Factors Affecting the Detection of an Imperiled and Cryptic Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Sites
2.2. Environmental Variables
2.3. Multicollinearity Assessment
2.4. Statistical Analyses
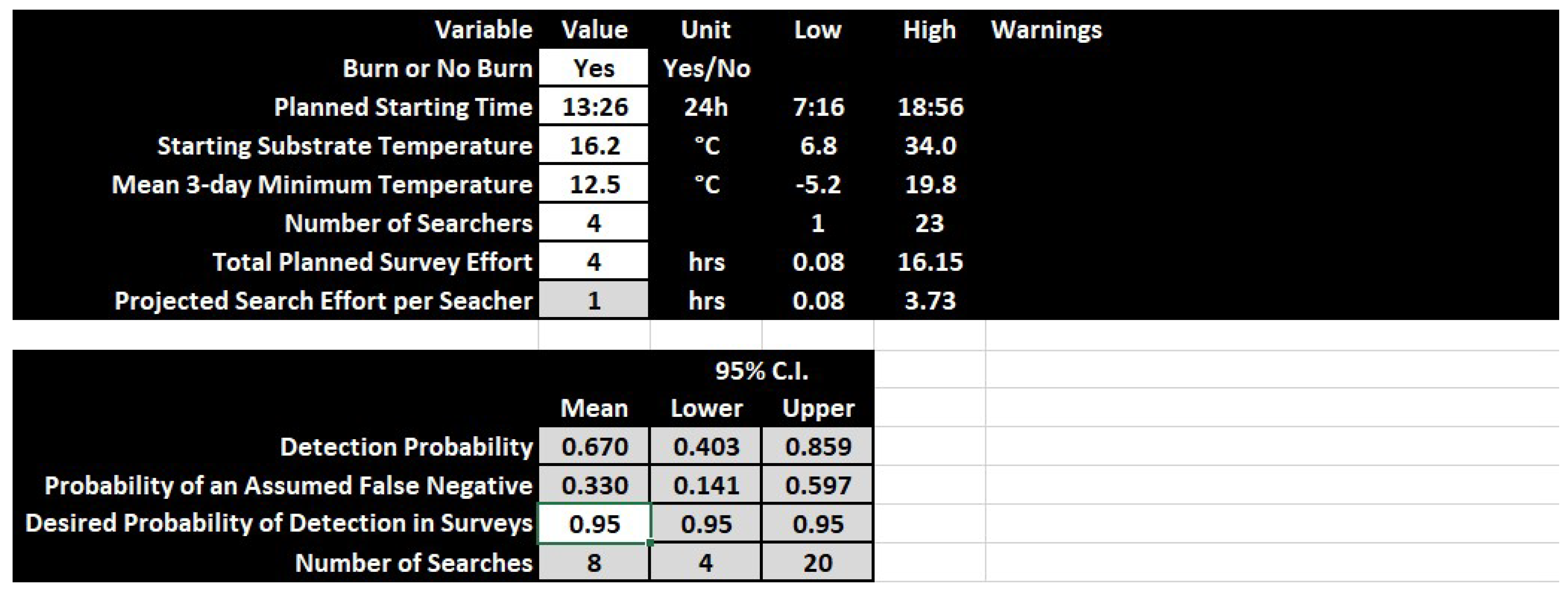
2.5. Conservation Applications
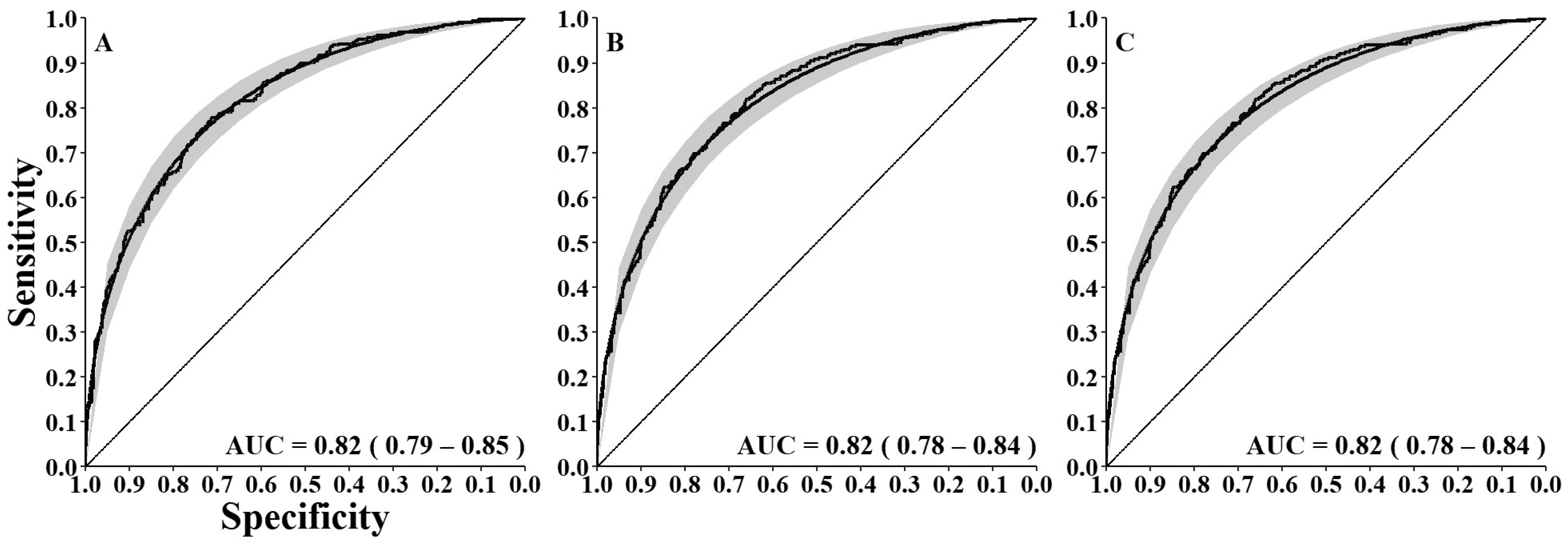
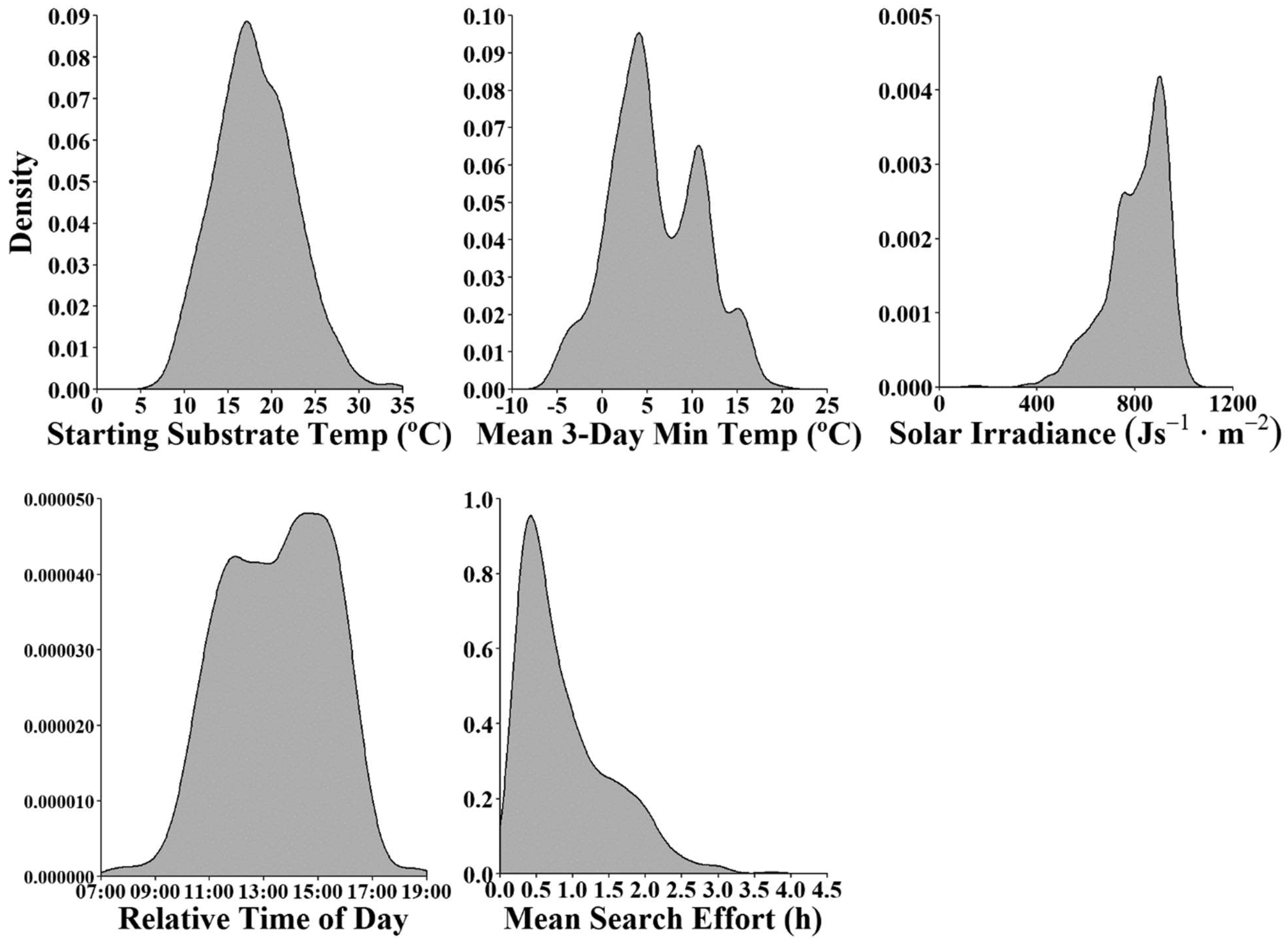
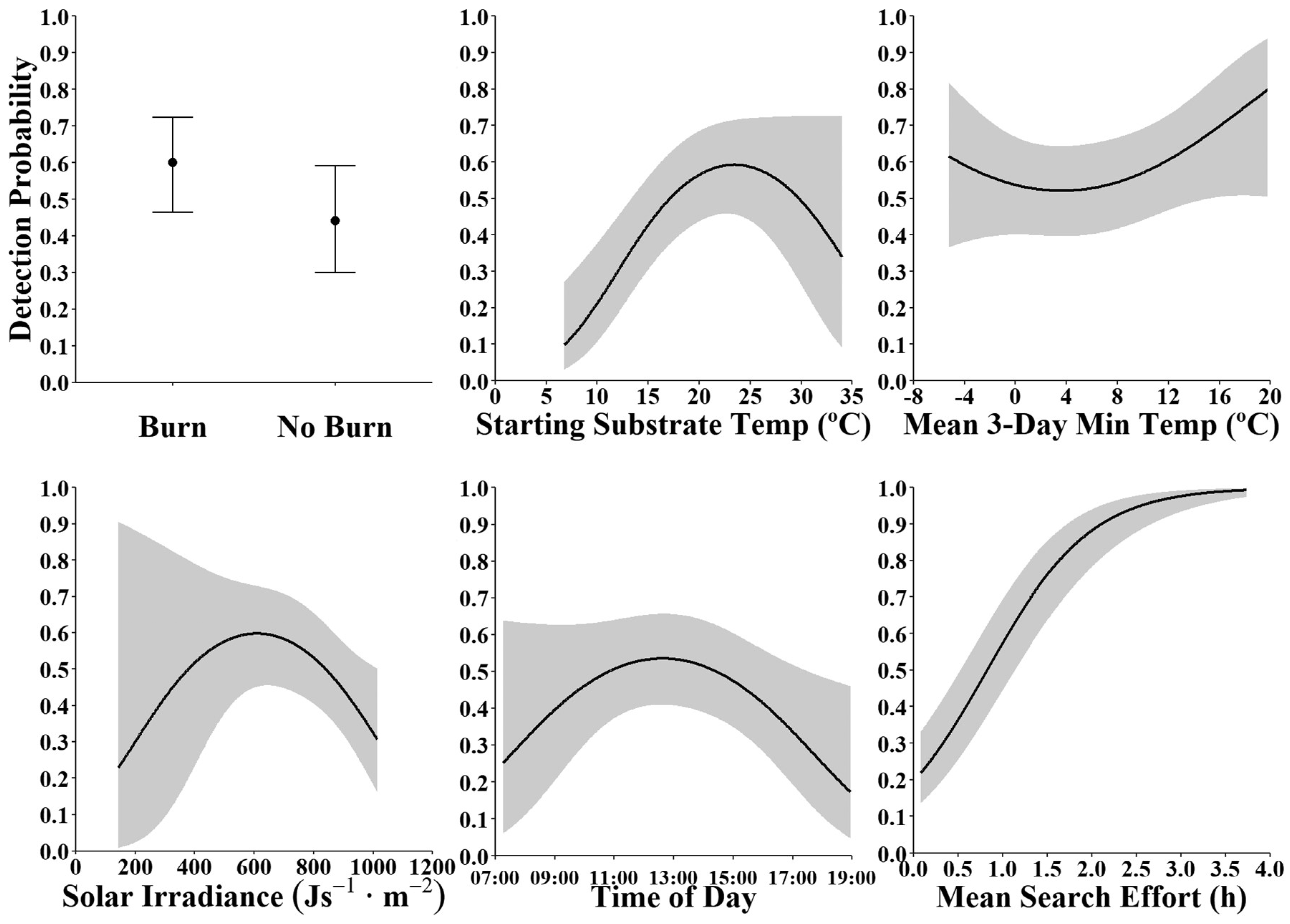
3. Results
3.1. Data Composition
3.2. Factors Affecting Detection Probabilities
3.3. Conservation Applications
4. Discussion
4.1. Modeled Effects
4.2. Conservation Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haughland, D.L.; Hero, J.M.; Schieck, J.; Castley, J.G.; Boutin, S.; Solymos, P.; Lawson, B.E.; Holloway, G.; Magnusson, W.E. Planning forwards: Biodiversity research and monitoring systems for better management. Trends Ecol. Evol. 2010, 25, 199–200. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.L. Sampling rare populations. In Sampling Rare or Elusive Species: Concepts, Designs, and Techniques for Estimating Population Parameters; Thompson, W.L., Ed.; Island Press: Washington, DC, USA, 2004; pp. 11–42. [Google Scholar]
- Rizkalla, C.E.; McCoy, E.D.; Britt, E.J.; Mushinsky, H.R. Indirect monitoring of a rare lizard: Effects of sampling intensity, season, and management practices. Herpetol. Conserv. Biol. 2015, 10, 894–903. [Google Scholar]
- Kéry, M. Inferring the absence of a species—A case study of snakes. J. Wildl. Manag. 2002, 66, 330–338. [Google Scholar] [CrossRef]
- Sewell, D.; Guillera-Arroita, G.; Griffiths, R.A.; Beebee, T.J.C. When is a species declining? Optimizing survey effort to detect population changes in reptiles. PLoS ONE 2012, 7, e43387. [Google Scholar] [CrossRef] [Green Version]
- Perkins, G.C.; Kutt, A.S.; Vanderduys, E.P.; Perry, J.J. Evaluating the costs and sampling adequacy of a vertebrate monitoring program. Aust. Zool. 2013, 36, 373–380. [Google Scholar] [CrossRef]
- Erb, L.A.; Willey, L.L.; Johnson, L.M.; Hines, J.E.; Cook, R.P. Detecting long-term population trends for an elusive reptile species. J. Wildl. Manag. 2015, 79, 1062–1071. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S. The global decline of reptiles, déjà vu amphibians. BioScience 2000, 50, 653–666. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Todd, B.; Willson, J.; Gibbons, J. The global status of reptiles and causes of their decline. In Ecotoxicology of Reptiles and Amphibians; Sparling, D., Linder, G., Bishop, C., Krest, S., Eds.; SETAC Press: Pensacola, FL, USA, 2010; pp. 48–61. [Google Scholar]
