Crenal Habitats: Sources of Water Mite (Acari: Hydrachnidia) Diversity
Abstract
1. Introduction
2. Materials and Methods
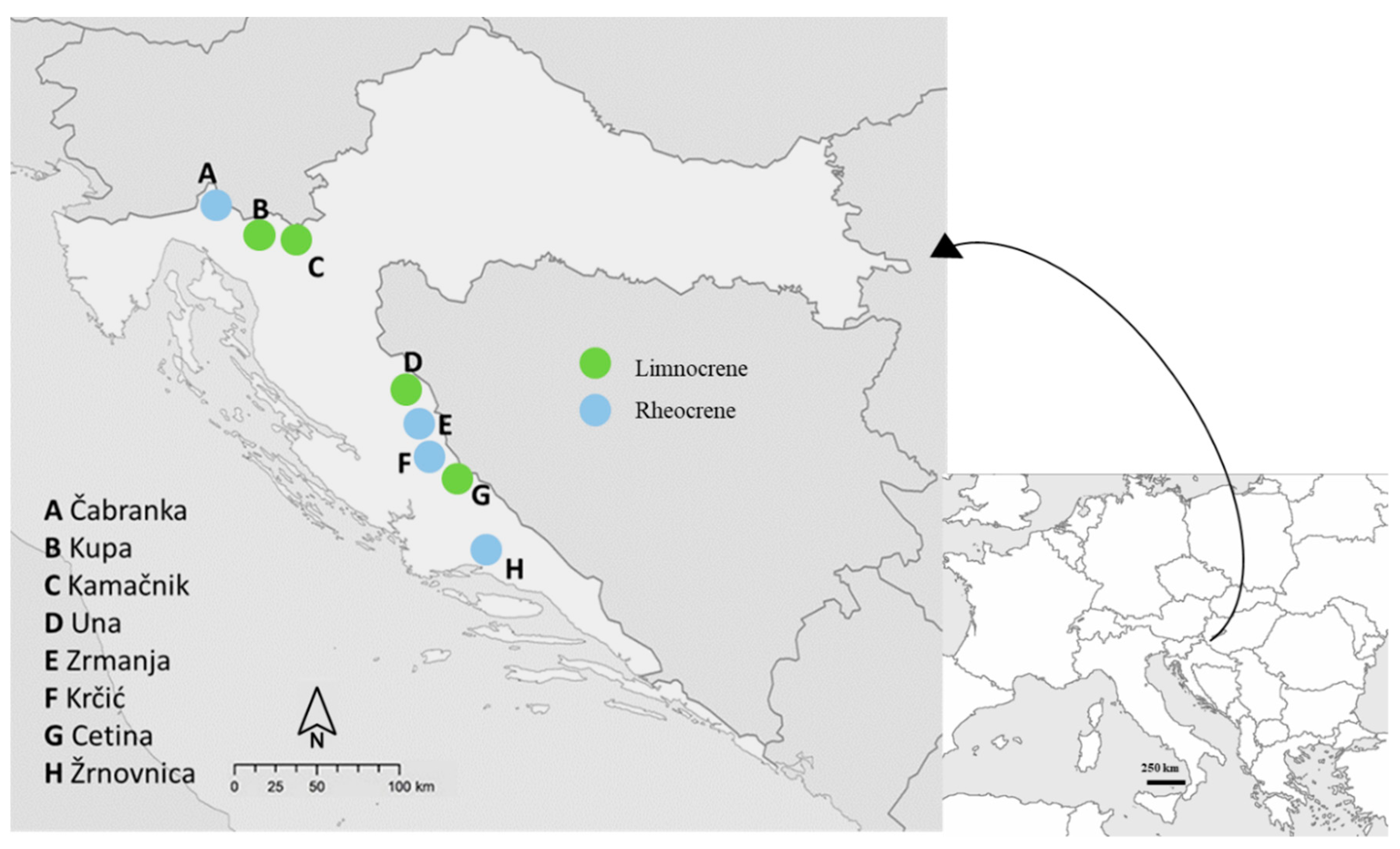
2.1. Study Area
2.2. Environmental Variables
2.3. Data Analysis
3. Results
3.1. Environmental Conditions in Spring Ecomorphotypes
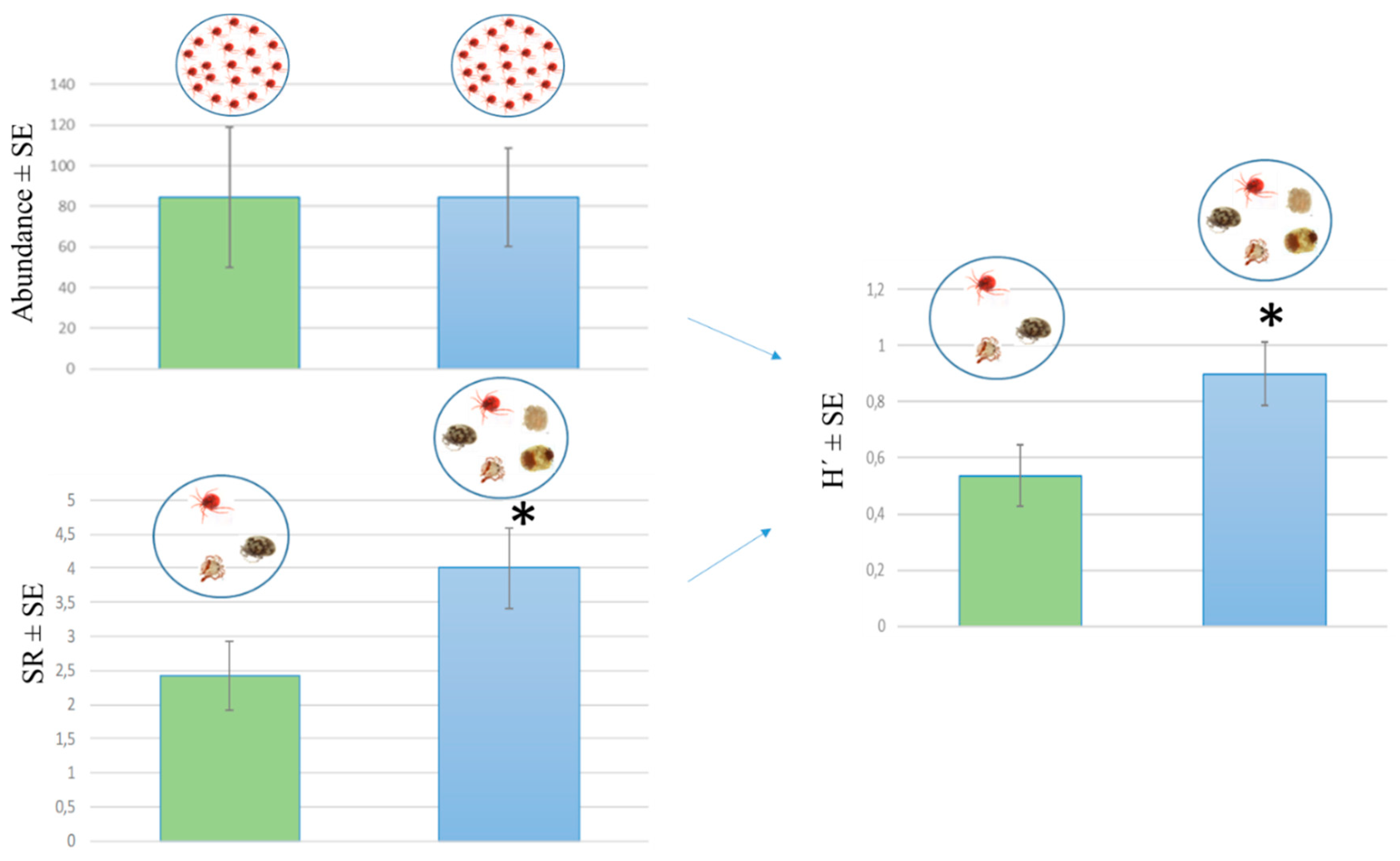
3.2. Water Mite Diversity in Spring Morphotypes
3.3. Synecological Water Mite Groups in Spring Morphotypes
3.4. Water Mite Assemblage Similarity within Spring Morphotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Spring (Morphotype) | Season | T (°C) | Con. O2 (mg/L) | Sat. O2 (%) | σ (μS/cm) | CaCO3 (mg/L) | COD (mgO2/L) |
|---|---|---|---|---|---|---|---|
| Čabranka | Fall | 8.75 | 11.39 | 105.73 | 436.00 | 232.50 | 2.01 |
| (Rheocrene) | Summer | 8.80 | 11.12 | 101.60 | 420.33 | 206.67 | 0.68 |
| Spring | 8.67 | 13.08 | 119.50 | 409.67 | 205.83 | 0.93 | |
| Winter | 8.38 | 11.09 | 101.50 | 378.75 | 192.50 | 1.81 | |
| Cetina | Fall | 8.40 | 12.25 | 104.80 | 331.00 | 160.00 | 1.18 |
| (Limnocrene) | Summer | 9.10 | 13.02 | 114.83 | 294.00 | 145.00 | 1.10 |
| Spring | 8.90 | 14.16 | 128.13 | 310.00 | 165.00 | 0.83 | |
| Winter | 8.90 | 12.81 | 111.38 | 338.00 | 175.00 | 0.24 | |
| Kamačnik | Fall | 7.40 | 11.74 | 102.10 | 336.00 | 180.00 | 2.36 |
| (Limnocrene) | Summer | 9.23 | 12.57 | 114.70 | 303.25 | 166.25 | 0.58 |
| Spring | 9.30 | 12.30 | 111.13 | 296.00 | 138.75 | 0.63 | |
| Winter | 7.20 | 11.95 | 103.70 | 322.00 | 162.50 | 1.02 | |
| Krčić | Fall | 9.10 | 10.29 | 97.25 | 395.00 | 200.00 | 1.49 |
| (Rheocrene) | Summer | 9.70 | 10.63 | 101.43 | 395.00 | 202.50 | 0.94 |
| Spring | 9.00 | 10.03 | 95.68 | 323.00 | 165.00 | 1.34 | |
| Winter | 8.90 | 9.90 | 94.30 | 406.00 | 195.00 | 1.49 | |
| Kupa | Fall | 7.73 | 10.73 | 96.10 | 283.25 | 153.13 | 1.79 |
| (Limnocrene) | Summer | 9.40 | 11.50 | 103.00 | 266.75 | 135.63 | 0.85 |
| Spring | 8.40 | 10.78 | 97.90 | 236.00 | 124.17 | 0.89 | |
| Winter | 7.57 | 11.49 | 103.80 | 256.33 | 125.83 | 1.55 | |
| Una | Fall | 9.50 | 11.80 | 104.20 | 414.00 | 227.50 | 1.57 |
