivore diet analysis resulting from misclassification of predator scats based on field identification. Wildl. Soc. Bull. 2016, 40, 669–677. [Google Scholar] [CrossRef]
- Moreira-Arce, D.; Vergara, P.M.; Boutin, S.; Carrasco, G.; Briones, R.; Soto, G.E.; Jimenez, J.E. Mesocarnivores respond to fine-grain habitat structure in a mosaic landscape comprised by commercial forest plantations in southern Chile. For. Ecol. Manag. 2016, 369, 135–143. [Google Scholar] [CrossRef]
- Noguera-Urbano, E.A.; Ramírez-Chaves, H.E.; Torres-Martínez, M.M. Análisis geográfico y conservación del zorro andino Lycalopex culpaeus (Mammalia, Canidae) en Colombia. Iheringia Sér. Zool. 2016, 106. [Google Scholar] [CrossRef]
- de Lamo, D.A.; Sanborn, A.F.; Carrasco, C.D.; Scott, D.J. Daily activity and behavioral thermoregulation of the guanaco (Lama guanicoe) in winter. Can. J. Zool. 1998, 76, 1388–1393. [Google Scholar] [CrossRef]
- Schai-Braun, S.C.; Rödel, H.G.; Hackländer, K. The influence of daylight regime on diurnal locomotor activity patterns of the European hare (Lepus europaeus) during summer. Mamm. Biol. 2012, 77, 434–440. [Google Scholar] [CrossRef]
- Zaccaroni, M.; Biliotti, N.; Buccianti, A.; Calieri, S.; Ferretti, M.; Genghini, M.; Riga, F.; Trocchi, V.; Dessì-Fulgheri, F. Winter locomotor activity patterns of European hares (Lepus europaeus). Mamm. Biol. 2013, 78, 482–485. [Google Scholar] [CrossRef]
- Durant, S.M. Competition refuges and coexistence: An example from Serengeti carnivores. J. Anim. Ecol. 1998, 67, 370–386. [Google Scholar] [CrossRef]
- Durant, S.M. Living with the enemy: Avoidance of hyenas and lions by cheetahs in the Serengeti. Behav. Ecol. 2000, 11, 624–632. [Google Scholar] [CrossRef]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Elbroch, L.M.; Wittmer, H.U. The effects of puma prey selection and specialization on less abundant prey in Patagonia. J. Mammal. 2013, 94, 259–268. [Google Scholar] [CrossRef]
- Wilson, P. Puma predation on guanacos in Torres del Paine National Park, Chile. Mammalia 1984, 48, 515–522. [Google Scholar] [CrossRef]
- Bank, M.S.; Sarno, R.J.; Campbell, N.K.; Franklin, W.L. Predation of guanacos (Lama guanicoe) by southernmost mountain lions (Puma concolor) during a historically severe winter in Torres del Paine National Park, Chile. J. Zool. 2002, 258, 215–222. [Google Scholar] [CrossRef]
- Cajal, J.L.; Lopez, N.E. The puma as a predator of wild camelids in the San Guillermo reserve, San-Juan, Argentina. Rev. Chil. Hist. Nat. 1987, 60, 87–91. [Google Scholar]
- Zanón Martínez, J.I.; Travaini, A.; Zapata, S.; Procopio, D.; Santillán, M.Á. The ecological role of native and introduced species in the diet of the puma Puma concolor in southern Patagonia. Oryx 2012, 46, 106–111. [Google Scholar] [CrossRef]
