Abstract
Epibiosis is a common phenomenon in marine systems. In marine environments, ciliates are among the most common organisms adopting an epibiotic habitus and nematodes have been frequently reported as their basibionts. In the present study, we report several new records of peritrich and suctorian ciliates-nematode association worldwide: from a deep-sea pockmark field in the NW Madagascar margin (Indian Ocean), from a shallow vent area in the Gulf of Naples (Mediterranean, Tyrrhenian Sea), in a MPA area in the Gulf of Trieste (Mediterranean, Adriatic Sea), from a mangrove system in French Guiana (South America, Atlantic Ocean), and from the Maldivian Archipelago. In addition, three new species of Suctorea from the Secca delle Fumose shallow vent area (Gulf of Naples) were described: Loricophrya susannae n. sp., Thecacineta fumosae n. sp. and Acinetopsis lynni n. sp. In the light of these new records and data from the existing literature, we discuss the suctorian–nematode epibiosis relationship as a lever to biodiversity.
1. Introduction
Epibiosis (greek epi “on top” and bios “life”) is a facultative spatial association between two organisms: the epibiont and the basibiont [1]. Epibionts are organisms that, during their sessile phase, remain attached to the surface of a living substratum, while the basibiont provides the support for the epibiont. Both concepts suggest ecological functions [2,3]. Epibiosis is a common phenomenon in marine systems and can be considered a direct consequence of surface limitation and/or a wave turbulence effect that obliges many lightweight organisms to evolve attachment systems to adhere to hard and relatively stable surfaces (e.g., of other living organisms; [4]). Epibiosis is the evolutionary result of an interaction between environmental factors and benthic life forms; it is a dynamic process and the ecological consequences for the basibiont and the colonizer (e.g., bacteria, fungi, algae and protozoans) can be of different nature (i.e., positive, negative or without effects for the host) depending on the environmental conditions and on the epibiotic assemblage composition and density [1,3,5]. Direct and indirect interactions among epibionts and with the host, and changing environmental conditions drive the dynamics of the epibiotic community [1]. Epibiosis can be temporary, i.e., linked to the seasonal presence of the basibiont and /or epibiont or it can represent a temporary colonization due to a decrease in basibiont defenses or to its fitness. Epibiosis may modify a number of interactions between the basibiont and the biotic and abiotic components of the environment [3,5,6]. This is the reason why epibiosis may act as an ecological lever by modifying and greatly amplifying or buffering biotic and abiotic stresses [5]. In some cases, epibionts are considered as commensals (e.g., [7]) because they are not harmful to the hosts; however, some of them can indirectly influence growth, survival rate and reproductive capability of basibionts, showing a negative impact on their fitness [5,8].
Epibiotic assemblages are rarely species-specific [2], and many colonizers are substratum generalists. Different basibiont species may also host different epibiotic communities (e.g., [9,10]). In most investigations, less than 20% of epibionts were reported as restricted to this mode of life, and less than 5% occur exclusively on one basibiont species [11,12]. Nevertheless, some exceptions were documented in previous studies (e.g., [7,13,14]) indicating species-specific host-epibiont relationships. In general, the epibiont must be able to cope with the basibiont lifestyle and its surface properties. The properties of the basibiont surface, i.e., its consistency, surface ornamentation, the presence of previous settlers (e.g., biofilms) and the deployment of defenses, determine which of the available potential epibionts will successfully settle and grow when a suitable substratum becomes available. Indeed, many basibionts have developed a variety of defense mechanisms to prevent epibiosis or to remove epibionts: these span from mechanical defenses (e.g., mucus secretion, burrowing behavior, movement in narrow caves for abrasion and epibiont elimination) to chemical methods (e.g., secretion of secondary metabolites such as antibacterial or antifungal compounds), if the nature of the relation is disadvantageous [3,15].
An important component of epibiont communities are ciliated protozoans. These organisms also constitute a significant component of the overall marine and freshwater ecosystems, and play an important role in the food chain [16]. Suctorian ciliates, together with peritrichs, are the most species-rich groups of Ciliophora. They live in all types of water bodies and they are epibionts on a wide diversity of hosts and substrates. Some species are ectoparasitic or endoparasitic species, but many of these ciliates are commensals of aquatic invertebrates or vertebrates [17]. Suctorian ciliates are quite selective by feeding principally on small ciliates, flagellates and amoebae that are captured by tentacles [17,18].
Many meiofaunal organisms such as Copepoda Harpacticoida, Ostracoda, Halacarida, Tanaidacea, Kinorhyncha and free-living Nematoda were found to be common basibionts for suctorian and peritrich ciliates and prevalent across estuarine to marine ecosystems [14,19,20,21]. However, many aspects of this relationship need to be clarified: the criteria for the host selection; adhesion mechanisms; the role of environmental variables in influencing the distribution and diversity of ciliates adhered to meiofauna, and the ecological significance of epibiont–basibiont interactions across different habitats [21,22].
Nematoda is the most abundant, ubiquitous and diverse meiofaunal marine phylum [23] and they cover a key ecological role in the ecosystem processes [24]. Thanks to their cuticle characteristics, often made by a thick and multi-layered collagenous covering, they are ideal basibionts for many suctorian ciliates (e.g., [22,25]). In particular, nematodes of the families Desmodoridae and Desmoscolecidae have found to be largely colonized due to the well-developed cuticular ornamentation that favors the adhesion of epibionts (e.g., [26]).
In a recent study based on published records, Chatterjee et al. [27] provided a checklist of suctorian epibionts on meiobenthic marine nematodes. Despite the amount of data presented from different geographical zones and types of environments, this phenomenon is still largely underestimated and the nematode-ciliate association might be more common than it actually appears to be. This is mainly due to three reasons: (i) in papers concerning nematode taxonomy and/or ecology the presence of epibionts was often overlooked or simply reported without a description of the ciliate(s), their number and distribution on the basibiont body surface; (ii) the methodology used for nematode extraction from the sediment (i.e., centrifugation) may induce the loss of some epibionts; (iii) specialists of ciliate or nematode taxonomy work separately and their focus of research is usually on the taxonomy and ecology of only one of the two groups. All these aspects have largely hampered a clear comprehension of this phenomenon.
In the present paper, we reported some new finds of Suctorea from the Secca delle Fumose shallow vent area (Naples, Italy) and we described three new species. Secca delle Fumose belongs to the degassing structure offshore of the Campi Flegrei caldera and its biology and ecology has been investigated only recently [28,29]. We reported also several new records of peritrich and suctorian ciliates-nematode association worldwide, providing an update of the check-list presented by Chatterjee et al. [27]. In the light of these new records and the literature data, we discussed the suctorian–nematode epibiosis relationship as a lever to biodiversity.
2. Material and Methods
2.1. Research Areas and Sampling Strategy
Sediment samples were collected from five different areas located worldwide: a deep-sea pockmark field in the northwestern Madagascar margin (Indian Ocean), a shallow vent area in the Gulf of Naples (Tyrrhenian Sea), a MPA area in the Gulf of Trieste (Adriatic Sea), a mangrove system in French Guiana (South America, Atlantic Ocean), and a coral reef system in the Maldivian Archipelago (Figure 1). Samples were collected either by a multi-corer (MUC), a manual corer or by SCUBA divers with the help of manual cylindrical corers (Table 1 for details). Hereafter, we briefly report the main characteristics of each study area.
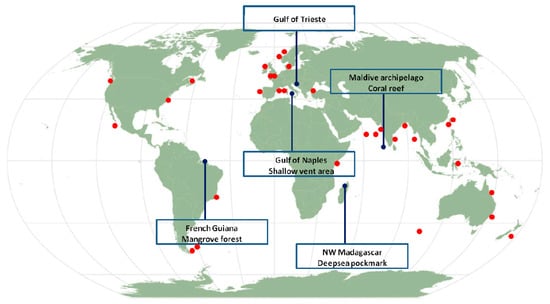
Figure 1.
Sampling locations in the present study (blue dots) and locations in which nematode–ciliate associations were reported from the available literature (red dots).

Table 1.
Geographical location and study sites, sediment features and methods used for each sampling and analysis activities.
2.1.1. Deep-Sea Pockmark: Madagascar Margin
This study was conducted on the northwestern part of the Madagascar along the Mahavavy slope to collect samples within a pockmark area (Site 1) and along the Betsiboka slope to collect samples outside the pockmark (Site 2) (PAMELA-MOZ01 cruise; [32]).
Nematodes with epibiont ciliates were found only at Site 1, which exhibited higher total sulfur concentrations (up to 4.7%), a lower dissolved oxygen penetration and the presence of CH4 (<1 µM) [33] compared to Site 2 outside the pockmark. Overall, higher sedimentation rates were observed at Site 1, with two or three main input events over the last 60 years [34], a period also characterized by a very high accumulation of total sulfur.