| (Limnocrene) | Summer | 11.80 | 11.72 | 102.70 | 420.00 | 215.00 | 1.18 |
| Spring | 10.20 | 11.33 | 104.10 | 394.00 | 205.00 | 0.71 | |
| Winter | 9.30 | 11.35 | 101.10 | 440.00 | 247.50 | 1.41 | |
| Zrmanja | Fall | 8.90 | 10.50 | 95.30 | 359.00 | 195.00 | 1.34 |
| (Rheocrene) | Summer | 9.80 | 11.84 | 108.70 | 347.00 | 170.00 | 1.10 |
| Spring | 9.50 | 10.85 | 98.90 | 319.00 | 175.00 | 1.81 | |
| Winter | 8.40 | 12.08 | 107.50 | 363.00 | 185.00 | 1.81 | |
| Žrnovnica | Fall | 12.52 | 11.26 | 102.30 | 413.80 | 190.00 | 1.20 |
| (Rheocrene) | Summer | 12.60 | 11.63 | 106.80 | 393.00 | 185.83 | 1.26 |
| Spring | 12.56 | 11.14 | 107.90 | 369.57 | 185.00 | 0.79 | |
| Winter | 12.50 | 11.37 | 103.40 | 373.33 | 189.17 | 1.09 |
| Spring | Latitude | Longitude | Microhabitat Ratio (%) | |||
|---|---|---|---|---|---|---|
| Spring | Summer | Fall | Winter | |||
| Čabranka (Rheocrene) | 45° 36′ 02.6″ | 14° 38′ 27.3″ | Microlithal + Mesolithal (40%), Phytal (60%) | Microlithal + Mesolithal (60%), Phytal (40%) | Microlithal + Mesolithal (55%), Phytal (45%) | Mesolithal (70%), Phytal (30%) |
| Cetina (Limnocrene) | 43° 58′ 50.9″ | 16° 25′ 81.4″ | Akal (15%), Macrolithal (50%), Phytal (35%) | Microlithal + Mesolithal + Macrolithal (30%), Phytal (30%), Psammal (40%) | Akal (15%), Mesolithal (45%), Phytal (40%) | Microlithal + Mesolithal (55%), Phytal (45%) |
| Kamačnik (Limnocrene) | 45° 20′ 49.3″ | 15° 03′ 38″ | Ksilal + Microlithal + Mesolithal (40%), Phytal (60%) | Macrolithal + Megalithal (10%), Phytal (90%) | Microlithal (10%), Phytal (90%) | Microlithal + Mesolithal (10%), Phytal (90%) |
| Krčić (Rheocrene) | 44° 25′ 48″ | 16° 33′ 61.9″ | Phytal (100%) | Mesolithal (40%), Phytal (60%) | Mesolithal (20%), Phytal (80%) | Phytal (100%) |
| Kupa (Limnocrene) | 45° 29′ 27.2″ | 14° 41′ 28.5″ | Megalithal (15%), Mesolithal + Macrolithal (30%), Phytal (50%) Psammal (5%) | Microlithal (20%), Mesolithal (40%), Phytal (40%) | Microlithal + Mesolithal (80%), Phytal (10%), Psammal (10%) | Phytal (50%), Mesolithal (50%) |