- IUCN Summary Statistics. Available online: http://www.iucnredlist.org/info/hold/facts-and-figures/summary-statistics (accessed on 6 September 2016).
- Mullin, S.J.; Seigel, R.A. Snakes: Ecology and Conservation; Cornell University Press: Ithaca, NY, USA, 2009. [Google Scholar]
- Illinois Department of Natural Resources Wildlife Action Plan. Available online: https://www.dnr.illinois.gov/conservation/IWAP/Pages/default.aspx (accessed on 12 September 2019).
- Godley, J.S. Foraging ecology of the striped swamp snake, Regina alleni, in southern Florida. Ecol. Monogr. 1980, 50, 411–436. [Google Scholar] [CrossRef]
- Dorcas, M.E.; Willson, J.D.; Reed, R.N.; Snow, R.W.; Rochford, M.; Miller, M.A.; Meshaka, W.E., Jr.; Andreadis, P.T.; Mazzotti, F.J.; Romagosa, C.M.; et al. Severe mammal declines coincide with python proliferation in Everglades National Park. Proc. Nat. Acad. Sci. USA 2012, 109, 2418–2422. [Google Scholar] [CrossRef] [Green Version]
- Willson, J.D.; Winne, C.T. Evaluating the functional importance of secretive species: A case study of aquatic snake predators in isolated wetlands. J. Zool. 2016, 29, 266–273. [Google Scholar] [CrossRef]
- Zipkin, E.F.; DiRenzo, G.V.; Ray, J.M.; Rossman, S.; Lips, K.R. Tropical snake diversity collapses after widespread amphibian loss. Science 2020, 367, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.J.; Luiselli, L.M.; Akani, G.C.; Bonnet, X.; Amori, G.; Ballouard, J.M.; Filippi, E.; Naulleau, G.; Pearson, D.; Rugiero, L. Are snake populations in widespread decline? Biol. Lett. 2010, 23, 777–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, W.S.; Plummer, M.V. Population ecology. In Snakes: Ecology and Evolutionary Biology; Seigel, R.A., Collins, J.T., Novak, S.S., Eds.; The Blackburn Press: Caldwell, NJ, USA, 1987; pp. 253–301. [Google Scholar]
- Steen, D.A. Snakes in the grass: Secretive natural histories defy both conventional and progressive statistics. Herpetol. Conserv. Biol. 2010, 5, 183–188. [Google Scholar]
- Durso, A.M.; Willson, J.D.; Winne, C.T. Needles in haystacks: Estimating detection probability and occupancy of rare and cryptic snakes. Biol. Conserv. 2011, 144, 1508–1515. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Peterson, C.R.; Kingsbury, B.A. Modeling snake distribution and habitat. In Snakes: Ecology and Conservation; Mullin, S.J., Seigel, R.A., Eds.; Cornell University Press: Ithaca, NY, USA, 2009; pp. 123–148. [Google Scholar]
- Böhm, M.; Collen, B.; Baillie, J.; Bowles, P.; Chanson, J.; Cox, N.; Hammerson, G.; Hoffmann, M.; Livingstone, S.; Ram, M.; et al. The conservation status of the world’s reptiles. Biol. Conserv. 2013, 157, 372–385. [Google Scholar]