2.1.2. Secca delle Fumose Shallow Vent: Gulf of Naples
The study area of Secca delle Fumose (SdF) is located in the northwestern side of the Gulf of Naples. SdF is a submarine relief consisting of a network of ancient Roman pillars, among which thermal vents releasing hot gas-rich hydrothermal fluids (9–14 m water depth range) occurred. In this study, we selected four sampling sites: one diffusive emission site (H) characterized by the presence of white microbial mats covering the soft bottom; one geyser site (G) at 65 m distance from the H site, with surrounding rocky substrate covered by yellow sulphur deposits and with hot water emissions reaching 80 °C at the sediment surface; two inactive sites (CN and CS) located at distance of 100 m from the active sites H and G. From site H we reported the highest sediment temperature (37.5 °C) and lowest pH value (7.56); site G was characterized by the presence of sulphur ion S2−, a pH of 8 and a temperature of 29.1 °C. From the inactive sites, CN and CS, we detected temperature (21.8 °C) and pH (8.1) values comparable to the background. Nematode with ciliates were only found at sites G, CN and CS [29].
2.1.3. Marine Protected Area: Gulf of Trieste
The study was carried out at the station C1, which is located ca. 200 m offshore nearby the outer border of the Marine Protected Area (MPA) of Miramare in the Gulf of Trieste (North Adriatic Sea). The Gulf is characterized by annual fluctuations of temperature (from 5 °C to ≥24 °C at the surface and from 6 °C to ≥20 °C at the bottom) and in summer the water column is usually stratified. Sedimentation is mainly controlled by river inputs rather than by marine currents [30]. Only one individual of nematode with ciliates was found from the sampling station C1.
2.1.4. Mangrove Forests: French Guiana
The study area was located in the vicinity of the Cayenne estuary, French Guiana (South America). Sediment samples were taken from three stations characterized by the presence of mangrove forests and situated on river edges in the polyhaline zone at an increasing distance from the Cayenne city. Station 1 was located near to a wastewater treatment plant which drained the waters from industrial, commercial and urban areas. Station 2 was located at the intersection between two rivers Cayenne and Montsinnery and Station 3 was 10 km from the estuary mouth and from the agricultural and urban environments. 4. Overall, Station 3 in downstream of the Cayenne estuary appeared to be more preserved from anthropogenic effects than the other two stations. Basibiont nematodes were found at St. 1 and St. 2 [Michelet et al., under review].
2.1.5. Coral Reefs: Archipelago of Maldives
The archipelago of Maldives is in the Indian Ocean, in the central part of the Chagos-Maldives-Laccadive Ridge [35]. The archipelago is formed by a single atoll chain in the northern and southern areas, and by a double atoll chain in the central area. All the Maldivian sediments are of coralline origin with a range of grain size very heterogeneous and poorly correlated to the level of hydrodynamic conditions [31]. This is mainly due to the short transport underwent by the sediments that are deposited almost immediately after their erosion by the reefs [36]. The archipelago is dominated by monsoons with southwest to northwestern winds (~225°–315°) from April to November (namely westerly monsoon); and northeast–eastern winds (~45°–90°) prevailing from November to March (e.g., northeastern monsoon) [35]. The samples were collected in the South and North Malé, and Felidhoo atolls (see [37] for details). A total of 20 sites were investigated: 10 outer (ocean-facing sides situated on the atoll rim) and 10 inner (lagoon sides of the atoll rim) reefs. Suctorians were found in the nematodes of the following locations: North Malé atoll, stations M1 and M4 located in two inner reefs at depths of 40 and 19 m, respectively; South Malé atoll, stations M8 and M9 located in two outer reefs at depths of 21 and 63 m of depths, respectively.
2.2. Samples Processing
Meiofauna organisms were extracted from the sediments by centrifugation with Ludox colloidal silica HS-40 [38]. From each sample, the first 100–110 nematodes encountered in the cuvette were hand-picked, transferred from fixative to glycerol through a series of ethanol-glycerol solutions and finally mounted on slides in anhydrous glycerin [39]. All nematodes on permanent slides were identified at the genus level using the pictorial keys of Platt and Warwick [40,41] and Warwick et al. [42], as well as the original species descriptions and identification keys available through NeMys [43]. An estimation of the percentage of nematode basibionts colonized was calculated based on the total nematode density in each sample.
The specimens showing epibionts attached to the body wall were isolated for an in-depth identification of the ciliate species. Measurements of ciliates were made using the program Toup View 3.7 for digital camera.
The terminology and systematic position of suctorian ciliates follow Dovgal [17,44].
A Permanent microscopic slide of the nematode specimen with the new suctorian species is deposited at REM/EEP/LEP of Ifremer Centre Brest, Brittany, France.
3. Results
3.1. New Records of Associations between Nematodes and Ciliates from Deep-Sea and Shallow Water Systems
The list of new records found in the present study is presented in Table 2; we also reported an estimated percentage of colonized nematodes at each sampling site, aware that the values may be underestimated in reality. A total of six genera and twelve species of epibiont ciliates were found on fifteen nematode genera and four families from five different habitats (Table 2 and Figure 1). Three ciliate species were recognized as new: Loricophrya susannae n. sp., Thecacineta fumosae n. sp., and Acinetopsis lynni n. sp.

Table 2.
Summary of data reported in the present study. Study sites and stations; estimated percentage of colonized nematodes (No. IN) on total nematodes found at each sampling site; B gender = gender of the basibiont; BG = basibiont genus; ES = epibiont species; No. E/B = number of epibiont found on basibiont; juv. = juvenile.
3.1.1. Deep-Sea Pockmark: Madagascar Margin
The highest percentage (15%) of colonized nematodes were reported from the deep-sea pockmark and only from the station inside the pockmark. All basibiont nematodes belonged to the genus Desmodora, the most abundant genus found at that station [Sanchez et al., unpublished data] and most of them were females (35 on a total of 48 specimens). Trematosoma rotunda and Paracineta homari were the two epibiont suctorian species on Desmodora sp., they were found attached to the middle and tail/cloaca region (P. homari) and in the head region (T. rotunda) from 1 to 4 individuals. T. rotunda was already reported as epibiont on Desmodora scaldensis (Supplementary Material, Table S1A; [45]) inhabiting a mangrove system in India, while the suctorian species Paracineta homari (Supplementary Material, Figure S1A) was reported for the first time as ciliate epibiont on nematodes [27].
3.1.2. Secca delle Fumose Shallow Vent: Gulf of Naples
We found from 2% (inactive sites CN and CS) to 5% (active site G) of nematodes with epibionts from the shallow vent area Secca delle Fumose (Table 2). Both females and males were found to be basibionts for ciliates and a minor number of juveniles (16 on a total of 90 nematodes). Basibionts belonged to ten different nematode genera and three families (i.e., Desmodoridae, Draconematidae and Epsilonematidae). Desmodoridae was the most represented family accounting for eight genera (Chromaspirina, Desmodora, Paradesmodora, Perspiria, Polysigma, Pseudochromadora, Pseudodesmodora and Sygmophoranema), followed by Draconematidae (Prochaetosoma) and Epsilonematidae (Perepsilonema) with only one genus. Seven species of suctorian ciliates were identified, and three of them are new species, Loricophrya susannae n. sp., Thecacineta fumosae n. sp., and Acinetopsis lynni sp. n. (Table 2, see below for taxonomic descriptions). From Secca delle Fumose we also found new associations between nematodes and suctorians, indeed we reported for the first time Thecacineta calix epibiont on four nematode genera Polysigma, Pseudodesmodora, Sygmophoranema and Prochaetosoma (Supplementary Material, Figure S1B–E) and Thecacineta fumosae n. sp. on Perepsilonema. The number of epibionts on the basibiont varied from a minimum of 1 to a maximum of 12 and they were usually attached along the entire body length of the nematodes or in the middle–posterior part of the body. Interestingly, we found nematodes with ciliates from all investigated sediment layers from the top 1 cm until 10 cm depth and in particular at site G the highest number of basibiont nematodes from the deepest layer were reported.
3.1.3. Marine Protected Area: Gulf of Trieste
In the Gulf of Trieste, only one nematode (0.2%) showed epibionts along its body (Supplementary Material, Figure S1F). The specimen belonged to the genus Pseudochromadora (Desmodoridae) and a total of 19 putative phoronts of Apostomatia (Oligohymenophorea, Ciliophora) were attached to the nematode along the entire length of its body. This was the first finding of phoronts of Apostomatia as epibionts on nematode and neither in the recent review by Chatterjee et al. [27] mentioned the representative of Apostomatia was as a nematode epibiont.
3.1.4. Mangrove Forests: French Guiana
In the mangrove forests of French Guiana, nematodes as basibionts of ciliates ranged from 0.5% (polluted area) to 2.7% (medium polluted area) of total abundance. Two suctorian (Paracineta homari and Loricophrya bosporica) and one peritrich (Cothurnia sp.) ciliates were found on specimens of three nematode genera: Desmodora, Spirinia (Desmodoridae) and Desmoscolex (Desmoscolecidae) (Table 2; Supplementary Material, Figure S2A) in a number of 1 to 6 along the entire body length or on the tail region. The associations Desmoscolex–L. bosporica and Spirinia–Cothurnia were not new (Supplementary Material Table S1; [27]). Differently, the associations Desmodora–L. bosporica (Supplementary Material, Figure S2B) and Desmodora–P. homari (see above) were reported for the first time in the present study.