| Una (Limnocrene) | 44° 24′ 12.9″ | 16° 06′ 41.6″ | Phytal (100 %) | Akal + Microlithal (10%), Mesolithal + Macrolithal (20%), Phytal (70%) | Microlithal + Mesolithal (80%), Phytal (20%) | Akal + Mesolithal (30%), Macrolithal + Mesolithal (50%), Phytal (20%) |
| Zrmanja (Rheocrene) | 44° 11′ 79.2″ | 16° 03′ 38.1″ | Microlithal + Mesolithal + Microlithal (55%), Phytal (45%) | Microlithal + Mesolithal (50%), Phytal (50%) | Microlithal + Mesolithal (40%), Phytal (60%) | Akal + Microlithal + Mesolithal (40%), Phytal (60%) |
| Žrnovnica (Rheocrene) | 43° 31′ 24.8″ | 16° 34′ 29.2″ | Mesolithal + Megalithal (60%), Phytal (40%) | Microlithal + Mesolithal (30%), Phytal (70%) | Microlithal + Mesolithal (40%), Phytal (60%) | Phytal (100%) |
| Spring | Season | Atractides Sp. | Atractides Gibberipapis | Atractides Latipapis | Atractides Loricatus | Atractides Nodipapis | Atractides Penatus | Atractides Walteri | Aturus Sp. | Feltria Sp. | Hydrodroma Reinhardii | Lebertia Sp. | Lethaxona Cavifrons | Ljania Macilenta | Partnunia Angusta |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Čabranka | Fall | + | |||||||||||||
| (Rheocrene) | Summer | ||||||||||||||
| Spring | |||||||||||||||
| Winter | + | ||||||||||||||
| Cetina | Fall | + | + | + | + | ||||||||||
| (Limnocrene) | Summer | + | + | + | |||||||||||
| Spring | + | + | + | + | |||||||||||
| Winter | + | + | |||||||||||||
| Kamačnik | Fall | + | |||||||||||||
| (Limnocrene) | Summer | ||||||||||||||
| Spring | + | + | |||||||||||||
| Winter | + | + | |||||||||||||
| Krčić | Fall | ||||||||||||||
| (Rheocrene) | Summer | + | + | ||||||||||||
| Spring | |||||||||||||||
| Winter | |||||||||||||||
| Kupa | Fall | + | + | ||||||||||||
| (Limnocrene) | Summer | + | + | + | + | + | + | ||||||||
| Spring | + | + | + | + | + | + | |||||||||
| Winter | + | + | + | + | + | + | |||||||||
| Una | Fall | + | + | + | + | + | |||||||||
| (Limnocrene) | Summer | + | + | + | |||||||||||
| Spring | + | + | |||||||||||||
| Winter | + | ||||||||||||||
| Zrmanja | Fall | + | + | + | + | + | + | + | + | + | |||||
| (Rheocrene) | Summer | + | + | + | + | + | + | + | + | ||||||
| Spring | + | ||||||||||||||
| Winter | + | + | + | + | |||||||||||
| Žrnovnica | Fall | + | + | ||||||||||||