- MacKenzie, D.I.; Nichols, J.D.; Sutton, N.; Kawanishi, K.; Bailey, L.L. Improving inferences in population studies of rare species that are detected imperfectly. Ecology 2005, 86, 1101–1113. [Google Scholar] [CrossRef] [Green Version]
- Mazerolle, M.J.; Bailey, L.L.; Kendall, W.L.; Royle, J.A.; Converse, S.J.; Nichols, J.D. Making great leaps forward: Accounting for detectability in herpetological field studies. J. Herpetol. 2007, 41, 672–689. [Google Scholar] [CrossRef]
- Kellner, K.F.; Swihart, R.K. Accounting for imperfect detection in ecology: A quantitative review. PLoS ONE 2014, 9, e111436. [Google Scholar] [CrossRef] [Green Version]
- Durso, A.M.; Seigel, R.A. A snake in the hand is worth 10,000 in the bush. J. Herpetol. 2015, 49, 503–506. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Royle, J.A. Designing occupancy studies: General advice and allocating survey effort. J. Appl. Ecol. 2005, 42, 1105–1114. [Google Scholar] [CrossRef]
- Ernst, C.H.; Ernst, E.M. Snakes of the United States and Canada; Smithsonian Institution: Washington, DC, USA, 2003; p. 668. [Google Scholar]
- Species Status Assessment for the Eastern Massasauga Rattlesnake (Sistrurus catenatus). Available online: https://www.scienceapplications.org/pluginfile.php/375/block_html/content/SSA_Eastern%20MassasaugaRattlesnake_201607.pdf (accessed on 23 June 2015).
- U.S. Fish and Wildlife Service. Endangered and threatened wildlife and plants: Threatened species status for the eastern massasauga rattlesnake. Fed. Regist. 2015, 80, 58688–58701. [Google Scholar]
- U.S. Fish and Wildlife Service. Endangered and threatened wildlife and plants; threatened species status for the eastern massasauga rattlesnake. Fed. Regist. 2016, 81, 67193–67214. [Google Scholar]
- Species at Risk Public Registry: Massasauga Species Profile. Available online: http://www.sararegistry.gc.ca/species/speciesDetails_e.cfm?sid=277 (accessed on 6 September 2016).
- Shepard, D.B.; Dreslik, M.J.; Jellen, B.C.; Phillips, C.A. Reptile road mortality around an oasis in the Illinois corn desert with emphasis on the endangered eastern massasauga. Copeia 2008, 2008, 350–359. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics., 4th ed.; Springer: New York, NY, USA, 2002; p. 498. [Google Scholar]
- R: A Language and Environment for Statistical Computing. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 10 February 2015).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 355. [Google Scholar]
- AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). Available online: https://cran.r-project.org/web/packages/AICcmodavg/index.html (accessed on 10 February 2015).
- Fox, J. Effect displays in R for generalised linear models. J. Stat. Soft. 2003, 8, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Hong, J. Effect displays in R for multinomial and proportional-odds logit models: Extensions to the effects package. J. Stat. Soft. 2009, 32, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Fitch, H.S. Methods of sampling snake populations and their relative success. Herpetol. Rev. 1992, 23, 17–19. [Google Scholar]
- Ryan, T.J.; Philippi, T.; Leiden, Y.A.; Dorcas, M.E.; Wigley, T.B.; Gibbons, J.W. Monitoring herpetofauna in a managed forest landscape: Effects of habitat types and census techniques. For. Ecol. Manag. 2002, 167, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Bartman, J.F.; Kudla, K.; Bradke, D.R.; Otieno, S.; Moore, J.A. Work smarter not harder: Comparison of visual and trap survey methods for the eastern Massasauga rattlesnake (Sistrurus catenatus). Herp. Cons. Biol. 2016, 11, 451–458. [Google Scholar]
- Durbian, F.F. Effects of mowing and summer burning on the massasauga (Sistrurus catenatus). Am. Midl. Nat. 2006, 155, 329–334. [Google Scholar] [CrossRef]
- Cross, M.D.; Root, K.V.; Mehne, C.J.; McGowan-Stinski, J.; Pearsall, D.; Gillinham, J.C. Multi-scale responses of eastern Massasauga rattlesnakes (Sistrurus catenatus) to prescribed fire. Am. Midl. Nat. 2016, 173, 346–362. [Google Scholar] [CrossRef]
- Harvey, D.S. Detectability of a large-bodied snake (Sistrurus c. catenatus) by time-constrained searching. Herpetol. Rev. 2005, 36, 413–415. [Google Scholar]
- Willson, J.D.; Winne, C.T.; Todd, B.D. Ecological and methodological factors affecting detectability and population estimation in elusive species. J. Wildl. Manag. 2011, 75, 36–45. [Google Scholar] [CrossRef]
- Hoare, J.M.; O’Donnell, C.F.J.; Westbrooke, I.; Hodapp, D.; Lettink, M. Optimising the sampling of skinks using artificial retreats based on weather conditions and time of day. Appl. Herpetol. 2009, 6, 379–390. [Google Scholar]
- Lewis, S.A.; Gould, W.R. Survey effort effects on power to detect trends in raptor migration counts. Wildl. Soc. Bull. 2000, 28, 317–329. [Google Scholar]
- Shirose, L.J.; Bishop, C.A.; Green, D.M.; MacDonald, C.J.; Brooks, R.J.; Helferty, N.J. Validation tests of an amphibian call count survey technique in Ontario, Canada. Herpetologica 1997, 53, 312–320. [Google Scholar]