3.1.5. Coral Reefs: Archipelago of Maldives
Five nematode specimens (1%) belonging to four different genera (Paradesmodora, Croconema, Desmodorella and Echinodesmodora) and one family (Desmodoridae) were found as basibionts for suctorian ciliates (five different species) inhabiting the coral reefs of Maldives (Table 2). Also in this study case, the majority of basibiont–epibiont associations were new and never reported before in the literature (Supplementary Material Table S1; [20,27]): Loricophrya sivertseni [46] and L. stresemanni [46] were found as epibionts on the same specimen of Paradesmodora (Supplementary Material, Figure S2C); Paradesmodora/Echinodesmodora–Thecacineta cothurnioides (Supplementary Material, Figure S2D) and Desmodorella–Paracineta sp. The number of epibionts hosted varied from 1 to 5 and they were mainly attached to the posterior part of the body of adults.
3.2. Taxonomic Account of Ciliates: Systematic Position
Class SUCTOREA Claparède et Lachmann, 1859
Subclass EXOGENIA Collin, 1912
Order METACINETIDA Jankowski, 1978
Family PARACINETIDAE Jankowski, 1975
Genus Loricophrya Matthes, 1956
Loricophrya susannae n. sp. (Figure 2A,B)
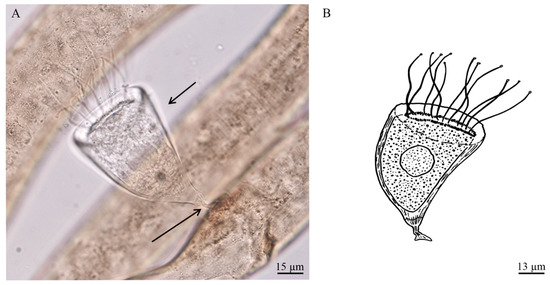
Figure 2.
(A) Bright field microscopy image of Loricophrya susannae n. sp. on Chromaspirina from the shallow vent area of Secca delle Fumose (Gulf of Naples, Italy); (B) drawing of Loricophrya susannae n. sp. (present study). The black arrows indicating the thin, striated pseudostyle (below) and conical, bent stylotheca (above).
Etymology: The specific name is in memoriam of Prof Susanna De Zio for her basic contribution to the taxonomy of marine meiobenthic Tardigrada.
Diagnosis: Suctorian ciliate covered with conical, smooth, transparent, weakly bent, stylotheca. Pseudostyle short, thin, curved, longitudinally striated (Figure 2A). The cell body entirely covered by stylotheca, attached to the lorica in the mouth area. There are about 15 thin, flexile tentacles. Macronucleus ovoid, centrally located.
Morphological description: Suctorian ciliate covered with conical, smooth, transparent, weakly bent, lorica (stylotheca 70 × 44 μm). The stalk-like protuberance of lorica (pseudostyle 10 μm in length) is shorter than the lorica itself, thin, curved, provided with longitudinally striae, expanded in plate in zone of contact with substrate (Figure 2A,B). The cell body unflattened, entirely covered by stylotheca, attached to the lorica in the area of aperture. The cytoplasm colorless, contains some dark inclusions (Figure 2A). There are about 15 thin, flexile tentacles (22–57 μm in length) with characteristic terminal knobs. Tentacles are evenly distributed at the apical body surface. Macronucleus relatively large, spherical (18 × 15 μm), centrally located. Reproduction not observed.
Measurements, based on two individuals (in μm): Stylotheca length 70, maximal stylotheca width 44, pseudostyle length 10, pseudostyle diameter 1, epicone length 6, width 6, body length 52, body width 36, macronucleus length 18, width 15, tentacle length 22–57.
Differential diagnosis: Genus Loricophrya Matthes, 1956 include 11 species (12, [47]), however the new species is relative to L. parva (Schulz, 1932), from which differs by presence of thin, striated pseudostyle and conical (nor urn-like), bent, stylotheca. In addition, in all known representatives of the genus the cell body is not entirely covered by stylotheca.
Type material: Permanent microscopic slides of nematodes with the new suctorian species were deposited at REM/EEP/LEP of Ifremer Centre Brest, Brittany, France.
Type locality: Secca delle Fumose, Gulf of Naples, Italy.
Type host: Chromaspirina sp. and Perspiria sp.
Order VERMIGEMMIDA Jankowski, 1973
Family THECACINETIDAE Matthes, 1956
Genus Thecacineta Collin, 1909
Thecacineta fumosae n. sp. (Figure 3A,B)
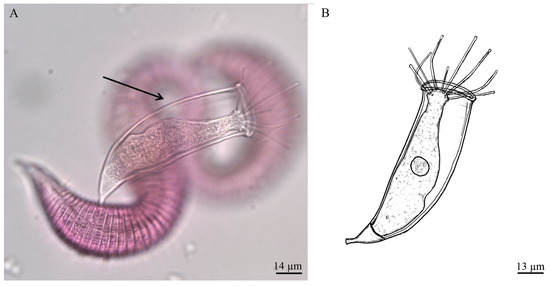
Figure 3.
(A) Bright field microscopy image of Thecacineta fumosae n. sp. on Perepsilonema from the shallow vent area of Secca delle Fumose (Gulf of Naples, Italy); (B) drawing of Thecacineta fumosae n. sp. (present study). The black arrow indicating the transparent lorica.
Etymology: The species name refers to the locality name, Secca delle Fumose.
Diagnosis: Marine loricate suctorian. Cell body attached to the bottom of lorica. The apical part of body narrowed, not protruded from lorica aperture. Up to 12 capitate tentacles placed at apical body surface. Macronucleus spherical, located in the middle of body. Lorica slightly curved, smooth, transparent, without any ribs. The mouth of lorica is some wider than the rest. Stalk short, curved, with good developed, conical epicone, which have the same width as bottom of lorica (Figure 3A). Reproduction not observed.
Morphological description: Marine. Cell body granulated, colorless, attached to the bottom of lorica. The basal part of two thirds of the cell body flared, then body to become narrow, but with weakly enlarged apical part, bearing tentacles. The cell body not protruded from lorica aperture. Up to 12 capitate tentacles (23–33 μm in length) evenly distributed at apical body surface. Macronucleus ovoid, located in the middle of body (Figure 3B). Lorica (82 μm in length and 27 μm in width) slightly curved, smooth, transparent, without any ribs or striae (Figure 3A). The aperture of lorica (29 μm in diameter) is some wider than the rest, with somewhat arched annular edge. The stalk (14 μm in length) is clearly delimited from lorica, short, weakly curved, without any folds or striae, with good developed, conical epicone, which have the same width as bottom of lorica in the area of contact with them (Figure 3B).
Measurements, based on one individual (in μm): Lorica length 82, lorica width 27, lorica aperture diameter 29, body length 78, body width 20, stalk length with epicone 14, diameter 11, maximal epicone diameter 33, length of tentacles 23–33.
Differential diagnosis: The new suctorian species differs from the relative species as Thecacineta cothurnioides (Collin, 1909) recorded on harpacticoid copepod Cletodes longicaudatus from Banyuls-sur-Mer at Mediterranean coast of France [48] and nematode Tricoma sp. from Ratnagiri, west coast of India, Indian Ocean [49] and Thecacineta urceolata [26] found on nematode Desmodora pontica from Ludao, Taiwan by its curved lorica, stalk with wide, conical epicone. From other species of the genus the new species differs by its transparent lorica.
Type material: Permanent slide of the nematode with the new suctorian species was deposited stored at REM/EEP/LEP of Ifremer Centre Brest, Brittany, France.
Type locality: Secca delle Fumose, Gulf of Naples, Italy.
Type host: Perepsilonema sp.
Subclass ENDOGENIA Collin, 1912
Order ACINETIDA Raabe, 1964
Family ACINETOPSIDAE Jankowski, 1978
Genus Acinetopsis Robin, 1879
Acinetid ciliates with tentacles of two types: hypertrophied, agile, prehensile ones, and regular feeding (sucking) ones [50]. The body is trapezium-like, laterally flattened, loricate and stalked. The macronucleus is spherical or ovoid.
In accordance with Dovgal [17], there are three species of the genus: Acinetopsis rara [51] (type species), A. tentaculata [52] and A. elegans [53].
However, the species found of Swarczewsky [53] on gills of amphipod crustacean Carinurus solskii from Baikal Lake were not provided with lorica and thus must be excluded from the genus Acinetopsis. It is Jankowski’s opinion [54] that the species is representative of genus Tokophrya Bütschli, 1899. A. rara (Figure 4(1)) was found [51] on hydroids from genus Sertularia collected near Concarneau (France). The ciliate is from 70 to 90 μm (in accordance with [51]), covered with stalked lorica, the height of which is one third less than its width. The stalk is very thin, 100 μm long. The body is uniformly granular, greyish, with a small contractile vacuole, a flat apical surface, from the centre of which one contractible tentacle extends. The body does not reach the bottom of the lorica. As observed by Grell and Meister [55] the A. rara feeds on representatives of genus Ephelota that is often far greater than the predator. The sucking tentacles of A. rara are much smaller and bear knobs devoid of haptocysts. The prehensile tentacles are ordinary in structure but, on the contrary, gigantic, very lively and enriched with haptocysts [55]. The original figure of Batisse [56] illustrates that, in addition to the central trapping tentacle, there are two groups of 12–13 thin, short sucking tentacles.
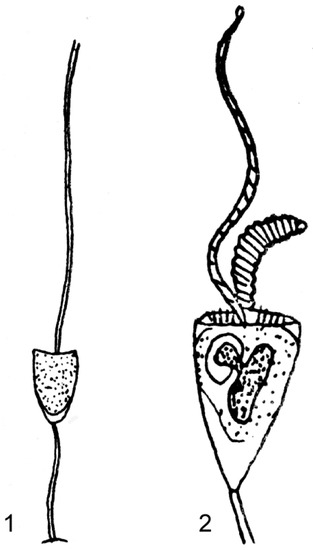
Figure 4.