| (Rheocrene) | Summer | + | + | ||||||||||||
| Spring | + | + | |||||||||||||
| Winter | + | + | |||||||||||||
| Spring | Season | Partnunia Steinmani | Protzia Eximia | Protzia Squamosa | Protzia Rugosa | Pseudotorrent-Icola Rhynchota | Sperchon Hibernicus | Sperchon Deticulatus Group | Sperchon Vaginousus | Sperchon Sp. | Sperchon Thienemanni | Torrenticola Eliptica | Torrenticola Sp. | Woolastookia Rotundifrons | Hydrachnidia (Larvae) |
| Čabranka | Fall | ||||||||||||||
| (Rheocrene) | Summer | + | |||||||||||||
| Spring | |||||||||||||||
| Winter | + | ||||||||||||||
| Cetina | Fall | + | + | + | + | ||||||||||
| (Limnocrene) | Summer | + | + | + | |||||||||||
| Spring | + | + | + | + | + | + | |||||||||
| Winter | + | + | + | ||||||||||||
| Kamačnik | Fall | + | |||||||||||||
| (Limnocrene) | Summer | ||||||||||||||
| Spring | + | ||||||||||||||
| Winter | + | + | |||||||||||||
| Krčić | Fall | ||||||||||||||
| (Rheocrene) | Summer | + | |||||||||||||
| Spring | |||||||||||||||
| Winter | |||||||||||||||
| Kupa | Fall | + | + | ||||||||||||
| (Limnocrene) | Summer | + | + | + | + | + | |||||||||
| Spring | + | + | + | + | + | ||||||||||
| Winter | + | + | + | + | + | + | |||||||||
| Una | Fall | + | + | + | |||||||||||
| (Limnocrene) | Summer | + | + | + | |||||||||||
| Spring | + | + | |||||||||||||
| Winter | + | ||||||||||||||
| Zrmanja | Fall | + | + | + | + | ||||||||||
| (Rheocrene) | Summer | + | + | + | + | ||||||||||
| Spring | + | + | |||||||||||||
| Winter | + | + | + | + | |||||||||||
| Žrnovnica | Fall | ||||||||||||||
| (Rheocrene) | Summer | + | + | + | |||||||||||
| Spring | |||||||||||||||
| Winter |
| Spring (Morphotype) | Season | Taxa Richness | Abundance (Individuals/m2) | Shannon Diversity Index Value | Ratio of Stygophilous Taxa | Ratio of Crenobiont and Crenophilous Taxa |
|---|---|---|---|---|---|---|
| Čabranka | Fall | 9 | 1280 | 1.446 | 1.82% | 7.27% |
| (Rheocrene) | Summer | 7 | 309 | 1.459 | 0.00% | 37.50% |
| Spring | 9 | 451 | 1.447 | 5.92% | 56.21% | |
| Winter | 5 | 53 | 1.359 | 0.00% | 79.17% | |
| Cetina | Fall | 2 | 57 | 0.693 | 0.00% | 0.00% |