- Tanadini, L.G.; Schmidt, B.R. Population size influences amphibian detection probability: Implications for biodiversity monitoring programs. PLoS ONE 2011, 6, e28244. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Royle, J.A.; Langtimm, C.A. Estimating site occupancy rates when detection probabilities are less than one. Ecology 2002, 83, 2249–2255. [Google Scholar] [CrossRef]
- Christy, M.T.; Adam, A.A.Y.; Rodda, G.H.; Savidge, J.A.; Tyrell, C. Modeling detection probability to evaluate management and control tolls for an invasive species. J. Appl. Ecol. 2010, 47, 106–113. [Google Scholar] [CrossRef]
- Steen, D.A.; McClure, C.J.W.; Brock, J.C.; Rudolph, D.C.; Pierce, J.B.; Lee, J.R.; Humphries, W.J.; Gregory, B.B.; Sutton, W.B.; Smith, L.L.; et al. Landscape-level influences of terrestrial snake occupancy within the southeastern United States. Ecol. Appl. 2012, 22, 1084–1097. [Google Scholar] [CrossRef] [Green Version]
- Bailey, L.L.; Simons, T.R.; Pollock, K.H. Estimating site occupancy and species detection probability parameters for terrestrial salamanders. Ecol. Appl. 2004, 14, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Edwards, E.; Pauley, T.K.; Waldron, J.L. Estimating spring salamander detection probability using multiple models. J. Herpetol. 2016, 50, 126–129. [Google Scholar] [CrossRef]
- Ernst, C.H.; Ernst, E.M. Venomous reptiles of the United States, Canada, and Northern Mexico; Volume 1 Heloderma, Micruroides, Micrurus, Pelamis, Agkistrodon, Sistrurus; The Johns Hopkins University Press: Baltimore, MD, USA, 2011; p. 352. [Google Scholar]
- Shaffer, S.A.; Roloff, G.J.; Campa III, H. Survey methodology for detecting Massasauga rattlesnakes in southern Michigan. Wildl. Soc. Bull. 2019, 43, 508–514. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [Green Version]
| Variable | Description | Unit |
|---|---|---|
| dSICA* | Presence or absence of an Eastern Massasauga in a search | binary |
| rTime* | Time calculated as the relative time of day on a 24-hour basis (hour + minute/60 + second/360)/24 | h |
| DayofYear | Day of the year the search was conducted | count |
| sArea | The total area of grassland that habitat searches were conducted in/patch size | ha |
| Burn | Whether or not a burn was conducted in the search area | binary |
| nSearch* | Number of searchers per search | count |
| mEffort* | Mean effort per searcher (tEffort/nSearch) | min |
| tEffort* | Total search effort per search (cumulative time per searcher) | min |
| Precip | Precipitation for the day of searching | cm |
| daRad* | Historic solar irradiance data | J/s·m2 |
