Abstract
Crustaceans are a key component of the fauna living in rhodoliths, but patterns in their distribution and abundance remain largely unknown. This paper assessed spatio-temporal variability of Brachyura associated with rhodoliths. A seasonal study was conducted at three depth layers (18, 25, and 40 m), throughout two years (December 2015 to October 2017) at Gran Canaria Island (eastern Atlantic Ocean). A total of 765 crabs belonging to 10 species were collected. A larger abundance and richness of crabs at 25 m correlated with a larger biomass of epiphytic algae attached to rhodoliths. A seasonal pattern was also observed, where a higher richness of crabs occurred in the summer. The Xanthid crab, Nanocassiope melanodactylus, dominated the assemblage (83%); juveniles of this species were more abundant in deeper waters (40 m), while adults were more abundant on the shallower depth layers (18 m and 25 m). The species Pilmunus hirtellus was restricted to 25 m. Nevertheless, Pisa carinimana and Achaeus cranchii did not show any spatio-temporal pattern. In summary, this study demonstrated that two conspicuous crabs, N. melanodactylus and P. hirtellus, associated with rhodolith beds are bathymetrically segregated.
1. Introduction
Rhodoliths are nodules of individual non-geniculate coralline red algae (Corallinales, Hapalidiales, Sporolithales: Corallinophycidae) with a calcified thallus []. They form relatively stable three-dimensional structures and large heterogeneous beds of biogenic substrates [,,,,,]. These beds are found worldwide, from the tropics to the poles, and from the intertidal zone to depths over 200 m [,]. Rhodoliths have relatively slow growth rates and a perennial life strategy; some thalli can even live >100 years [].
As they are not attached to a fixed surface, individual thalli are exposed to the action of waves and currents and have the potential to be rolled over or moved on the seafloor. Hydrodynamic conditions and light are pivotal factors determining their growth, morphology, and, therefore, the overall heterogeneity of rhodolith beds [,]. In terms of area covered, rhodolith beds may be one of the Earth’s “Big Four” benthic communities dominated by marine macrophytes, including kelp beds, seagrass meadows, and non-geniculate coralline reefs []. Rhodolith beds occur in large extensions in the Canary Islands (Central-East Atlantic Ocean), but few studies addressing basic ecological questions have been carried out []. These habitats can be found from 15 m all the way down to 100 m depth, so large variation in the spatial configuration is expected across bathymetry [].
Rhodolith beds have considerable ecological significance because they provide large amounts of internal space providing habitat for other algae and invertebrates, including abundant juvenile stages [,,,,]. Hence, rhodoliths are considered habitat modifiers or “bioengineers” due to their wide variety of ecological niches and resources, compared to surrounding habitats, e.g., sandy bottoms []. In addition, rhodoliths provide a basal habitat for epiphytes (and fauna) to attach, which greatly increase the complexity of the entire ecosystem.
Typically, a higher biodiversity of epifauna is found in rhodolith ecosystems than over other common substrata (e.g., sandy substrates) [,,,]. Crustaceans are one of the most abundant faunal groups, which have appeared to be the dominant fauna in rhodolith beds [,,,,]. However, few studies mention the infraorder Brachyura (i.e., true crabs) in these habitats [,,,,], and none assessed the spatio-temporal distribution of these animals living in these three-dimensional structures.
Brachyuran crabs, also known as true crabs, are found both in marine environments and fresh waters worldwide, from coastal waters to the deep ocean [,]. There is an updated literature on shallow and deep brachyuran crabs from the Canary Islands [,]. However, few or scarce information is already published about the distribution of this faunal group in rhodolith beds []. The overall objective of this study was, therefore, to understand the spatio-temporal variability of brachyuran crabs associated with rhodoliths. In particular, rhodolith beds were seasonally studied (spring, summer, autumn, winter) at three depths layers through two years (i.e., eight times). Despite the fact that the effect of depth over the biology and morphology of rhodoliths has been widely studied, few works have analyzed whether the distribution and abundance of associated invertebrates change through bathymetrical gradients [], and no study has concurrently explored variability at seasonal scales.