Acinetopsis rara (1) (modified from Robin, 1879) and Acinetopsis tentaculata (2) (modified from Root, 1922).
Acinetopsis tentaculata [52] (Figure 4(2)) has a body enclosed in a flattened, cup-shaped lorica, borne on a slender stalk, of which some are longer than the lorica. The body is irregularly flattened-ovoid in shape, bearing one or two agile prehensile tentacles and two groups of small sucking tentacles on its apical surface. The macronucleus is ovoid; there are also one or more micronuclei. A single contractile vacuole is located near base of the body. Reproduction occurs by endogenous budding.
Measurement (in μm, in accordance with Root, [52]): Lorica length 187, width 105, body length 138, width 100, thick 73, length of stalk 287, extended prehensile tentacle length 500.
Locality: Woods Hole, USA.
Host: Ephelota coronata Wright, 1858 found on hydroids Obelia commissuralis and O. geniculata.
Jankowski [54] believed that the A. tentaculata is a younger synonym of A. rara.
Acinetopsis lynni n. sp. (Figure 5A–D).
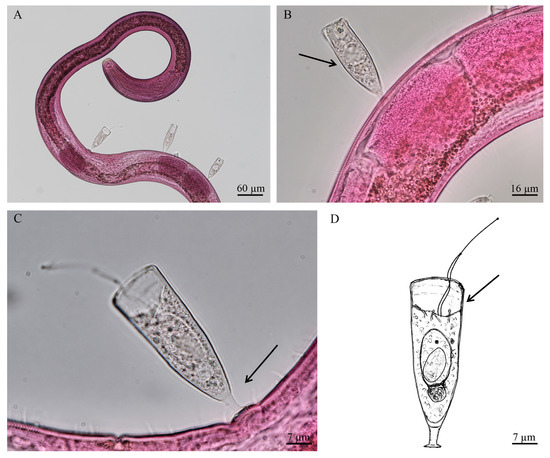
Figure 5.
(A–C) Bright field microscopy images of Acinetopsis lynni n. sp. on Desmodora from the shallow vent area of Secca delle Fumose (Gulf of Naples, Italy); (D) drawing of Acinetopsis lynni n. sp (present study). The black arrows indicating the narrow, elongated lorica (B), the short stalk (C) and presence only a three to four contractile sucking tentacles (D).
Etymology: The specific name is in honor of outstanding protistologist Denis Lynn (1947–2018).
Diagnosis: Suctorian ciliate enclosed in a flattened, narrow, elongate, smooth lorica with short extended upward stalk. Body attached to the bottom of lorica and fills about two thirds or three fourths of it. There are two agile hunting tentacles and from three to four short, contractile sucking tentacles. The macronucleus is ovoid, positioned near foot of the body. Reproduction occurs by endogenous budding with formation of single protomite (Figure 5A,B).
Morphological description: Marine suctorian ciliate enclosed in stalked lorica. The cell body is attached to the bottom of lorica, which fills about two thirds or three fours of it. Cytoplasm colorless and faintly granulated, with numerous inclusions (Figure 5B,C). The apical body surface, which bearstentacles, is noticeably concave downward. Lorica elongate (49–62 μm × 17–19 μm), somewhat expanded, slightly flattened, smooth, but with two or three annular striae on the inside of walls in the top. Stalk short (5–7 μm × 2–4 μm), straight, some extended upward, very weakly longitudinally striated, with adhesive disc. There are two extremely long (about the same length as body), agile hunting tentacles (27–46 μm) and from three to four short, contractile sucking tentacles (5–10 μm × 1–2 μm). Macronucleus ovoid, positioned near foot of the body. Reproduction by endogenous budding with the formation of single protomite. Protomite relatively large (24 μm × 11 μm), its length makes up more than half of the body length (Figure 5B–D).
Measurement, based on four individuals (in μm): Lorica length 49–62, lorica width 17–19, lorica aperture diameter 12–20, body length 36–41, body width 14–18, macronucleus diameter 7–9, stalk length 5–7, stalk diameter 2–4, prehensile tentacle length 27–46, sucking tentacle length 5–10, sucking tentacle diameter 1–2, protomite length 24, width 11.
Differential diagnosis: The new species differs from other representatives of the genus by narrow, elongated lorica, short stalks, and the presence of only three to four contractile sucking tentacles. In addition, the cell body of the new species does not fill all lorica.
Type material: Permanent slide of the nematode with the new suctorian species was deposited and stored at REM/EEP/LEP of Ifremer Centre Brest, Brittany, France
Type locality: Secca delle Fumose, Gulf of Naples, Italy.
Type host: Desmodora sp.
3.3. Nematode-Ciliate Association: An Analysis
A total of 22 species of epibionts—among these 20 suctorian ciliates, 1 peritrich ciliate and 1 apostome ciliate—have been reported until now to infest different species of nematodes inhabiting deep-sea and shallow systems worldwide (Figure 1; Supplementary Material, Tables S1 and S2). Figure 6 documents the number of nematode basibionts (n. Genus and n. Species) that each epibiont is able to infest along with the number of habitats (e.g., mangrove forests, coral reef, meadows, open slope systems, sandy beach, etc.) where those associations were recorded. The graph was built up considering all the available literature and the new records from the present study. Thecacineta calix was the suctorian ciliate with the wider distribution both in term of basibiont genera/species and kind of habitats, followed by Trematosoma rotunda, Thecacineta cothurnioides, Loricophrya bosporica and Paracineta homari. All the other ciliates were characteristic of 1 to 2 nematode species or genera and recovered from 1 to 2 habitats at most maximum.
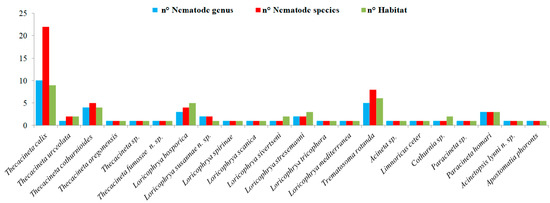
Figure 6.
Number of nematode basibionts (n. Genera and n. Species) for each epibiont ciliate and number of habitats (n. Habitat) from where these associations have been reported (data from the literature and present study).
A total of 33 identified species, 23 genera and 5 families of nematodes, have been reported to be ideal basibionts for ciliates (Supplementary Material, Tables S1 and S3). The family with the highest number of nematode basibionts was that of Desmodoridae, accounting for 27 identified species, followed by Epsilonematidae (3), Desmoscolecidae (2), Comesomatidae (1) and Draconematidae (1). Figure 7 analyzes the nematode–ciliates association, considering the number of epibiont species per basibiont and the number of habitats where the association was recorded. There were some nematode genera/species that clearly constituted a more attractive alive substrate for the epibionts (i.e., they can be colonized by different ciliate species), such as Desmodora sp., Paradesmodora sp., Spirinia parasitifera, Chromaspirina sp., Pseudochromadora sp., and Tricoma sp. All the other nematodes were found to be colonized by 1 to 2 ciliate species. The nematode genera that could be basibiont for the higher number of epibiont species, were reported also from a wide range of habitat types. Moreover, if we grouped all the habitats in which these associations have been reported in three main categories: deep-sea/extreme environments, polluted/impacted and pristine environments (see also Table 2 and Table S1 for details), most of the records were found in pristine areas (e.g., sandy beaches, coastal waters, seagrass meadows, coral reefs) and deep-sea/extreme environments (e.g., hypoxic/anoxic sediments, seamounts, open slopes, pockmark areas, hydrothermal vents, deep sediments from Antarctica). Only a minority of cases were reported from polluted systems (e.g., mangrove forests, sandy beaches near domestic sewage). The graphs presented in Figure 6 and Figure 7 were realized according to the available literature and our new records.
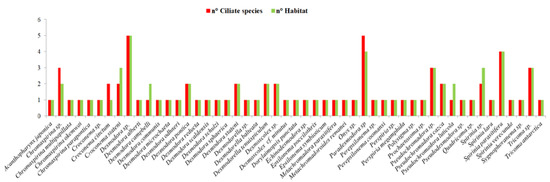
Figure 7.
Number of epibiont species for each basibiont and the habitat distribution (n. Habitat) from where these associations were reported (data from the literature and present study).
4. Discussion
4.1. Nematodes as ‘Promoters’ of Biodiversity
In the present study, we reported three new suctorian species—i.e., Acinetopsis lynni n. sp., Loricophrya susannae sp. n. and Thecacineta fumosae sp. n.—as epibionts of four different basibiont nematode genera (i.e., Desmodora, Perspiria, Chromaspirina and Perepsilonema). Moreover, we also reported many new interactions between nematodes and ciliates never documented before [27]. In detail, we found the suctorian ciliates Paracineta homari and Paracineta sp. as epibionts of nematodes from different environments. Until now, P. homari and species of the genus Paracineta were reported as epibionts of crustaceans such as pagurid crabs [50] and copepods [57]. The same for the representative of subclass Apostomatia, reported here for the first time as epibiont of Pseudochromadora from the Gulf of Trieste, but until now found mainly on crustaceans [58].