| sHum* | Relative humidity at the ground level as the start of searching | % |
| eHum* | Relative humidity at the ground level as the end of searching | % |
| sWind | Wind speed ~1.5–2 m above ground level at the start of searching | m/s |
| eWind | Wind speed ~1.5–2 m above ground level at the end of searching | m/s |
| sSAT* | Shaded air temperature at the start of searching | °C |
| eSAT* | Shaded air temperature at the end of searching | °C |
| dSAT* | Change in shaded air temperature during searching (sSAT − eSAT) | °C |
| sSUB* | Substrate temperature ~2 cm in the substrate at the start of searching | °C |
| eSUB* | Substrate temperature at the end of searching | °C |
| dSUB* | Change in substrate temperature during searching (sSUB − eSUB) | °C |
| pMean* | Mean temperature for the previous day | °C |
| pMax* | Maximum temperature for the previous day | °C |
| pMin* | Minimum temperature for the previous day | °C |
| p3Mean* | Average mean temperature for the previous three days | °C |
| p3Max* | Average maximum temperature for the previous three days | °C |
| p3Min* | Average minimum temperature for the previous three days | °C |
| Model | Variables | Description |
|---|---|---|
| Global | All Variables | Global additive |
| Null | Intercept | Intercept only |
| 3DayTemps | m3Min, m3Max, m3Mean | Recent temperature |
| Burn | Burn | Habitat management |
| Calendar | rTime, DayofYear | Seasonality |
| EvapCooling | sHUM, sWind, sSAT, Precip, daRad | Evaporative cooling |
| m3Mean + daRad | m3Mean, daRad | Recent mean temps |
| m3Mean + Precip | m3Mean, Precip | Recent mean temps and precipitation |
| m3Min + daRad | m3Min, daRad | Recent min temps |
| m3Min + Precip | m3Min, Precip | Recent min temps and precipitation |
| tEffort + Burn | tEffort, Burn | Search effort and management |
| tEffort + sArea | tEffort, sArea | Search effort and area |
| tEffort + sArea + Burn | tEffort, sArea, Burn | Effort, area, and management |
| PDayTemps | pMin, pMax, pMean | Previous days temps |
| pMean + daRad | pMean, daRad | Previous mean and radiation |
| pMean + Precip | pMean, Precip | Previous mean temp and precipitation |
| pMin + daRad | pMin, daRad | Previous min and radiation |
| pMin + Precip | pMin, Precip | Previous min and precipitation |
| ProximateTemps | sSUB, sSAT | Proximate temps |
| SearchEffort | tEffort, nSearch, sArea | Total effort |
| sSUB + Burn | sSUB, Burn | Substrate and management |
| sSUB + daRad | sSUB, daRad | Substrate and radiation |
| sSUB + Precip | sSUB, Precip | Substrate and precipitation |
| sSUB + sArea | sSUB, sArea | Substrate and area |