2. Materials and Methods
The location of this study was the east coast of Gran Canaria Island (Canary Islands, eastern Atlantic Ocean, Figure 1a) on a large rhodolith bed near Gando Bay (27°55′54″ N, 15°21′11″ W) (Figure 1b). The SST of the study site seasonally ranged around ca. 5.5 °C, with a mean (± SE) of 21.2 °C (± 0.16). Maximum SSTs were measured in early autumn, while minimums were recorded at the end of winter []. PAR seasonally varied ca. 42.05 E m−2 d−1, with a mean of 42.13 E m−2 d−1 (± 1.26). Maximum PAR occurred before summer, while minimum PAR occurred in winter []. In term of levels of hydrodynamism, the mean wave direction was predominantly from the NE (50.18°), with larger wave peak periods in winter []. The rhodolith bed is mainly composed by several species of red calcareous algae, e.g., Lithothamnion spp. and Phymatolithon spp. [,]. Typically, the size of rhodoliths (longest axis) is between 2–4 cm []. A previous study in the study site demonstrated that depth affected the size and morphology of rhodoliths, with bigger and mainly spherical nodules at 25, relative to those at depths of 18 m and 40 m. The biomass of epiphytic algae was generally larger at 18 and 25 m depth than at 40 m (Figure 2) []. Rhodolith samples (Figure 1c) were taken at each season (i.e., spring, summer, autumn and winter) for two years (2015–2017) at three different depths (18 m, 25 m, and 40 m).
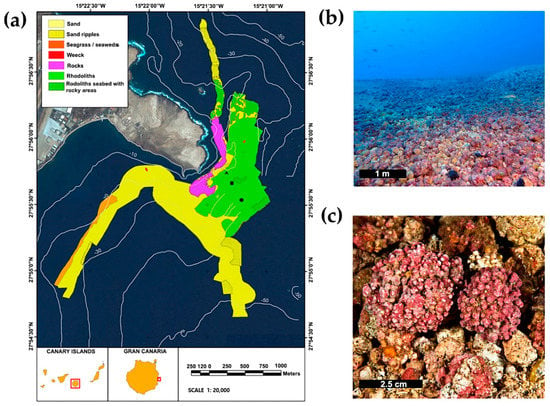
Figure 1.
(a) Location of the study area at Gran Canaria Island, showing the three sampling sites at 18 m (triangle), 25 m (square), and 40 m (circle); figure adapted from []. (b) Rhodolith beds. (c) Nodules of rhodoliths.
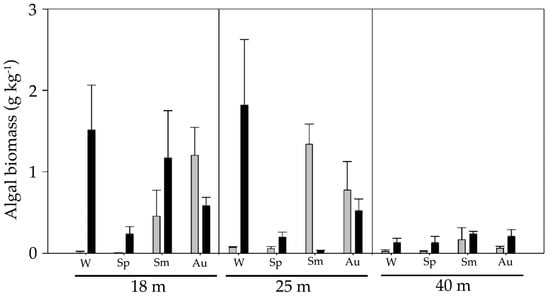
Figure 2.
Total epiphytic algal biomass (dry weigh) (+ SE of means) at different depths (18, 25, and 40 m) and seasons (W: winter, Sp: spring, Sm: summer, Au: autumn) from 2015–2016 (grey bars) and 2017 (black bars). Data from [].
More specifically, sampling took place at winter (December 2015 and 2016), spring (March 2016 and 2017), summer (July 2016 and 2017), and autumn (October 2016 and 2017). Samples were collected by SCUBA divers. On each depth layer, n = 5 random replicates (25 × 25 cm) were taken at each time (n = 120 samples for the entire study) by collecting all rhodolithic nodules up to 5 cm below the first layer of rhodoliths. SCUBA divers collected the samples by hand, which were enclosed in cloth bags and preserved in a freezer at –20 °C until sorting.
In order to remove sand and debris from rhodoliths, each sample was initially defrosted and filtered through a 0.5 mm sieve. All crabs retained by this mesh sieve were subsequently identified under a stereomicroscope (Leica, EZ4W) and preserved in alcohol. Three identification keys [,,] and a checklist [] were used in this sense. Epiphytic macroalgae were removed and subsequently dried at 70 °C for 48 h to obtain their dry weight.
For the most abundant crab species, their distribution and abundance patterns were analyzed using the average of the five replicates from each depth layer and time. To shed light into potential recruitment seasons, the carapace width was considered for the most abundant species, Nanocassiope melanodactylus (Milne-Edwards, 1867). A Chi-square then tested for differences in the size structure of these crab species between the three bathymetrical layers.
Univariate responses (taxonomic richness and abundance of the most conspicuous species and the mean size of N. melanodactylus, as well as the epiphytic algal biomass) were analyzed by means of mixed-effects generalized linear models (GLMs) to test the effect of “Depth,” “Season,” and “Year.” The first two factors were considered fixed, while year was typified as a random factor. A “negative binomial” family error structure was selected, which is ideal for over-dispersed count data []. In all cases, we assessed model performance (i.e., assumptions of linearity and homogeneity of variances) by visually inspecting graphical model diagnoses (QQ-plots, residuals histograms, residuals versus fitted values, and fitted values versus observed values) []. To select models with the largest parsimony, i.e., with or without the random effect, the AIC (Akaike Information Criterion) was obtained. Because the AIC was larger when the random effect (“Year”) was considered for univariate responses, this factor was finally not included. All modelling and testing were implemented in the R.3.6.0. statistical package, using the default packages and the “lmertest” library [].