| (Limnocrene) | Summer | 2 | 8 | 0.693 | 0.00% | 100.00% |
| Spring | 0 | 0 | 0 | *** | *** | |
| Winter | 3 | 90 | 0.822 | 0.00% | 92.86% | |
| Kamačnik | Fall | 3 | 7 | 1 | 0.00% | 100.00% |
| (Limnocrene) | Summer | 0 | 0 | 0 | *** | *** |
| Spring | 3 | 144 | 0.958 | 0.00% | 0.00% | |
| Winter | 5 | 43 | 1.355 | 0.00% | 45.83% | |
| Krčić | Fall | 0 | 0 | 0 | *** | *** |
| (Rheocrene) | Summer | 3 | 32 | 0.856 | 0.00% | 0.00% |
| Spring | 0 | 0 | 0 | *** | *** | |
| Winter | 0 | 0 | 0 | *** | *** | |
| Kupa | Fall | 4 | 14 | 1.091 | 0.00% | 14.29% |
| (Limnocrene) | Summer | 8 | 220 | 1.528 | 7.27% | 7.27% |
| Spring | 10 | 1667 | 1.583 | 44.44% | 44.44% | |
| Winter | 12 | 856 | 1.479 | 53.08% | 53.08% | |
| Una | Fall | 8 | 360 | 1.485 | 0.00% | 27.27% |
| (Limnocrene) | Summer | 5 | 162 | 1.044 | 0.00% | 76.54% |
| Spring | 5 | 22 | 1.221 | 0.00% | 35.71% | |
| Winter | 2 | 32 | 0.693 | 0.00% | 0.00% | |
| Zrmanja | Fall | 13 | 900 | 1.711 | 13.39% | 0.00% |
| (Rheocrene) | Summer | 11 | 630 | 1.552 | 1.52% | 0.00% |
| Spring | 4 | 9 | 1.242 | 0.00% | 0.00% | |
| Winter | 8 | 288 | 1.444 | 0.00% | 0.00% | |
| Žrnovnica | Fall | 3 | 5 | 1.04 | 0.00% | 50.00% |
| (Rheocrene) | Summer | 5 | 25 | 1.274 | 36.36% | 36.36% |
| Spring | 3 | 12 | 1.011 | 33.33% | 33.33% | |
| Winter | 3 | 35 | 0.986 | 27.27% | 100.00% |
References
- Gerecke, R.; Meisch, C.; Stoch, F.; Acri, F.; Franz, H. Eucrenon-hypocrenon ecotone and spring typology in the Alps of Berchtesgaden (Upper Bavaria, Germany). A study of microcrustacea (Crustacea: Copepoda, Ostracoda) and water mites (Acari: Halacaridae, Hydrachnellae). In Studies in Crenobiology; The Biology of Springs and Springbrooks; Botosaneanu, L., Ed.; Backhuys Publisher: Leiden, The Netherlands, 1998; pp. 167–182. [Google Scholar]
- Knight, R.L.; Notestein, S.K. Effects of nutrients on spring ecosystems. In Summary and Synthesis of the Available Literature on the Effects of Nutrients on Spring Organisms and Systems; Florida Department of Environmental Protection: Tallahassee, FL, USA, 2008; pp. 271–304. [Google Scholar]