- Baldi, R.; PELLIZA-SBRILLER, A.; Elston, D.; Albon, S. High potential for competition between guanacos and sheep in Patagonia. J. Wildl. Manag. 2004, 68, 924–938. [Google Scholar] [CrossRef]
- Remonti, L.; Balestrieri, A.; Raubenheimer, D.; Saino, N. Functional implications of omnivory for dietary nutrient balance. Oikos 2016, 125, 1233–1240. [Google Scholar] [CrossRef]
- Lanszki, J.; Zalewski, A.; Horváth, G. Comparison of red fox Vulpes vulpes and pine marten Martes martes food habits in a deciduous forest in Hungary. Wildl. Biol. 2007, 13, 258–271. [Google Scholar] [CrossRef]
- Moss, W.E.; Alldredge, M.W.; Logan, K.A.; Pauli, J.N. Human expansion precipitates niche expansion for an opportunistic apex predator (Puma concolor). Sci. Rep. 2016, 6, 39639. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.W.; Logan, K.A.; Sweanor, L.L.; Boyce, W.M. Scavenging behavior in puma. Southwest Nat. 2005, 50, 466–471. [Google Scholar] [CrossRef]
- Aranda, M.; Sánchez-Cordero, V. Prey spectra of jaguar (Panthera onca) and puma (Puma concolor) in tropical forests of Mexico. Stud. Neotrop. Fauna Environ. 1996, 31, 65–67. [Google Scholar] [CrossRef]
- Moreno, R.S.; Kays, R.W.; Samudio, R. Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. J. Mammal. 2006, 87, 808–816. [Google Scholar] [CrossRef]
| Model | AICc | ΔAICc | wi | L | K | |
|---|---|---|---|---|---|---|
| Puma | Ψ 1(Le30 2),p 3(Le24 4+D.river 5) | 477.28 | 0.00 | 0.725 | 1.000 | 5 |
| Ψ(.),p(Le24+D.river) | 483.48 | 5.59 | 0.035 | 0.061 | 4 | |
| Ψ(Le30),p(Le24) | 493.90 | 16.01 | 0.000 | 0.000 | 4 | |
| Ψ(.),p(.) 6 | 540.10 | 62.21 | 0.000 | 0.000 | 2 | |
| Culpeo Fox | Ψ(.),p(Elev 7) | 1551.40 | 0.00 | 0.710 | 1.000 | 3 |
| Ψ(.),p(Pc30 8+Elev) | 1553.33 | 1.93 | 0.271 | 0.482 | 4 | |
| Ψ(.),p(Habitat) | 1559.61 | 8.21 | 0.012 | 0.017 | 4 | |
| Ψ(.),p(.) 6 | 1604.21 | 52.81 | 0.000 | 0.000 | 2 |
| β (95% CI) | ||
|---|---|---|
| Puma | Ψ 1(Le30 2) | 2.53 (−0.14; 5.21) |
| p3(Le24 4) | 0.11 (0.45; 0.90) | |
| p(D.river 5) | −1.48 (−2.23; −0.73) | |
| Culpeo Fox | p(Elev 6)top model | −0.47 (−0.59; −0.34) |
| p(Elev)second model | −0.45 (−0.58; −0.33) | |
| p(Pc30 7)second model | −0.06 (−0.20; 0.09) |
| Model | AICc | ΔAICc | wi | L | K |
|---|---|---|---|---|---|
| ΨP(Le30 1), ΨFP(.) = ΨFp(.), pP(Le24 2), pF(Elev 3 + Pc30 4), rP(Le24), rFP(Elev + Pc30), rFp(Elev + Pc30) | 1952.1 | 0 | 0.91 | 1 | 11 |
| ΨP(Le30), ΨFP(.) = ΨFp(.), pP = rP(Le24), pF(Elev + Pc30), rFP(Elev+Pc30), rFp(Elev + Pc30) | 1957.9 | 5.74 | 0.05 | 0.06 | 10 |
| ΨP(.), ΨFP(.) ≠ ΨFp(.), pP(.), pF(.), rP(.), rFP(.) ≠ rFp(.) 5 | 2126.7 | 174.5 | 0.00 | 0.00 | 8 |
| Species | JSInight (95% CI) | JSIdawn (95% CI) | JSIday (95% CI) | JSIdusk (95% CI) |