We also documented the presence of well-known ciliate epibionts of some nematode genera and/or species colonizing new nematode basibionts. This was the case of the widespread suctorian Thecacineta calix [20], found here as epibiont on four new nematode basibionts, i.e., Polysigma, Pseudodesmodora, Sygmophoranema and Prochaetosoma inhabiting the shallow vent area in the Gulf of Naples. This finding confirmed the ubiquitous nature of this ciliate adapted to different environments and its ability to colonize many hosts. Loricophrya bosporica, previously reported on the nematodes Desmoscolex cf. minutus and Metachromadoroides remanei [59,60] inhabiting the polluted anoxic Black Sea sediments and methane seeps, was reported here on Desmodora from the polluted mangrove forests in French Guiana. Loricophrya bosporica defined as extremophile [27], showed a wider range of distribution and number of suitable nematode basibionts. The same consideration could be made for two other species of Loricophrya: L. sivertseni and L. stresemanni. We found both species sharing the same micro-niche on the tail of one specimen of Paradesmodora from the Maldivian coral reef. These two ciliates were reported from Norwegian fjords on Spirinia parasitifera (L. stresemanni) and on other nematodes that were not identified (L. sivertseni) [25,27].
Thecacineta cothurnioides was previously reported on Chromaspirina sp., C. parapontica and Tricoma sp. nematodes [21,22,53] from shallow continental shelf and polluted mangrove systems and on copepods from the Mediterranean coast of France [20]. In the present study, T. cothurnioides was found as epibiont on Paradesmodora and Echinodesmodora inhabiting the Maldivian coral reef broadening the geographical range of distribution of this ciliate and their suitable hosts.
Previous findings from the existing literature and new records from the present study, showed evidences that nematodes are ‘promoters’ of biodiversity for ciliates, a biodiversity of which we have only a small perception since it remains largely underestimated. Sartini et al. [61] suggested that freshwater snails could be regarded as a source of biodiversity in limnic environments, since with their shells they offer a range of microhabitats that peritrich ciliates can occupy. Moreover, freshwater gastropods show tolerance to a wide range of environmental conditions and they are commonly found inhabiting many freshwater systems. Similarly, nematodes constitute a suitable substratum colonized by new ciliates species and in addition to that, they support new nematode–ciliate associations in different habitats worldwide. Nematodes are good basibiont candidates, being among the most abundant, diversified, and ubiquitous meiofauna groups, able to colonize many environments, from shallow to deep-sea waters and survived in adverse and extreme conditions [23].
It is known that the basibiont highly enhances epibiont dispersal, being a motile living substratum for sessile marine organisms, and that an epibiont actively selects the colonized species and the attachment site on the basibiont [62]. Indeed, epibiotic associations represent an excellent framework with which to examine diversity patterns among geographical regions on a variety of scales [1].
4.2. Advantages and Disadvantages of Nematode–Ciliate Association
The debate is still open on whether for nematodes to be promoters of biodiversity is more of a cost than a benefit. Previous studies conducted mainly on crustaceans as basibionts for various epibionts (e.g., [1,3,15,63]), analyzed in detail costs and benefits for both the actors involved in this association (Table 3).

Table 3.
Costs vs. benefits in the epibiont–basibiont relationship of epibiosis (summarized from Key et al., 1999; Harder, 2008; Wahl, 2009; Fernandez-Leborans, 2010).
Epibiosis has a number of effects on both the epibiont and the basibiont, and these include advantages for the epibiont such as: (i) new available surface to colonize; (ii) dispersal and geographical expansion; (iii) increase in the supply of nutrients; (iv) protection against predation [3]. In general, the epibiont must be able to cope with all aspects of the basibiont lifestyle and surface properties, and, frequently, older individuals or older parts of an individual tend to be more heavily covered by epibionts [1].
On the other hand, epibiosis can be disadvantageous for the epibiont since the basibiont is a biologically unstable, variable, and non-durable living organism [1]. Epibionts can be: (i) removed from the basibiont by abrasion or molt, (ii) exposed to some detrimental host defense, (iii) exposed to inadequate environmental conditions, and (iv) captured by some basibiont predator [1,3,15].
The nature of epibiont impact on the basibiont is variable and strong context-specific [15]. In some way, epibiosis can be beneficial for the basibiont by providing: (i) both mimetic protection and cleansing, (ii) defense mechanisms against predators, and (iii) nutrient flow [3]. On the other hand, epibiosis disadvantages for the basibiont are numerous (Table 3). Epibionts may: (i) restrict the mobility of the basibiont, (ii) affect growth and molting, (iii) affect the functions of several organs (e.g., eyes, gills), (iv) cause an increase in predation risk, (v) compete for nutrients with basibiont, and (vi) mask the chemical identity of the host [1,3,15]. Wahl [5] sustained that epibiosis acts as an ecological lever by amplifying or buffering the basibiont–environment interactions (i.e., amplifying or buffering biotic and abiotic stress). The way the epibiosis influences an interaction may be of two dissimilar kinds: one called exploitative (i.e., epibiont exerts a stress on the basibiont) and the other called interference mediation (i.e., the susceptibility of a basibiont may be increased or decreased according to the identity or quantity of epibionts) [5]. Harder [15], reporting some examples among basibionts such as algae, molluscs, cnidaria or echinoderms, stated that any potential basibiont must either tolerate epibiosis or employ some sort of defense against this phenomenon.
Could all these costs vs. benefits listed above be applicable to nematode–ciliate association? If we consider the costs for the basibiont, they can be all potentially valid for nematodes particularly when epibiont ciliates exceed a certain number (e.g., Supplementary Material Figure S1F) and/or the size of a single epibiont is almost equal to the size of the nematode (e.g., Supplementary Material Figure S2A). Under those conditions, every vital activity of the basibiont may became difficult, for instance: motility, mating, gas exchange and perception of the external environment, reduction in defense and competition capacities. This is the case of the exploitative nature of the epibiosis [5].
Harder [15] suggested a variety of beneficial effects for the basibiont induced by the presence of epibionts (Table 3); however, we think they are hardly applicable in the case of nematodes. The positive effects of camouflage, or of epibiont mechanisms against predators and associational resistance may be effective in the presence of a certain number of epibionts covering the basibiont surface; however, in the case of nematodes (organisms usually smaller than 0.5 mm) higher is the number of epibionts greater is the negative effect for the host (= interference mediation; [15]). Our studied case of colonized nematodes from polluted sediments of mangrove system (French Guiana) could be an example of ecological lever. Under an environmental stress condition, the vulnerability of the basibionts favored the epibionts’ colonization amplifying the abiotic stress. Ansari and Bhadury [21] reported a high number of infested nematodes inhabiting polluted mangrove systems (India). The authors explained that in such stress conditions nematodes were easily colonized due to a defense lowering, while for the epibionts, this association was even more advantageous for survivors. The overall feeling is that, for nematodes, the cost of epibiosis exceeds the benefits.
4.3. The Cost for Biodiversity
Previous authors (e.g., [22,26,64]) suggested that the success of nematodes as basibionts was related to some of their morphological and physiological features coupled with their lifestyle, to which the number and type of epibiont ciliates are related.
Among their morphological features, a thick, heavily ornamented or annulated cuticle was reported as the primary characteristic to be a suitable basibiont nematode for ciliates [27,65]. This characteristic presents in certain nematodes body surface shows a similitude with the calcified body surface of many crustaceans, usually reported as good habitat for epibionts [8,16].
Similarly, all nematode genera we found with ciliate epibionts presented the typical thick ornamented cuticle. We reported nematodes usually found as basibionts for ciliates (e.g., Desmodora, Desmoscolex, Perepsilonema) and new colonized genera (e.g., Polysigma, Pseudodesmodora, Sygmophoranema and Prochaetosoma) increasing the number of ‘available’ nematode basibionts and the perception that this number can be even higher.
The importance of the cuticle for the epibiont settlement was confirmed once we considered the Secca delle Fumose (Gulf of Naples) case study. We reported colonized nematodes from all sites, with the only exception being the active site H, dominated by Oncholaimus and followed by Daptonema. Both these nematodes presented a thin, smooth or weakly striated cuticle. It is also true that site H presented the highest temperature and lowest pH values, conditions that might be conducive to the survival of ciliates. Nevertheless, is well known the existence of suctorian ciliates that can survive under extreme conditions [27,59]. Indeed, we think that it was the absence of ‘good’ basibionts, instead of the environmental conditions, that consequently determined the absence of the epibionts [8].
If the properties of the body surface play a crucial role in most interactions between nematodes and ciliates, for nematodes it represents one of the highest prices to pay—particularly when the epibionts are of a conspicuous number. In nematodes, the cuticle is involved in many vital functions: exchange of gasses and nutrients, info-chemicals and defense metabolites, mediation of many processes of recognition of an organism by a partner, a parasite, an epibiont or predator, transmission of many types of biotic and abiotic stress [66]. The properties and functions of this interface may be modified substantially by the presence and activities of epibiotic communities, with consequences for its interaction with the environment and for the relative fitness of the host organism [5]. In our samples, we reported a number of epibionts per nematode ranging from 1 to 19. If we consider that all our nematodes showed a length less than 500 µm and ciliates’ length was ≥30–40 µm, it is reasonable to think that in a number of 2–3 epibionts start being a cause of stress for the basibiont. In a number exceeding 5–6 epibionts, we think that the condition of stress was even increased due to a significant increase in the energetic demand of individuals for locomotion [8].