3. Results
In this study, a total of 765 brachyuran crabs, corresponding to 10 different species, were identified (Table 1). Nanocassiope melanodactylus accounted for more than 80% of the total abundance. Pisa carinimana (Miers, 1879), Achaeus cranchii (Leach, 1817), and Pilumnus hirtellus (Linnaeus, 1761) contributed with another ca. 10%.

Table 1.
Total abundance (dominance, as a proportion of the total) and number of individuals of each species for the overall study (n = 120 samples).
At 25 m, the number of crab species was higher than at 18 and 40 m (Figure 3, Table 2, Estimate = 0.8754, p = 0.0116). A seasonal pattern was also observed; a higher richness of crabs was observed in Autumn (October, Figure 3, Table 2, estimate = 0.7419, p = 0.0443).
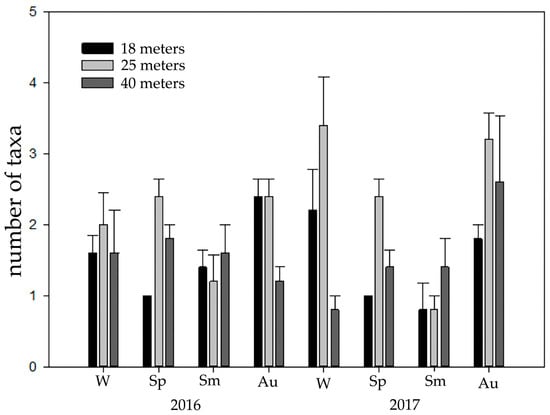
Figure 3.
Richness (+SE of means) of brachyuran species at different depths (18, 25, and 40 m) and seasons (W: winter, Sp: spring, Sm: summer, Au: autumn) in 2015–2016 and 2017.

Table 2.
Summary of the mixed-effects GLM testing the effects of the fixed factors “Depth” (18, 25, and 40 m) and “Season” on the richness of brachyuran species. The contribution of “Year” is not presented, as this was a random source of variation. Reference values were 18 m for “Depth” and winter for “Season”.
The abundance of N. melanodactylus was lower in summer (July 2016 and 2017) than in the other seasons (Figure 4a, Table 3, estimate = −1.0986, p = 0.0010). This pattern was irrespective of depth (Figure 4a, Table 3). The distribution of P. carinimana and A. cranchii was random, with no discernible spatio-temporal pattern (Figure 4b,c; Tables S2 and S3, respectively). Pilumnus hirtellus was present only at 25 m (Figure 4d, Table S4, estimate = 21.74687, p < 0.0001.
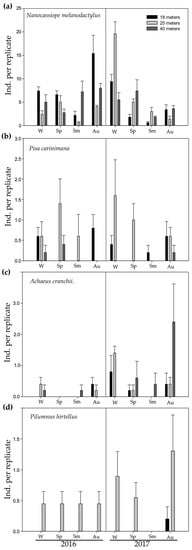
Figure 4.
Abundance (+SE of means) of (a) Nanocassiope melanodactylus, (b) Pisa carinimana, (c) Achaeus cranchii, and (d) Pilumnus hirtellus at different depths (18, 25, and 40 m) and seasons (W: winter, Sp: spring, Sm: summer, Au: autumn) in 2015–2016 and 2017. Scales are different among panels.

Table 3.
Summary of the mixed-effects GLM testing the effects of the fixed factors “Depth” (18, 25 and 40 m) and “Season” on the abundance of Nanocassiope melanodactylus. The contribution of “Year” is not presented, as this was a random source of variation. Reference values were 18m for “Depth” and winter for “Season”.
The size of N. melanodactylus, the most abundant crab species, changed with depth; individuals at 40 m were smaller than those at shallower waters (Figure 5c; Table 4; estimate = –0.4960, p = 0.0099). Seasonal bathymetric segregation by size occurs at summer, where large-sized crabs were only found at 25 m (Figure 5b; X2 = 42.106, df = 18, p = 0.0011), while a broad range sizes occurs at 18 m (2.5–6.0 mm CL). Conversely, the lowest mean size of crabs in autumn indicated the absence of adults in this season at all depth layers (Figure 5a–c; Table 4; estimate = −0.7900, p = 0.0003).