|---|---|---|---|---|
| Puma | 0.34 (0.24; 0.45) + | 0.16 (−0.01; 0.35) | −0.56 (−0.67; −0.44) − | 0.10 (−0.07; 0.32) |
| Culpeo fox | 0.60 (0.56; 0.63) + | −0.28 (−0.37; −0.19) | −0.65 (−0.69; −0.61) − | −0.15 (−0.24; −0.06) − |
| Guanaco | −0.77 (−0.87; −0.67) − | −0.35 (−0.65; 0.03) | 0.77 (0.69; 0.86) + | −0.42 (−0.72; −0.04) − |
| Hare | 0.47 (0.41; 0.53) + | 0.20 (0.10; 0.31) + | −0.80 (−0.85; −0.74) − | 0.13 (0.02; 0.25) + |
| Rabbit | 0.55 (0.47; 0.62) + | 0.12 (−0.02; 0.29) | −0.81 (−0.88; −0.74) − | 0.00 (−0.17; 0.18) |
| Small mammals | 0.99 (0.98; 1.00) + | −1.00 (−1.00; −1.00) − | −1.00 (−1.00; −1.00) − | −0.92 (−1.07; −0.74) − |
| Puma | Culpeo Fox | ||||||
|---|---|---|---|---|---|---|---|
| Item | N | FO 1 | RPO% 2 | RBM% 3 | N | FO 1 | RPO% 2 |
| Lagomorphs | 120 | 76 | 63 | 70 | 12 | 25 | 12 |
| Small mammals | 41 | 26 | 22 | 2 | 36 | 75 | 36 |
| Viscacha | 2 | 1 | 1 | 1 | 0 | 0 | 0 |
| Ungulates | |||||||
| Cattle | 5 | 3 | 3 | 8 | 2 | 4 | 2 |
| Horse | 10 | 6 | 5 | 15 | 2 | 4 | 2 |
| Guanaco | 2 | 1 | 1 | 3 | 0 | 0 | 0 |
| Total ungulates | 17 | 11 | 9 | 26 | 4 | 8 | 4 |
| Carnivores | |||||||
| Culpeo fox | 1 | 1 | 1 | 1 | - | - | - |
| Hog-nosed skunk | 0 | 0 | 0 | 0 | 1 | 2 | 1 |
| Total carnivores | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| Birds | 4 | 3 | 2 | - | 4 | 8 | 4 |
| Reptiles | 1 | 1 | 1 | - | 0 | 0 | 0 |
| Seeds | |||||||
| Peumo 4 | - | - | - | - | 2 | 4 | 2 |
| Litre 5 | - | - | - | - | 28 | 58 | 28 |
| Molle 6 | - | - | - | - | 4 | 8 | 4 |
| Drupes | - | - | - | - | 5 | 10 | 5 |
| Unknown seeds | - | - | - | - | 4 | 8 | 4 |
| Total seeds | - | - | - | - | 43 | 88 | 43 |
| Undetermined | 3 | 2 | 2 | - | - | - | - |
| Total | 189 | 120 | 100 | 100 | 100 | 206 | 100 |
| Total scats | 158 | 48 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio, C.; Muñoz, A.; Guarda, N.; Bonacic, C.; Kelly, M. Exotic Prey Facilitate Coexistence between Pumas and Culpeo Foxes in the Andes of Central Chile. Diversity 2020, 12, 317. https://doi.org/10.3390/d12090317
Osorio C, Muñoz A, Guarda N, Bonacic C, Kelly M. Exotic Prey Facilitate Coexistence between Pumas and Culpeo Foxes in the Andes of Central Chile. Diversity. 2020; 12(9):317. https://doi.org/10.3390/d12090317
Chicago/Turabian StyleOsorio, Christian, Ana Muñoz, Nicolás Guarda, Cristian Bonacic, and Marcella Kelly. 2020. "Exotic Prey Facilitate Coexistence between Pumas and Culpeo Foxes in the Andes of Central Chile" Diversity 12, no. 9: 317. https://doi.org/10.3390/d12090317
APA StyleOsorio, C., Muñoz, A., Guarda, N., Bonacic, C., & Kelly, M. (2020). Exotic Prey Facilitate Coexistence between Pumas and Culpeo Foxes in the Andes of Central Chile. Diversity, 12(9), 317. https://doi.org/10.3390/d12090317