At the cuticle level, there is mucus production that characterizes many of the basibiont nematodes. Mucus production and the release of other physiological secretions/excretions enhance the attachment of bacteria and food particles on the basibiont body surface and ciliates may take advantage of this available direct or indirect food source [25]. For instance, the desmoscolecid nematodes, in adults, possess an annulated body cuticle covered with a fine granular substance (which may be a secretion) and imbedded mineral particles and aggregations of bacteria [67]. Nevertheless, the use of bacteria and particles as food sources for ciliates may be strictly linked to the feeding strategy of the epibiont. Since the majority of ciliates found as epibionts on our nematodes were predator suctorian ciliates, the theory of ciliates feeding on the nematode body surface may be hardly attributed to these epibionts [18,27]. What is certain is that the attachment on a living mobile body surface—like that of nematodes—highly facilitates the ciliate feeding [3,5].
The position of the epibiont may be linked to several factors, such as burrowing behavior, locomotion, the presence of chemical inductors, bacterial exudates, pH and microtexture [5]. Moreover, the area along the body devoted to the secretions/excretions release such as cloaca, anus and vulva regions has been found to attract the epibionts and often their settlement occurred around those body regions (e.g., [58]). It has been hypothesized that these attachment preferences may be connected with the mode of reproduction of some ciliates (i.e., vermigemmic budding) with a migratory stage unable to swim [27,58]. We found that half of the studied basibiont nematodes were colonized on the posterior body region (anus-cloaca) and on the vulva region; however, in all the other cases, ciliates were attached everywhere on the body as previously reported [21,26,64]. We also noticed that the majority of basibionts were adult nematodes, as reported from other authors (e.g., [26,68]), which may be explained by an epibiont strategy to avoid molt and with no distinction between sex. The role of gender and maturity stage (adult vs. juvenile) in the selection criteria by an epibiont remains yet to be clarified [21].
As for the living mode of nematodes and their ability to penetrate into the sediment, Chatterjee et al. [27] reported that available data on epibiont ciliates on nematodes living in the deeper sediment layers are very few. Surprisingly, we found colonized nematodes until the deepest layers (5–10 cm) in a number comparable with colonized nematodes inhabiting the upper sediment layers. However, in this case, we can hypothesize a damage for the epibionts due to abrasion—a mechanical defense [3] probably also adopted by nematodes against the epibionts.
Mikac et al. [7] defined the association between peritrich ciliates and polychaetes as ectocommensalism—where the ciliates have the advantages of increased food availability but the polychaetes do not have any benefits and are not harmed. Authors stated that the major advantage which ciliates gain from being associated with a motile substratum is increased food availability, assured by the free transport to a variety of habitats, and by increased water flow. Similarly, Key et al. [63] defined the relationship between blue-crab and bryozoa as ‘phoretic’. Phoresis, or phoresy, it is used to describe a non-permanent, commensalistic interaction in which one organism attaches itself to another (the host) solely for the purpose of travel. Actually, we think that the ciliate–nematode relationship cannot be defined, neither as ectocommensal nor phoretic, since the cost for carrying this diverse population of ciliates appears high for the basibiont. We are aware that further investigations are needed to analyze the effects for the basibionts in depth.
Very little is known about nematode mechanisms of defense. As mentioned above, we can suppose that abrasion may be a strategy to remove epibionts when they move deeper into the sediment. Only recently, new researches have started and are developing, focused on the nematode immune defenses [69]. We know that nematodes can produce several classes of AMPs (antimicrobial peptides) as natural response to fungal, bacteria and yeast attack [70]. Moreover, some AMPs’ gene expressions were specifically found at the epidermis level to avoid the infection of certain spores [69]. Many nematodes produce AMPs, but neither their activity nor relative gene expression have been investigated for most of them. Understanding how different nematode species defend themselves against potential pathogens in their environment(s) requires the characterization of the defense molecules from each species [70]. The presence of AMPs involved in the defense against ciliate epibionts is quite probable, but for now with no specific, supporting data.
4.4. Host–Epibiont Species–Specificity and the Environment
Epibiotic relationships are rarely species-specific [1], nevertheless, that is not confirmed for polichaete–peritrich ciliate relationships since species-specific relationships were documented [7,13]. Results of the present study and of the literature highlighted that the majority of ciliates have been reported as epibiont on only one genus and/or species of nematode and with a limited range of distribution (Figure 6). Nevertheless, eight species of ciliates were found colonizing different nematode species, and Thecacineta. calix confirmed its ability to colonize a wide number of hosts also among nematodes (22 species of nematodes) and to inhabit different kind of environments [20]. Talking about a host species-specificity may also be premature at the moment in cases of ciliates found only on one nematode, considering the scarcity of data. However, we can confirm that most of the suctorian species we report in this study were found only on nematodes [27], with the only exceptions being T. calix and Paracineta homari. At the genus level, we reported two ciliates that were also found on other hosts (i.e., crustaceans): Acineta, and Cothurnia [8] even with different species.
Conversely, looking at a possible epibiont species-specificity (Figure 7) for the basibiont, we reported the same result: most nematodes were found to be basibiont only for one epibiont species and only a few nematodes could recruit different epibionts in different environments. It has been proved that processes of colonization are mediated by specific settlement cues (e.g., surface features) and biogenetic signals from the basibiont [15].
Environmental conditions are also important in favoring (or not favoring) epibiotic relationships [5], since they can determine the presence or absence of the host and/or of the epibiont, as well as enhance the colonization process. From this research, we reported nematode–ciliate relationships from extreme shallow-water and deep-sea environments to impacted and pristine systems, and spanning different geographical areas: the results support the idea that this association might be highly common and diversified in nature. Our study added three new species of suctorian ciliates epibionts on nematodes, pointing out nematodes as a living biotic substrate source of diversity.
In conclusion, our analysis and results suggest that the nematode–ciliate relationship deserves further investigation to elucidate all aspects of this association: diversity, costs and benefits for the basibiont and epibiont, and the ecological meaning of this phenomenon. Subsequently, we can think to apply the epibiosis in the environmental monitoring.
Supplementary Materials
The following materials are available online at https://www.mdpi.com/1424-2818/12/6/224/s1, Figure S1: Bright field microscopy image of (A) Paracineta homari on Desmodora sp. from deep-sea pockmark (Madagascar margin); (B) Thecacineta calix on Polysigma sp. from the shallow vent area of Secca delle Fumose (Gulf of Naples, Italy); (C) Thecacineta calix on Pseudodesmodora sp. from the shallow vent area of Secca delle Fumose (Gulf of Naples, Italy); (D) Thecacineta calix on Sygmophoranema sp. from the shallow vent area of Secca delle Fumose (Gulf of Naples, Italy); (E) Thecacineta calix on Prochaetosoma sp. from the shallow vent area of Secca delle Fumose (Gulf of Naples, Italy); (F) Apostomatia on Pseudochromadora sp. from the Gulf of Trieste. Figure S2: Bright field microscopy image of (A) Loricophrya bosporica on Desmoscolex sp. from mangrove forests (French Guiana); (B) Loricophrya bosporica on Desmodora sp. from mangrove forests (French Guiana); (C) Loricophrya sivertseni and L. stresemanni on Paradesmodora sp. from Maldivian coral reefs; (D) Thecacineta cothurnioides on Echinodesmodora sp. from Maldivian coral reefs. Table S1: Summary of all documented nematode–ciliate associations from the available literature. Table S2: List of epibiont ciliates of nematodes. Listed are the number of nematode genera and species colonized by each ciliate and the number of habitats where these associations were documented. Table S3: List of basibiont nematodes. Listed are the number of ciliate species found to be epibiont for each nematode and the number of habitats where these associations were documented.
Author Contributions
Conceptualization E.B., I.D., F.S.; methodology E.B., I.D., A.A., C.M., A.F., E.G., L.C., F.S.; writing E.B., I.D.; revisions E.B., I.D., D.Z., A.F., E.M., L.G., M.B., R.S., F.S. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by the project “Prokaryote-nematode Interaction in marine extreme envirONments: a uniquE source for ExploRation of innovative biomedical applications” (PIONEER) funded by the Total Foundation and IFREMER (2016–2019). The work of second (ID) and fourth (AA) authors was made within the framework of research issue of A.O. Kovalevsky Institute of Biology of the Southern Seas #AAAA-A19-119060690014-5. French Guiana sampling and working were funded by “Office de l’Eau de la Guyane” (OEG) and “Agence française pour la biodiversité” (AFB). The authors are grateful to the working group “Mangroves” and the LEEISA laboratory (Cayenne) for its help in the fieldwork made within the European Water Framework Directive.