| sSUB + sArea + Burn | sSUB, sArea, Burn | Substrate, area, and management |
| post hoc Additive | Burn, sSUB, m3Min, rTime, mEffort, daRad | post hoc |
| Manager Additive | Burn, sSUB, m3Min, rTime, mEffort | Manager variables |
| Rank | Model | K | −2LL | AICC | ΔAICC | ωi | r2marg | r2cond |
|---|---|---|---|---|---|---|---|---|
| 1 | post hoc | 13 | −399.65 | 825.82 | 0.00 | 0.83 | 0.28 | 0.40 |
| 2 | Manager | 11 | −403.31 | 828.99 | 3.17 | 0.17 | 0.27 | 0.39 |
| 3 | Global | 30 | −389.07 | 840.87 | 15.05 | 0.00 | 0.33 | 0.47 |
| 4 | tEffort + Burn | 5 | −416.62 | 843.33 | 17.51 | 0.00 | 0.25 | 0.34 |
| 5 | tEffort + sArea + Burn | 6 | −415.90 | 843.92 | 18.10 | 0.00 | 0.25 | 0.35 |
| 6 | SearchEffortAdd | 6 | −418.55 | 849.21 | 23.39 | 0.00 | 0.23 | 0.35 |
| 7 | tEffort + sArea | 5 | −420.73 | 851.55 | 25.72 | 0.00 | 0.22 | 0.33 |
| 8 | Calander | 7 | −444.23 | 902.62 | 76.79 | 0.00 | 0.11 | 0.33 |
| 9 | sSUB + sArea + Burn | 7 | −450.72 | 915.61 | 89.78 | 0.00 | 0.08 | 0.31 |
| 10 | sSUB + Burn | 6 | −452.62 | 917.36 | 91.54 | 0.00 | 0.06 | 0.29 |
| 11 | m3Min + daRad | 6 | −453.60 | 919.32 | 93.50 | 0.00 | 0.03 | 0.28 |
| 12 | sSUB + daRad | 7 | −452.58 | 919.33 | 93.50 | 0.00 | 0.04 | 0.31 |
| 13 | sSUB + Precip | 6 | −453.84 | 919.81 | 93.98 | 0.00 | 0.03 | 0.29 |
| 14 | 3DayTemps | 9 | −450.85 | 919.96 | 94.14 | 0.00 | 0.04 | 0.29 |
| 15 | m3Min + Precip | 7 | −452.94 | 920.03 | 94.21 | 0.00 | 0.04 | 0.29 |
| 16 | sSUB + sArea | 6 | −454.19 | 920.50 | 94.68 | 0.00 | 0.03 | 0.29 |
| 17 | m3Mean + daRad | 7 | −454.20 | 922.56 | 96.73 | 0.00 | 0.03 | 0.28 |
| 18 | pMin + Precip | 6 | −455.34 | 922.79 | 96.97 | 0.00 | 0.02 | 0.26 |
| 19 | ProximateTemps | 7 | −454.74 | 923.65 | 97.82 | 0.00 | 0.03 | 0.28 |
| 20 | m3Mean + Precip | 6 | −455.87 | 923.86 | 98.03 | 0.00 | 0.02 | 0.26 |
| 21 | pMin + daRad | 7 | −455.15 | 924.45 | 98.63 | 0.00 | 0.03 | 0.27 |
| 22 | Burn | 4 | −458.28 | 924.62 | 98.80 | 0.00 | 0.03 | 0.25 |
| 23 | pMean + Precip | 6 | −456.38 | 924.87 | 99.05 | 0.00 | 0.02 | 0.25 |
| 24 | PDayTemps | 9 | −453.71 | 925.67 | 99.85 | 0.00 | 0.03 | 0.27 |
| 25 | pMean + daRad | 7 | −456.24 | 926.64 | 100.82 | 0.00 | 0.02 | 0.26 |
| 26 | Null | 3 | −461.16 | 928.36 | 102.53 | 0.00 | 0.00 | 0.24 |
| 27 | EvapCooling | 10 | −457.74 | 935.79 | 109.96 | 0.00 | 0.01 | 0.26 |
| Variable | Mean | SD | Min | Max | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| sSUB | 18.12 | 4.52 | −0.10 | 34.00 | 17.79 | 18.45 |
| daRad | 806.34 | 121.91 | 144.00 | 1014.00 | 797.35 | 815.25 |
| m3Min | 5.84 | 4.98 | −7.20 | 19.80 | 5.46 | 6.19 |