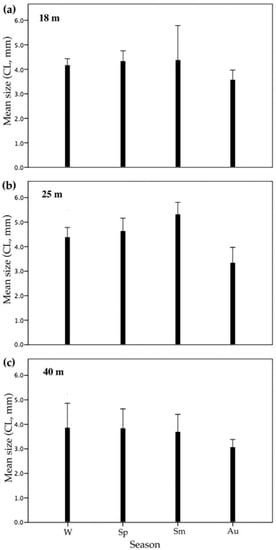
Figure 5.
Mean size (carapace length, +SE of means) of N. melanodactylus at each season (W: winter, Sp: spring, Sm: summer, Au: autumn) and at (a) 18 m, (b) 25 m, and (c) 40 m. Carapace length (CL) was measured in mm.

Table 4.
Summary of the mixed-effects GLM testing the effects of the fixed factors “Depth” (18, 25, and 40 m) and “Season” on the mean size of Nanocassiope melanodactylus. The contribution of “Year” is not presented, as this was a random source of variation. Reference values were 18 m for “Depth” and winter for “Season”.
4. Discussion
Understanding patterns of spatial and temporal variability in the abundances of biota provides basic ecological insight, which is essential for determining priority areas for conservation in a given habitat. Our work is the first specific, though exploratory, survey on brachyuran crabs inhabiting rhodolith beds. Other studies have also annotated that crustaceans, including crabs, contribute significantly to the macrofaunal community from these habitats [,].
The present study has first evidenced that the assemblage of brachyuran crabs associated with rhodoliths in Gran Canaria Island is dominated by Nanocassiope melanodactylus. A basic premise of crustacean ecology states that the numerically dominant species within a biotope are generally those that are best adapted to efficiently exploit food and habitat resources []. From the 10 brachyurans species we found here, N. melanodactylus was the dominant species, accounting for ca. 83% of the total abundance of crabs. Rhodolith beds in the east coast of Gran Canaria Island seem, therefore, to provide an optimal habitat for the development of this Xanthid crab. In the Canary Islands, Xanthid crabs from shallow waters are a valuable resource, mainly as a bait for traditional fishing []. In other regions, crabs of Family Xanthidae are dominant species in intertidal rocky bottoms (e.g., Xantho poressa) []. Our results now highlight that another species of Xanthid crab, here, N. melanodactylus is a dominant species in rhodolith beds from Gran Canaria Island.
Second, we demonstrated that the abundance of certain crab species varied according to the sampling season (e.g., N. melanodactylus) and depth of collection (e.g., N. melanodactylus and Pilummnus hirtelus). Recent studies have revealed that ecological drivers of macrofauna associated with rhodoliths include depth (i.e., variation in light, sedimentation and turbulence across bathymetric gradients), which affect the average diameter, biomass of epiphytic macroalgae, and the density of rhodoliths [,,]. In the study area, previous research has observed an increase in rhodolith size from 18 m to 25 m, followed by decrease from 25 m to 40 m []. As observed in this study, the biomass of epiphytic algae also varies with depth, probably linked with a larger light availability at shallower depths [,,]. Increases in the heterogeneity and complexity of the available habitat through epiphytic algae attached to rhodoliths underpins a “habitat cascade” effect [], inducing an increment in the biomass of invertebrates, e.g., amphipods []. Consequently, it is plausible that increased epiphytic algae can promote larger abundances of prey for brachyurans. Despite prey availability and habitat complexity may be suitable for brachyuran crabs at the shallower depth layer, the relationship between abundances of crabs and preys are difficult to describe, because other factors come into play, e.g., as agonistic behavior or reproduction [].
At 25 m, brachyuran taxonomic richness was higher than in the shallowest (18 m) and deepest layers (40 m). This pattern somehow follows that of the most important environmental factors driving the growth of rhodolith nodules: hydrodynamic conditions and light [,]. The action of waves and currents are stronger at 18 m, relative to deeper waters, promoting overturning and the breakage of rhodoliths. At a depth of 40 m, even though wave disturbance is reduced, conditions are not ideal for rhodoliths to grow due to low irradiance at this depth []. Reductions in rhodolith epiphytic algal biomass with increasing depth was observed in the study area [] and in other studies worldwide [,,,]. Rhodolith complexity have appeared to be a good predictor of epifaunal abundance and taxonomic richness [,,,,,,], including the abundance and richness of amphipods in the study area []. These results support the hypothesis that increasing structural complexity leads to enhanced species diversity [].