Acknowledgments
We thank the chief scientists, the scientific party and the crew during the cruises PAMELA-MOZ01 cruise; in the framework of the Passive Margin Exploration Laboratories (PAMELA) project, funded by TOTAL and Ifremer. The authors EB, DZ and RS are grateful to Soprintendenza of the Underwater Archeological Park of Baia (Gulf of Naples) (prot. 5667, 24/10/2016) for the authorization to sampling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ecological Studies. Marine Hard Bottom Communities; Wahl, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 206, p. 61. [Google Scholar] [CrossRef]
- Wahl, M.; Hay, M.E.; Enderlein, P. Effects of epibiosis on consumer-prey interactions. In Interactions and Adaptation Strategies of Marine Organisms. Proceedings of the 31st European Marine Biology Symposium, held in St. Petersburg, Russia, 9–13 September 1996; Kluwer Academic Publishers: New York, NY, USA, 1997; Volume 355, pp. 49–59. [Google Scholar] [CrossRef]
- Fernandez-Leborans, G. Epibiosis in Crustacea: An overview. Crustaceana 2010, 83, 549–640. [Google Scholar] [CrossRef]
- Fernandez-Leborans, G. Ciliate—Decapod epibiosis in two areas of the north-west Mediterranean coast. J. Nat. Hist. 2003, 37, 1655–1678. [Google Scholar] [CrossRef]
- Wahl, M. Ecological lever and interface ecology: Epibiosis modulates the interactions between host and environment. Biofouling 2008, 24, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.; Mark, O. The predominantly facultative nature of epibiosis: Experimental and observational evidence. Mar. Ecol. Prog. Ser. 1999, 187, 59–66. [Google Scholar] [CrossRef]
- Mikac, B.; Semprucci, F.; Guidi, L.; Ponti, M.; Abbiati, M.; Balsamo, M.; Dovgal, I. Newly discovered associations between peritrich ciliates (Ciliophora: Peritrichia) and scale polychaetes (Annelida: Polynoidae and Sigalionidae) with a review of polychaete–peritrich epibiosis. Zoöl. J. Linn. Soc. 2019, 188, 939–953. [Google Scholar] [CrossRef]
- Fernandez-Leborans, G.; Chatterjee, T.; Grego, M. New records of epibiont ciliates (Ciliophora) on Harpacticoida (Copepoda, Crustacea) from the Bay of Piran (Gulf of Trieste, Northern Adriatic). Cah. Biol. Mar. 2012, 53, 53–63. [Google Scholar]
- Chiavelli, D.A.; Mills, E.L.; Threlkeld, S.T. Host preference, seasonality, and community interactions of zooplankton epibionts. Limnol. Oceanogr. 1993, 38, 574–583. [Google Scholar] [CrossRef]
- Davis, A.; White, G.A. Epibiosis in a guild of sessile subtidal invertebrates in south-eastern Australia: A quantitative survey. J. Exp. Mar. Biol. Ecol. 1994, 177, 1–14. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Clarke, A. Epibiotic communities on sublittoral macroinvertebrates at Signy Island, Antarctica. J. Mar. Biol. Assoc. UK 1995, 75, 689–703. [Google Scholar] [CrossRef]
- Cook, J.A.; Chubb, J.C. Veltkamp Epibionts of Asellus aquaticus (L.) (Crustacea, Isopoda): An SEM study. Freshw. Biol. 1998, 39, 423–438. [Google Scholar] [CrossRef]
- Magagnini, G.; Verni, F. Epibiosis of Scyphidia sp. (Ciliophora, Peritrichida) on Nerilla antennata (Archiannelida, Nerillidae). Boll. Zoöl. 1988, 55, 185–189. [Google Scholar] [CrossRef][Green Version]
- Ansari, K.G.M.T.; Guidi, L.; Dovgal, I.; Balsamo, M.; Semprucci, F. Some epibiont suctorian ciliates from meiofaunal organisms of Maldivian archipelago with description of a new ciliate species. Zootaxa 2017, 4258, 375. [Google Scholar] [CrossRef] [PubMed]
- Harder, T. Marine Epibiosis: Concepts, Ecological Consequences and Host Defence. In Marine and Industrial Biofouling; Springer: Berlin/Heidelberg, Germany, 2009; pp. 219–231. [Google Scholar]
- Chatterjee, T.; Fernandez-Leborans, G.; Ramteke, D.; Ingole, B.S. New records of epibiont Ciliates (Ciliophora) from Indian coast with descriptions of six new species. Cah. Biol. Mar. 2013, 54, 143–159. [Google Scholar]
- Dovgal, I.V. Evolution, phylogeny and classification of Suctorea (Ciliophora). Protistology 2002, 2, 194–270. [Google Scholar]
- Chatterjee, T.; Fernandez-Leborans, G.; Chan, B.K.K. New record of ciliate Thecacineta calix (Ciliophora: Suctorea) epibiont on Agauopsis halacarid mite (Acari, Halacaridae) from Taiwan. Scr. Sci. Nat. 2012, 2, 121–127. [Google Scholar]
- Dovgal, I.; Chatterjee, T.; Ingole, B. An overview of Suctorian ciliates (Ciliophora, Suctorea) as epibionts of halacarid mites (Acari, Halacaridae). Zootaxa 2008, 1810, 60–68. [Google Scholar] [CrossRef]
- Chatterjee, T.; Nanajkar, M.; Dovgal, I.; Sergeeva, N.; Bhave, S. New records of epibiont Thecacineta calix (Ciliophora, Suctorea) from the Caspian Sea and Angira Bank, Arabian Sea. Cah. Biol. Mar. 2019, 60, 445–451. [Google Scholar]
- Ansari, K.G.M.T.; Bhadury, P. Occurrence of epibionts associated with meiofaunal basibionts from the world’s largest mangrove ecosystem, the Sundarbans. Mar. Biodivers. 2016, 47, 539–548. [Google Scholar] [CrossRef]
- Bhattacharjee, D. Suctorian epibionts on Chromaspirina sp. (Nematoda: Desmodoridae) from the shallow continental shelf of the Bay of Bengal, northern Indian Ocean. Mar. Biodivers. Rec. 2014, 7, 1–3. [Google Scholar] [CrossRef]
- Zeppilli, D.; LeDuc, D.; Fontanier, C.; Fontaneto, D.; Fuchs, S.; Gooday, A.J.; Goineau, A.; Ingels, J.; Ivanenko, V.N.; Kristensen, R.M.; et al. Characteristics of meiofauna in extreme marine ecosystems: A review. Mar. Biodivers. 2017, 48, 35–71. [Google Scholar] [CrossRef]
- Schratzberger, M.; Ingels, J. Meiofauna matters: The roles of meiofauna in benthic ecosystems. J. Exp. Mar. Biol. Ecol. 2018, 502, 12–25. [Google Scholar] [CrossRef]
- Fernandez-Leborans, G.; Román, S.; Martin, D. A New Deep-Sea Suctorian-Nematode Epibiosis (Loricophrya-Tricoma) from the Blanes Submarine Canyon (NW Mediterranean). Microb. Ecol. 2017, 74, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-X.; Dovgal, I. A new Thecacineta species (Ciliophora, Suctorea) on Desmodora pontica (Nematoda, Desmodorida) from a seagrass bed in Taiwan. Protistology 2015, 9, 75–78. [Google Scholar]
- Chatterjee, T.; Dovgal, I.; Fernandez-Leborans, G. A checklist of suctorian epibiont ciliates (Ciliophora) found on aquatic meiobenthic nematodes. J. Nat. Hist. 2019, 53, 2133–2143. [Google Scholar] [CrossRef]
- Donnarumma, L.; Appolloni, L.; Chianese, E.; Bruno, R.; Baldrighi, E.; Guglielmo, R.; Russo, G.F.; Zeppilli, D.; Sandulli, R. Environmental and Benthic Community Patterns of the Shallow Hydrothermal Area of Secca Delle Fumose (Baia, Naples, Italy). Front. Mar. Sci. 2019, 6, 685. [Google Scholar] [CrossRef]
- Baldrighi, E.; Zeppilli, D.; Appolloni, L.; Donnarumma, L.; Chianese, E.; Russo, G.F.; Sandulli, R. Meiofaunal communities and nematode diversity characterizing the Secca delle Fumose shallow vent area (Gulf of Naples, Italy). PeerJ 2020, 8, e9058. [Google Scholar] [CrossRef]
- Franzo, A.; Guilini, K.; Cibic, T.; Del Negro, P. Interactions between free-living nematodes and benthic diatoms: Insights from the Gulf of Trieste (northern Adriatic Sea). Mediterr. Mar. Sci. 2018, 19, 538–554. [Google Scholar] [CrossRef]
- Semprucci, F.; Balsamo, M. New records and distribution of marine free-living nematodes in the Maldivian Archipelago. Proc. Biol. Soc. Wash. 2014, 127, 35–46. [Google Scholar] [CrossRef]
- Olu, K. PAMELA-MOZ01 Cruise. 2014. Available online: https://campagnes.flotteoceanographique.fr/campagnes/14001000/ (accessed on 1 March 2020). [CrossRef]
- Fontanier, C.; Garnier, E.; Brandily, C.; Dennielou, B.; Bichon, S.; Gayet, N.; Eugene, T.; Rovere, M.; Grémare, A.; Deflandre, B. Living (stained) benthic foraminifera from the Mozambique Channel (eastern Africa): Exploring ecology of deep-sea unicellular meiofauna. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 115, 159–174. [Google Scholar] [CrossRef]