- Stoch, F.; Gerecke, R.; Pieri, V.; Rossetti, G.; Sambugar, B. Exploring species distribution of spring meiofauna (Annelida, Acari, Crustacea) in the south-eastern Alps. J. Limnol. 2011, 70 (Suppl. 1), 65–76. [Google Scholar] [CrossRef]
- Reiss, M.; Chifflard, P. Hydromorphology and Biodiversity in headwaters: An Eco-faunistic substrate preference assessment in forest springs of the German subdued mountains. In Biodiversity in Ecosystems—Linking Structure and Function; Lo, Y.-H., Blanco, J.A., Roy, S., Eds.; InTech Open: Rijeka, Croatia, 2015; pp. 223–258. [Google Scholar]
- Gottstein, M.S.; Bakran-Petricioli, T.; Bedek, J.; Bukovec, D.; Buzjak, S.; Franičević, M.; Tvrtković, N. An overview of the cave and interstitial biota in Croatia. Nat. Croat. 2002, 11 (Suppl. 1), 1–112. [Google Scholar]
- Bonacci, O. Karst springs hydrographs as indicators of karst aquifers. Hydrol. Sci. J. 1993, 38, 51–62. [Google Scholar] [CrossRef]
- Smart, C.; Worthington, S.R.H. Springs. In Encyclopedia of Caves and Karst Science; Gunn, J., Ed.; Taylor & Francis: London, UK, 2004; pp. 1495–1505. [Google Scholar]
- Prelovšek, M. Hydrology. In Introduction to the Dinaric Karst; Mihevc, A., Prelovšek, M., Zupan, H.N., Eds.; Karst Research Institute: Postojna, Slovenija, 2010; pp. 14–19. [Google Scholar]
- Glazier, D.S. Springs. In Likens GE (ur.) Encyclopedia of Inland Waters; Elsevier: Oxford, UK, 2009; Volume 1, pp. 734–755. [Google Scholar]
- Goldschmidt, T. Water mites (Acari, Hydrachnidia): Powerful but widely neglected bioindicators—A review. Neotrop. Biodivers. 2016, 2, 12–25. [Google Scholar] [CrossRef]
- Gerecke, R.; Martin, P.; Gledhill, T. Water mites (Acari: Parasitengona: Hydrachnidia) as inhabitants of groundwater-influenced habitats—Considerations following an update of Limnofauna Europaea. Limnologica 2018, 69, 81–93. [Google Scholar] [CrossRef]
- Pešić, V.; Savić, A.; Jabłońska, A.; Michoński, G.; Grabowski, M.; Bańkowska, A.; Zawal, A. Environmental factors affecting water mite assemblages along eucrenon-hypocrenon gradients in Mediterranean karstic springs. Exp. Appl. Acarol. 2019, 77, 71–486. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Water mites (Acari: Parasitengona: Hydrachnida) of spring habitats in Canada. Mem. Entomol. Soc. Can. 1991, 155, 141–167. [Google Scholar] [CrossRef]