This work pointed out that juveniles of the dominant brachyuran species, N. melanodactylus, were majorly located on the deepest waters (40 m). The distribution of brachyuran crabs through bathymetric ranges is heterogeneous and may be caused by several factors, such as the type of habitat, oceanographic conditions, seasonal effects, spawning migrations, or agonistic intra- and interspecific competition [,,]. N. melanodactylus has a broad bathymetric distribution (mainly between 3 m and 150 m), inhabiting a range of nearshore habitats, including rhodolith beds, muddy and sandy bottoms, gravels, stones and corals []. Our results showed differences in the size of N. melanodactylus according to the depth layer and season of collection. Size segregation majorly occurs at summer, where bigger crabs dominate at 25 m, while a broad range of sizes occurs at 18 m. Hence, adults have a major presence at shallow waters (i.e., 18 m and 25 m). Juveniles of N. melanodactylus, however, seem to live in deeper waters (i.e., 40 m). A possible explanation for this outcome is the agonistic behavior of Xanthid crabs, where large-sized male crabs are more aggressive than females [,,], inducing habitat partitioning at reproductive seasons. The large range of crab sizes at the shallowest depths in summer has been attributed to the need of females (medium sizes) to seek warmer waters due to advantageous conditions for ovarian development and eggs maturation []. As a result, habitat partitioning by depth between juveniles and adults of N. melanodactylus could result from these reasons. Finally, the absence of adults in autumn, at all the depth layers, and the parallel large abundances of juveniles, could be indicative of adult displacement to nearby habitats. This is noteworthy because it may suggest the influence of surrounding habitats on the composition and structure of faunal assemblages (here, crabs) inhabiting rhodoliths, as it occurs in seagrass meadows [,].
5. Conclusions
This study has contributed to the understanding of spatio-temporal variability of the brachyuran fauna associated with rhodoliths in the Canary Islands. Here, we demonstrated that the assemblage of brachyuran crabs was largely dominated by Nanocassiope melanodactylus, which seems to majorly recruit at 40 m. It turns out that, at 25 m, rhodolith beds provide ideal conditions in terms of provision of habitat for associated assemblages of brachyuran crabs. Despite this pattern being observed for certain species, e.g., Pilumnus hirtellus, other crab species, e.g., Pisa carinimana or Achaeus cranchii, did not show any spatio-temporal pattern at all. Due to the low number of individuals of these species, caution is necessary for the interpretation of a lack of depth pattern. As this is the first study of the spatio-temporal variability of Brachyura from Gran Canaria Island, further research is needed.
Supplementary Materials
The following material is available online at https://www.mdpi.com/1424-2818/12/6/223/s1, Table S1. Epiphytic algal biomass (GML); Table S2. Pisa carinimana abundance (GML); Table S3. Achaeus cranchii abundance (GML); Table S4. Pilumnus hirtellus abundance (GML).
Author Contributions
Conceptualization, F.T. and F.O.-F.; Methodology, all authors; Formal analysis, C.S.-L., R.T.-P., F.T., and F.O.-F.; Investigation, all authors; Writing—original draft preparation, C.S.-L., F.T., and F.O.-F.; Writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially financed by the Excellence International Campus of the Canary Islands (CEI-Canarias), the Agency for Research, Innovation and Information Society of the Canary Islands, and the EcoAqua European project (ERA CHAIR program—Grant Agreement no. 621341).
Acknowledgments
We acknowledge T. Sánchez and M. Cosme de Esteban for their extraordinary logistical support during sampling and “Puertos del Estado” (Spain) for the supply of some oceanographic data. The authors are indebted with anonymous reviewers for their comments, corrections, and recommendations, which improved the manuscript during the reviewing process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Riosmena-Rodríguez, R.; Nelson, W.; Aguirre, J. Rhodolith/maërl Beds: A Global Perspective; Springer International Publishing: Basel, Switzerland, 2017. [Google Scholar]
- Ginsburg, R.N.; Bosellini, A. Form and internal structure of recent algal nodules (Rhodolites) from Bermuda. J. Geol. 1971, 79, 669–682. [Google Scholar] [CrossRef]
- Steller, D.L.; Riosmena-Rodríguez, R.; Foster, M.S.; Roberts, C.A. Rhodolith bed diversity in the Gulf of California: The importance of rhodolith structure and consequences of disturbance. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S5–S20. [Google Scholar] [CrossRef]
- Sciberras, M.; Rizzo, M.; Mifsud, J.R.; Camilleri, K.; Borg, J.A.; Lanfranco, E.; Schembri, P.J. Habitat structure and biological characteristics of a maerl bed off the northeastern coast of the Maltese Islands (central Mediterranean). Mar. Biodivers. 2009, 39, 251–264. [Google Scholar] [CrossRef]
- Steller, D.L.; Cáceres-Martínez, C. Coralline algal rhodoliths enhance larval settlement and early growth of the pacific calico scallop Argopecten ventricosus. Mar. Ecol. Prog. Ser. 2009, 396, 49–60. [Google Scholar] [CrossRef]
- Basso, D.; Babbini, L.; Kaleb, S.; Bracchi, V.A.; Falace, A. Monitoring deep Mediterranean rhodolith beds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 549–561. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Mannarà, E.; Cosme, M.; Falace, A.; Montiel-Nelson, J.A.; Espino, F.; Tuya, F. Early-faunal colonization patterns of discrete habitat units: A case study with rhodolith-associated vagile macrofauna. Estuar. Coast. Shelf Sci. 2019, 218, 9–22. [Google Scholar] [CrossRef]
- Foster, M.S. Rhodoliths: Between rocks and soft places. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Nelson, W.A.; New Zealand Ministry for Primary Industries. Rhodolith Beds in Northern New Zealand: Characterisation of Associated Biodiversity and Vulnerability to Environmental Stressors. 2012. Available online: http://fs.fish.govt.nz/Doc/23064/AEBR_99.pdf.ashx (accessed on 2 February 2020).