- Fontanier, C.; Mamo, B.; Toucanne, S.; Bayon, G.; Schmidt, S.; Deflandre, B.; Dennielou, B.; Jouet, G.; Garnier, E.; Sakai, S.; et al. Are deep-sea ecosystems surrounding Madagascar threatened by land-use or climate change? Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 131, 93–100. [Google Scholar] [CrossRef]
- Kench, P.S.; Brander, R.W. Wave Processes on Coral Reef Flats: Implications for Reef Geomorphology Using Australian Case Studies. J. Coast. Res. 2006, 221, 209–223. [Google Scholar] [CrossRef]
- Semprucci, F.; Colantoni, P.; Baldelli, G.; Rocchi, M.B.; Balsamo, M. The distribution of meiofauna on back-reef sandy platforms in the Maldives (Indian Ocean). Mar. Ecol. 2010, 31, 592–607. [Google Scholar] [CrossRef]
- Semprucci, F.; Frontalini, F.; Losi, V.; Du Châtelet, E.A.; Cesaroni, L.; Sandulli, R.; Coccioni, R.; Balsamo, M. Biodiversity and distribution of the meiofaunal community in the reef slopes of the Maldivian archipelago (Indian Ocean). Mar. Environ. Res. 2018, 139, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Heip, C.; Vincx, M.; Vranken, G. The ecology of marine nematodes. Oceanogr. Mar. Biol. Annu. Rev. 1985, 23, 399–489. [Google Scholar]
- Seinhorst, J.W. A rapid method for the transfer of nematodes from fixative toanhydrous glicerine. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes. Part. I. British Enoplids; Synopses of the British Fauna, Volume 28; Cambridge University Press: Cambridge, UK, 1983; p. 307. [Google Scholar]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes. Part II. British Chromadorids; Synopses of the British Fauna, Volume 38; E.J Brill: Leiden, The Netherlands, 1988; p. 502. [Google Scholar]
- Warwick, R.M.; Platt, H.M.; Somerfield, P.J. Free-Living Marine Nematodes. Part III. Monhysterids; Synopses of the British Fauna, Volume 53; Field Studies Council: Shrewsbury, UK, 1988; p. 296. [Google Scholar]
- Bezerra, T.N.; Decraemer, W.; Eisendle-Flöckner, U.; Hodda, M.; Holovachov, O.; Leduc, D.; Miljutin, D.; Mokievsky, V.; Peña Santiago, R.; Sharma, J.; et al. Nemys: World Database of Nematodes. 2019. Available online: http://nemys.ugent.be (accessed on 7 October 2019). [CrossRef]
- Dovgal, I.V. Fauna of Ukraine: In 40 Vol. Volume 36: Ciliates—Ciliophora. Issue 1: Class Suctorea; Naukova Dumka: Kyiv, Ukraine, 2013; p. 267. [Google Scholar]
- Ghosh, M.; Mandal, S. Living with Nematode: An Epibiont Trematosoma rotunda Associated with Basibiont Desmodora scaldensis from Matla Estuary, Sundarbans, India. Thalassas 2019, 35, 619–624. [Google Scholar] [CrossRef]
- Chen, C.; Golovatch, S.I.; Chang, H.-W. Identity of the east Asian millipede Habrodesmus inexpectatus Attems, 1944 (Diplopoda: Polydesmida: Paradoxosomatidae). J. Nat. Hist. 2008, 42, 2547–2556. [Google Scholar] [CrossRef]
- Chatterjee, T.; Nanajkar, M.; Dovgal, I. New record of Loricophrya stresemanni (Ciliophora, Suctorea) as epibiont on nematodes from the India Ocean and notes on the genus Loricophrya. Cah. Biol. Mar. 2019, 60, 283–288. [Google Scholar]
- Collin, B. Etudes monographiques sur les Acinetiens. II. Morphologie, physiologie, systematique. Arch. Zool. Exp. Gen. 1912, 51, 1–457. [Google Scholar]
- Dovgal, I.; Chatterjee, T.; Ingole, B. New records of Thecacineta cothurnioides and Trematosoma rotunda (Ciliophora, Suctorea) as epibionts on nematodes from Indian Ocean. Protistology 2009, 6, 19–23. [Google Scholar]
- Lynn, D.H. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2008; p. 605. [Google Scholar]
- Robin, M.C. Mémoire sur la structure et la reproduction de quelques infusoires tentaculés suceurs et flagellés. Journal l’Anatomie Physiologie Normale Pathologiques l’homme Animaux 1979, 529–583. [Google Scholar]
- Root, F.M. A New Suctorian from Woods Hole. Trans. Am. Microsc. Soc. 1922, 41, 77. [Google Scholar] [CrossRef]
- Swarczewsky, B. Sur Kenntnis der Baikalprotistenfauna. Die an der Baikalgammariden lebeden Infusorien. 1–6. 4. Acinetidae. Archive Protistenkunde 1929, 63, 362–449. [Google Scholar]
- Jankowski, A.V. Review of Taxa Phylum Ciliophora Doflein, 1901; Protista: Handbook on Zoology. Volume Part. 2; Alimov, A.F., Ed.; Nauka: St. Petersburg, Russia, 2007; pp. 415–993. [Google Scholar]
- Grell, K.G.; Meister, A. Die Ultrastruktur von Acinetopsis rara Robin (Suctoria). I. Tentakeln und Nahrungsaufnahnme. Protistologica 1982, 18, 67–84. [Google Scholar]
- Batisse, A. Sous-Classe des Suctoria Claparede et Lachmann, 1858; Traite de Zoologie. Anatomie, Systematique, Biologie. Tome II. Infusoires Cilies. Fascicule 2. Systematique; De Puytorac, P., Ed.; Masson: Paris, France; Milan, Italy; Barcelone, Spain, 1994; pp. 493–563. [Google Scholar]
- Sergeeva, N.; Dovgal, I. First finding of epibiont peritrich and suctorian ciliates (Ciliophora) on oligochaetes and harpacticoid copepods from the deep-water hypoxic/anoxic conditions of the Black Sea. Ecol. Montenegr. 2014, 1, 49–54. [Google Scholar]
- Lynn, D.H.; Gómez-Gutiérrez, J.; Strüder-Kypke, M.; Shaw, C.T. Ciliate species diversity and host-parasitoid codiversification in the apostome genus Pseudocollinia (Ciliophora, Apostomatia, Pseudocollinidae) that infect krill, with description of Pseudocollinia similis n. sp., a parasitoid of the krill Thysanoessa spinifera. Dis. Aquat. Org. 2014, 112, 89–102. [Google Scholar]
- Sergeeva, N.; Dovgal, I. Loricophrya bosporica n. sp. (Ciliophora, Suctorea) epibiont of Desmoscolex minutus (Nematoda, Desmoscolecida) from oxic/anoxic boundary of the Black Sea Istanbul Strait’s outlet area. Zootaxa 2016, 4061, 596. [Google Scholar] [CrossRef]
- Ivanova, E.; Dovgal, I.V.; Newton, A. First records of epibiont ciliates (Ciliophora) in methane enriched sediments with species redescriptions. Ecol. Montenegr. 2017, 10, 51–57. [Google Scholar]
- Sartini, B.; Marchesini, R.; D’ávila, S.; D’Agosto, M.; Dias, R.J.P. Diversity and Distribution of Peritrich Ciliates on the Snail Physa acuta Draparnaud, 1805 (Gastropoda: Physidae) in a Eutrophic Lotic System. Zool. Stud. 2018, 57, 2018–2057. [Google Scholar]
- Fernandez-Leborans, G.; Gabilondo, R. Inter-annual variability of the epibiotic community on Pagurus bernhardus from Scotland. Estuar. Coast. Shelf Sci. 2006, 66, 35–54. [Google Scholar] [CrossRef]
- Key, M.M.; Winston, J.E.; Volpe, J.W.; Jeffries, W.B.; Voris, H.K. Bryozoan fouling of the blue crab Callinectes sapidus at Beaufort, North Carolina. Bull. Mar. Sci. 1999, 64, 513–533. [Google Scholar]
- Fisher, R. Ciliate Hitch-hikers—Nematode ecto-commensals from tropical Australian sea grass meadows. J. Mar. Biol. Assoc. UK 2003, 83, 445–446. [Google Scholar] [CrossRef]
- Panigrahi, S.; Bindu, V.K.; Bramha, S.N.; Samantara, M.K.; Mohanty, A.K.; Satpathy, K.K.; Dovgal, I. Report of Thecacineta calix (Ciliophora: Suctoria) on nematode Desmodora from the intertidal sediments of Southwest Bay of Bengal. Indian J. Geo-Mar. Sci. 2015, 44, 1840–1843. [Google Scholar]
- Decraemer, W.; Coomans, A.; Baldwin, J. Morphology of Nematoda. Nematoda 2013, 2, 1–60. [Google Scholar]
- Riemann, F.; Riemann, O. The enigmatic mineral particle accumulations on the cuticular rings of marine desmoscolecoid nematodes—Structure and significance explained with clues from live observations. Meiofauna Mar. 2010, 18, 1–10. [Google Scholar]
- Ingole, B.; Singh, R.; Sautya, S.; Dovgal, I.; Chatterjee, T. Report of epibiont Thecacineta calix (Ciliophora: Suctorea) on deep-sea Desmodora (Nematoda) from the Andaman Sea, Indian Ocean. Mar. Biodivers. Rec. 2010, 3, 3. [Google Scholar] [CrossRef]
- Pujol, N.; Davis, P.; Ewbank, J.J. The Origin and Function of Anti-Fungal Peptides in C. elegans: Open Questions. Front. Immunol. 2012, 3, 237. [Google Scholar] [CrossRef]
- Tarr, D.E.K. Nematode antimicrobial peptides. Invert. Surv. J. 2012, 9, 122–133. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).