- Gerecke, R.; Di Sabatino, A. Water mites (Acari, Hydrachnellae) and spring typology in Sicily. Crunoecia 1996, 5, 35–41. [Google Scholar]
- Illies, J. Limnofauna Europaea; Gustav Fischer: Stuttgart, Germany; New York, NY, USA, 1978; pp. 1–532. [Google Scholar]
- Pešić, V.; Asadi, M.; Cimpean, M.; Dabert, M.; Esen, Y.; Gerecke, R.; Martin, P.; Savic, A.; Smit, H.; Stur, E. Six species in one: Evidence of cryptic speciation in the Hygrobates fluviatilis complex (Acariformes, Hydrachnidia, Hygrobatidae). Syst. Appl. Acarol. 2017, 22, 1327–1377. [Google Scholar] [CrossRef]
- AQEM Consortium. Manual for the application of the AQEM system. In A Comprehensive Method to Assess European Streams Using Benthic Macroinvertebrates Developed for the Purpose of the Water Framework Directive; Version 1.0; European Commission: Duisburg, Germany, 2020. [Google Scholar]
- Davids, C.; Di Sabatino, A.; Gerecke, R. Chelicerata: Araneae, Acari I. In Chelicerata: Araneae, Acari I. Süßwasserfauna von Mitteleuropa 7/2-1; Bartsch, I., Davids, C., Deichsel, R., Eds.; Spektrum Akademischer: Heidelberg, Germany, 2007; pp. 241–333. [Google Scholar]
- Di Sabatino, A.; Gerecke, R.; Gledhill, T.; Smit, H. Chelicerata: Acari II. In Süßwasserfauna von Mitteleuropa 7/2-2; Gerecke, R., Ed.; Spektrum: Heidelberg, Germany, 2010; pp. 1–216. [Google Scholar]
- Gerecke, R.; Gledhill, T.; Pešić, V.; Smit, H. Chelicerata: Acari III. In Süßwasserfauna von Mitteleuropa 7/2-3; Spektrum: Heidelberg, Germany, 2016; pp. 1–417. [Google Scholar]
- Tuzovskij, P. Key to Deutonymphs of Water Mites; Akademia Nauka Moscow, UdSSR: Moscow, Russia, 2000. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER V6: User manual/tutorial. In Plymouth: Primer-E; Plymouth Marine Laboratory: Plymouth, UK, 2006; pp. 1–192. [Google Scholar]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. Available online: http://statistica.io (accessed on 7 May 2019).