- Hernandez-Kantun, J.J.; Hall-Spencer, J.M.; Grall, J.; Adey, W.; Rindi, F.; Maggs, C.A.; Peña, V. North Atlantic Rhodolith Beds. In Coastal Research Library; Springer: Cham, Switzerland, 2017; Volume 15. [Google Scholar]
- Rebelo, C.; Johnson, M.E.; Quartau, R.; Rasser, M.W.; Melo, C.S.; Neto, A.I.; Ávila, S.P. Modern rhodoliths from the insular shelf of Pico in the Azores (Northeast Atlantic Ocean). Estuar. Coast. Shelf Sci. 2018, 210, 7–17. [Google Scholar] [CrossRef]
- Riera, R.; Delgado, J.D.; Rodríguez, M.; Monterroso, Ó.; Ramos, E. Macrofaunal communities of threatened subtidal maërl seabeds on Tenerife (Canary Islands, north-east Atlantic Ocean) in summer. Acta Oceanol. Sin. 2012, 31, 98–105. [Google Scholar] [CrossRef]
- Hinojosa-Arango, G.; Riosmena-Rodriguez, R. Influence of Rhodolith-Forming Species and Growth-Form on Associated Fauna of Rhodolith Beds in the Central-West Gulf of California, Mexico. Mar. Ecol. 2014, 25, 109–127. [Google Scholar] [CrossRef]
- Kamenos, N.A.; Moore, P.G.; Hall-Spencer, J.M. Maerl grounds provide both refuge and high growth potential for juvenile queen scallops (Aequipecten opercularis L.). J. Exp. Mar. Biol. Ecol. 2004, 313, 241–254. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Maneveldt, G.; Manso, R.C.C.; Marins-Rosa, B.V.; Pacheco, M.R.; Guimaraes, S.M. Estructura de los mantos de rodolitos de 4 a 55 metros de profundidad en la costa sur del estado de Espírito Santo, Brazil. Cienc. Mar. 2007, 33, 399–410. [Google Scholar] [CrossRef]
- De Grave, S. The Influence of Sedimentary Heterogeneity on Within Maerl Bed Differences in Infaunal Crustacean Community. Estuar. Coast. Shelf Sci. 1999, 49, 153–163. [Google Scholar] [CrossRef]
- Veras, P.C.; Pierozzi, I.; Lino, J.B.; Amado-Filho, G.M.; de Senna, A.R.; Santos, C.S.G.; Pereira-Filho, G.H. Drivers of biodiversity associated with rhodolith beds from euphotic and mesophotic zones: Insights for management and conservation. Perspect. Ecol. Conserv. 2020, in press. [Google Scholar] [CrossRef]
- Bouzon, J.L.; Freire, A.S. The Brachyura and Anomura fauna (Decapoda; Crustacea) in the Arvoredo Marine Biological Reserve on the southern brazilian coast. Braz. J. Biol. 2007, 67, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Borg, J.A.; Howege, H.M.; Lanfranco, E.; Micallef, S.; Mifsud, C.; Schembri, P.J. The macrobenthic species of the infralittoral to circalittoral transition zone off the northeastern coast of Malta (Central Mediterranean). Xjenza 1998, 3, 16–24. [Google Scholar]
- Koettker, A.G.; Lopes, R.M. Meroplankton spatial structure and variability on Abrolhos Bank and adjacent areas, with emphasis on brachyuran larvae. Cont. Shelf Res. 2013, 70, 97–108. [Google Scholar] [CrossRef]
- Herrera-Martínez, P.; Álvarez-Hernández, S.; del Carmen Méndez-Trejo, M.; Riosmena-Rodríguez, R. Invertebrates: Structure of the Community and Biodiversity Associated to Rhodolith-Sponge Complex at Magdalena Bay. South Baja California. In Invertebrates: Classification, Evolution and Biodiversity; Nova Science Publishers: New York, NY, USA, 2013; pp. 107–130. [Google Scholar]
- Abele, L.G. Species diversity of decapod crustaceans in marine habitats. Ecology 1974, 55, 156–161. [Google Scholar] [CrossRef]
- Hines, A.H. Larval patterns in the life histories of brachyuran crabs (Crustacea, Decapoda, Brachyura). Bull. Mar. Sci. 1986, 39, 444–466. [Google Scholar]