| rTime | 0.56 | 0.08 | 0.30 | 0.79 | 0.56 | 0.57 |
| mEffort | 53.19 | 37.38 | 5.00 | 224.00 | 50.41 | 55.90 |
| Parameter | post hoc 95% CI | Manager 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Lower | Upper | Estimate | SE | Lower | Upper | |
| Intercept | 0.40 | 0.28 | −0.15 | 0.96 | 0.31 | 0.27 | −0.22 | 0.85 |
| Burn | −0.65 | 0.28 | −1.19 | −0.10 | −0.65 | 0.28 | −1.19 | −0.11 |
| sSUB | 0.47 | 0.12 | 0.22 | 0.71 | 0.31 | 0.27 | −0.22 | 0.85 |
| sSUB2 | −0.20 | 0.07 | −0.34 | −0.06 | −0.65 | 0.28 | −1.19 | −0.11 |
| daRad | −0.36 | 0.13 | −0.62 | −0.10 | - | - | - | - |
| daRad2 | −0.11 | 0.07 | −0.25 | 0.03 | - | - | - | - |
| m3Min | 0.11 | 0.11 | −0.11 | 0.32 | 0.31 | 0.27 | −0.22 | 0.85 |
| m3Min2 | 0.12 | 0.09 | −0.05 | 0.30 | −0.65 | 0.28 | −1.19 | −0.11 |
| rTime | −0.14 | 0.11 | −0.35 | 0.07 | 0.31 | 0.27 | −0.22 | 0.85 |
| rTime2 | −0.16 | 0.08 | −0.32 | 0.00 | −0.65 | 0.28 | −1.19 | −0.11 |
| mEffort | 1.07 | 0.13 | 0.81 | 1.33 | 0.31 | 0.27 | −0.22 | 0.85 |
| Year | Probability Missed but Present | Odds Missed but Present | ||||
|---|---|---|---|---|---|---|
| Mean | Lower | Upper | Mean | Lower | Upper | |
| 2008 | 2.54 × 10−28 | 1.29 × 10−16 | 1.46 × 10−50 | 1:3.94 × 1027 | 1:7.77 × 1015 | 1:6.84 × 1049 |
| 2009 | 2.56 × 10−5 | 4.40 × 10−4 | 7.92 × 10−11 | 1:3.91 × 104 | 1:2.27 × 103 | 1:1.26 × 1010 |
| 2010 | 5.23 × 10−6 | 1.74 × 10−3 | 6.88 × 10−9 | 1:1.91 × 105 | 1:5.73 × 102 | 1:1.45 × 108 |
| 2015 | 1.80 × 10−8 | 3.95 × 10−4 | 1.46 × 10−15 | 1:5.57 × 107 | 1:2.53 × 103 | 1:6.84 × 1014 |
| Overall | 6.09 × 10−46 | 3.90 × 10−26 | 1.17 × 10−83 | 1:1.64 × 1045 | 1:2.57 × 1025 | 1:8.58 × 1082 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, J.A.; Dreslik, M.J.; Baker, S.J.; Phillips, C.A.; Peterman, W.E. Factors Affecting the Detection of an Imperiled and Cryptic Species. Diversity 2020, 12, 177. https://doi.org/10.3390/d12050177
Crawford JA, Dreslik MJ, Baker SJ, Phillips CA, Peterman WE. Factors Affecting the Detection of an Imperiled and Cryptic Species. Diversity. 2020; 12(5):177. https://doi.org/10.3390/d12050177
Chicago/Turabian StyleCrawford, John A., Michael J. Dreslik, Sarah J. Baker, Christopher A. Phillips, and William E. Peterman. 2020. "Factors Affecting the Detection of an Imperiled and Cryptic Species" Diversity 12, no. 5: 177. https://doi.org/10.3390/d12050177
APA StyleCrawford, J. A., Dreslik, M. J., Baker, S. J., Phillips, C. A., & Peterman, W. E. (2020). Factors Affecting the Detection of an Imperiled and Cryptic Species. Diversity, 12(5), 177. https://doi.org/10.3390/d12050177