- Goldschmidt, T. Environmental parameters determining the water mite communities in Costa Rican freshwater habitats. Exp. Appl. Acarol. 2004, 34, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, T. Water mites (Acari, Hydrachnidia) in tropical springs—Diversity, specificity, monitoring possibilities. Verh. Des. Int. Ver. Limnol. 2009, 30, 669–672. [Google Scholar] [CrossRef]
- Martin, P.; Stur, E. Parasite-host associations and life cycles of spring-living water mites (Hydrachnidia, Acari) from Luxembourg. Hydrobiologia 2006, 573, 17–37. [Google Scholar] [CrossRef]
- Pozojević, I.; Pešić, V.; Gottstein, S. Two water mite species (Acari: Hydrachnidia) from karst springs new for the fauna of Croatia with notes on distribution and environmental preferences. Nat. Croat. 2019, 28, 417–424. [Google Scholar] [CrossRef]

| Water Mite Species | Preferences to Crenal Habitats | Preferences to Groundwater Habitats |
|---|---|---|
| Atractides latipalpis | Stygophilous | |
| Atractides pennatus | Crenobiont | |
| Atractides walteri | Crenobiont | |
| Lethaxona cavifrons | Stygophilous | |
| Ljania macilenta | Stygophilous | |
| Partnunia angusta | Crenophilous | Stygophilous |
| Partnunia steinmanni | Crenobiont | |
| Protzia eximia | Crenophilous | |
| Protzia squamosa | Crenobiont | |
| Pseudotorrenticola rhynchota | Stygophilous | |
| Sperchon thienemanni | Crenophilous | |
| Torrenticola elliptica | Crenophilous | Stygophilous |
| Species | Average Abundance (Individuals/m2) | Contribution to Group Similarity (%) |
|---|---|---|
| Group R-Rheocrenes | ||
| Average similarity: 20.12 | ||
| Atractides gibberipalpis | 1.73 | 20.63 |
| Lebertia sp. | 1.07 | 20.31 |
| Atractides nodipalpis | 1.22 | 10.15 |
| Torrenticola sp. | 1.1 | 9.44 |
| Atractides sp. | 1.15 | 8.87 |
| Protzia squamosa | 1.02 | 6.28 |
| Sperchon sp. | 0.9 | 5.01 |
| Protzia rugosa | 0.68 | 4.05 |
| Group L-Limnocrenes | ||
| Average similarity: 19.44 | ||
| Sperchon sp. | 1.77 | 34.46 |
| Atractides sp. | 1.21 | 27.98 |
| Atractides nodipalpis | 0.82 | 7.31 |
| Atractides gibberipalpis | 0.9 | 5.23 |
| Atractides pennatus | 0.46 | 4.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozojević, I.; Pešić, V.; Goldschmidt, T.; Gottstein, S. Crenal Habitats: Sources of Water Mite (Acari: Hydrachnidia) Diversity. Diversity 2020, 12, 316. https://doi.org/10.3390/d12090316
Pozojević I, Pešić V, Goldschmidt T, Gottstein S. Crenal Habitats: Sources of Water Mite (Acari: Hydrachnidia) Diversity. Diversity. 2020; 12(9):316. https://doi.org/10.3390/d12090316
Chicago/Turabian StylePozojević, Ivana, Vladimir Pešić, Tom Goldschmidt, and Sanja Gottstein. 2020. "Crenal Habitats: Sources of Water Mite (Acari: Hydrachnidia) Diversity" Diversity 12, no. 9: 316. https://doi.org/10.3390/d12090316
APA StylePozojević, I., Pešić, V., Goldschmidt, T., & Gottstein, S. (2020). Crenal Habitats: Sources of Water Mite (Acari: Hydrachnidia) Diversity. Diversity, 12(9), 316. https://doi.org/10.3390/d12090316