- González, J.A. Brachyuran crabs (Crustacea: Decapoda) from the Canary Islands (eastern Atlantic): Checklist, zoogeographic considerations and conservation. Sci. Mar. 2016, 80, 89–102. [Google Scholar] [CrossRef]
- González, J.A.; Triay-Portella, R.; Martins, A.; Lopes, E. Checklist of brachyuran crabs (Crustacea: Decapoda) from the Cape Verde Islands, with a biogeographic comparison with the Canary Islands (Eastern Atlantic). Cah. Biol. Mar. 2017, 58, 137–151. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Cosme, M.; Tuya, F.; Espino, F.; Haroun, R. Effect of depth and seasonality on the functioning of rhodolith seabeds. Estuar. Coast. Shelf Sci. 2020, 106579. [Google Scholar] [CrossRef]
- Haroun, R.J.; Gil-Rodríguez, M.C.; de Castro, J.D.; Prud’Homme Van Reine, W.F. A checklist of the marine plants from the Canary Islands (central eastern Atlantic Ocean). Bot. Mar. 2002, 45, 139–169. [Google Scholar] [CrossRef]
- Pardo, C.; Lopez, L.; Peña, V.; Hernández-Kantún, J.; Le Gall, L.; Bárbara, I.; Barreiro, R. A multilocus species delimitation reveals a striking number of species of coralline algae forming maerl in the OSPAR maritime area. PLoS ONE 2014, 9, e104073. [Google Scholar] [CrossRef] [PubMed]
- Monod, T. Hippidea et Brachyura ouest-africains. Ifan 1956, 45, 1–674. [Google Scholar]
- Zariquiey, A.R. Crustáceos decápodos ibéricos. Investig. Pesq. 1968, 32, 1–510. [Google Scholar]
- Manning, R.B.; Holthuis, L.B. West African Brachyura Crabs (Crustacea: Decapoda); Smithsonian Institution Press: Washington, DC, USA, 1981. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef]
- Abele, L.G. Comparative habitat diversity and faunal relationships between the Pacific and Caribbean Panamanian decapod Crustacea: A preliminary report with remarks on the crustacean fauna of Panama. Bull. Biol. Soc. Wash. 1972, 2, 125–138. [Google Scholar]
- Spivak, E.D.; Arévalo, E.; Cuesta, J.A.; González-Gordillo, J.I. Population structure and reproductive biology of the stone crab Xantho poressa (Crustacea: Decapoda: Xanthidae) in the ‘Corrales de Rota’ (south-western Spain), a human-modified intertidal fishing area. J. Mar. Biol. Assoc. U. K. 2010, 90, 323–334. [Google Scholar] [CrossRef][Green Version]
- Connell, S.D. Assembly and maintenance of subtidal habitat heterogeneity: Synergistic effects of light penetration and sedimentation. Mar. Ecol. Prog. Ser. 2005, 289, 53–61. [Google Scholar] [CrossRef]
- Pascelli, C.; Riul, P.; Riosmena-Rodríguez, R.; Scherner, F.; Nunes, M.; Hall-Spencer, J.M.; de Oliveira, E.C.; Horta, P. Seasonal and depth-driven changes in rhodolith bed structure and associated macroalgae off Arvoredo island (southeastern Brazil). Aquat. Bot. 2013, 111, 62–65. [Google Scholar] [CrossRef]
- McConnico, L.A.; Carmona, G.H.; Morales, J.S.M.; Rodríguez, R.R. Temporal variation in seaweed and invertebrate assemblages in shallow rhodolith beds of Baja California Sur, México. Aquat. Bot. 2017, 139, 37–47. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Wernberg, T.; Altieri, A.; Tuya, F.; Gulbransen, D.; McGlathery, K.J.; Holmer, M.; Silliman, B.R. Habitat cascades: The conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr. Comp. Biol. 2010, 50, 158–175. [Google Scholar] [CrossRef]
- Navarro-Mayoral, S.; Fernandez-Gonzalez, V.; Otero-Ferrer, F.; Tuya, F. Spatio-temporal variability of amphipod assemblages associated with rhodolith seabeds. Mar. Freshw. Res. 2020, 71, 1–8. [Google Scholar] [CrossRef]
- Arnott, G.; Elwood, R.W. Assessment of fighting ability in animal contests. Anim. Behav. 2009, 77, 991–1004. [Google Scholar] [CrossRef]
- Steller, D.L.; Foster, M.S. Environmental factors influencing distribution and morphology of rhodoliths in Bahía Concepción, BCS, México. J. Exp. Mar. Biol. Ecol. 1995, 194, 201–212. [Google Scholar] [CrossRef]
- Riul, P.; Lacouth, P.; Pagliosa, P.R.; Christoffersen, M.L.; Horta, P.A. Rhodolith beds at the easternmost extreme of South America: Community structure of an endangered environment. Aquat. Bot. 2009, 90, 315–320. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Maneveldt, G.; Pereira-Filho, G.; Manso, R.; Bahia, R.; Barros-Barreto, M.; Guimaraes, S.M. Seaweed diversity associated with a Brazilian tropical rhodolith bed. Cienc. Mar. 2010, 36, 371–391. [Google Scholar] [CrossRef]
- Weber-Van Bosse, A.; Foslie, M. The corallinaceae of the Siboga Expedition. Leid. Bull. Rep. 1904, 61, 1–110. [Google Scholar]
- Turner, H.V.; Wolcott, D.L.; Wolcott, T.G.; Hines, A.H. Post-mating behavior, intramolt growth, and onset of migration to Chesapeake Bay spawning grounds by adult female blue crabs, Callinectes sapidus Rathbun. J. Exp. Mar. Biol. Ecol. 2003, 295, 107–130. [Google Scholar] [CrossRef]
- Aguilar, R.; Hines, A.H.; Wolcott, T.G.; Wolcott, D.L.; Kramer, M.A.; Lipcius, R.N. The timing and route of movement and migration of post-copulatory female blue crabs, Callinectes sapidus Rathbun, from the upper Chesapeake Bay. J. Exp. Mar. Biol. Ecol. 2005, 319, 117–128. [Google Scholar] [CrossRef]
- Fransen, C.H.J.M. Preliminary Report on Crustacea Collected in the Eastern Part of the North Atlantic during the CANCAP and Mauritania Expeditions of the Former Rijksmuseum van Natuurlijke Historie, Leiden; Nationaal Naturhistorisch Museum: Leiden, The Netherlands, 1991. [Google Scholar]
- Sinclair, M.E. Agonistic behavior of the stone crab, Menippe mercenaria (Say). Anim. Behav. 1977, 25, 193–207. [Google Scholar] [CrossRef]
- Silliman, B.R.; Layman, C.A.; Geyer, K.; Zieman, J.C. Predation by the black-clawed mud crab, Panopeus herbstii, in mid-Atlantic salt marshes: Further evidence for top-down control of marsh grass production. Estuaries 2004, 27, 188–196. [Google Scholar] [CrossRef]
- Brown, K.M.; Keenan, S.F.; Banks, P.D. Dominance hierarchies in xanthid crabs: Roles in resource-holding potential and field distributions. Mar. Ecol. Prog. Ser. 2005, 291, 189–196. [Google Scholar] [CrossRef]
- Thatje, S.; Hall, S. The effect of temperature on the evolution of per offspring investment in a globally distributed family of marine invertebrates (Crustacea: Decapoda: Lithodidae). Mar. Biol. 2016, 163, 48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mateo-Ramírez, Á.; García Raso, J.E. Temporal changes in the structure of the crustacean decapod assemblages associated with Cymodocea nodosa meadows from the Alboran Sea (Western Mediterranean Sea). Mar. Ecol. 2012, 33, 302–316. [Google Scholar] [CrossRef]
- Mateo-Ramírez, Á.; Urra, J.; Marina, P.; Rueda, J.L.; García Raso, J.E. Crustacean decapod assemblages associated with fragmented Posidonia oceanica meadows in the Alboran Sea (Western Mediterranean Sea): Composition, temporal dynamics and influence of meadow structure. Mar. Ecol. 2016, 37, 344–358. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).