Niche Models Differentiate Potential Impacts of Two Aquatic Invasive Plant Species on Native Macrophytes
Abstract
1. Introduction
2. Materials and Methods
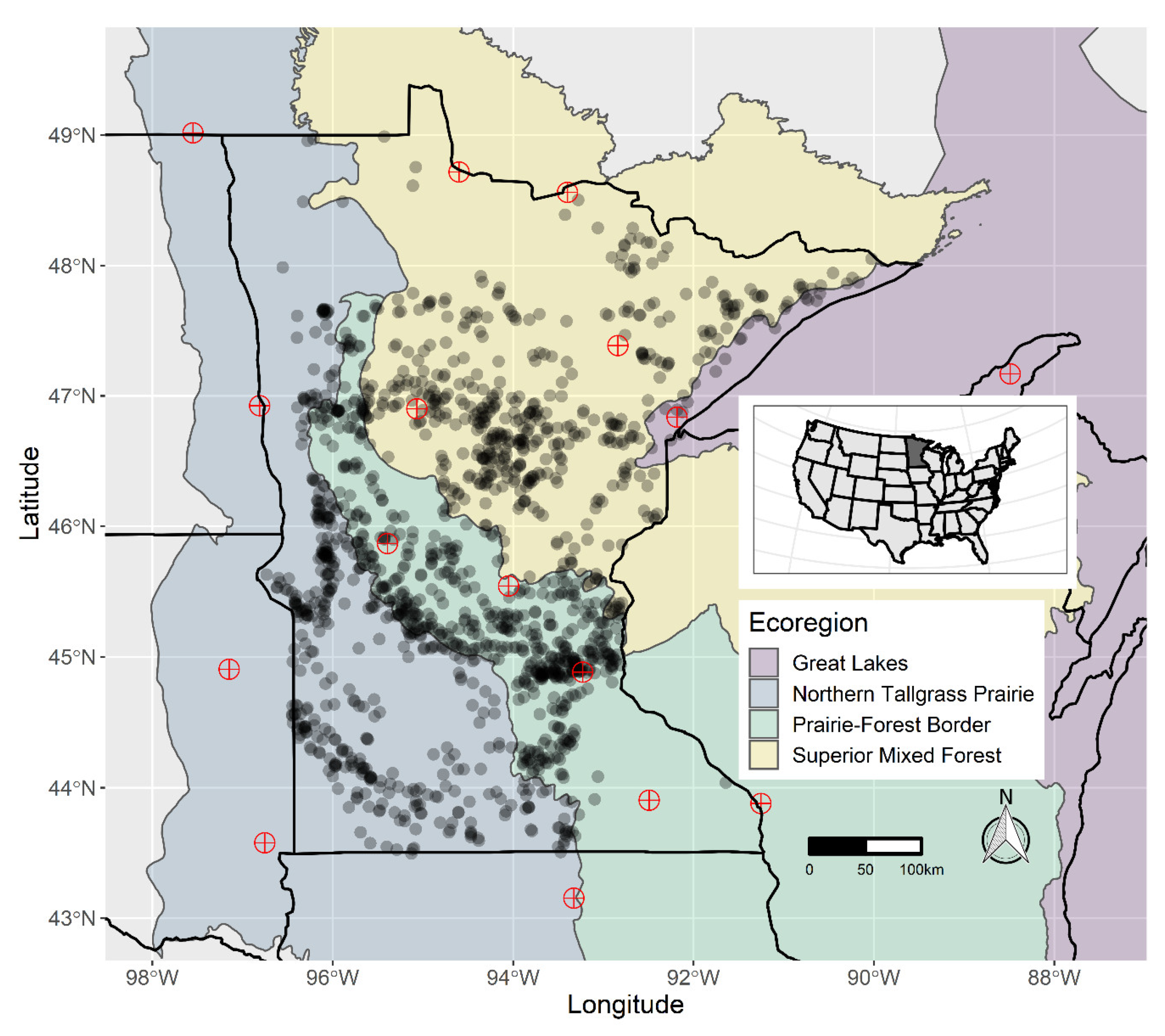
2.1. Macrophyte Occurrence Data
2.2. Environmental Data
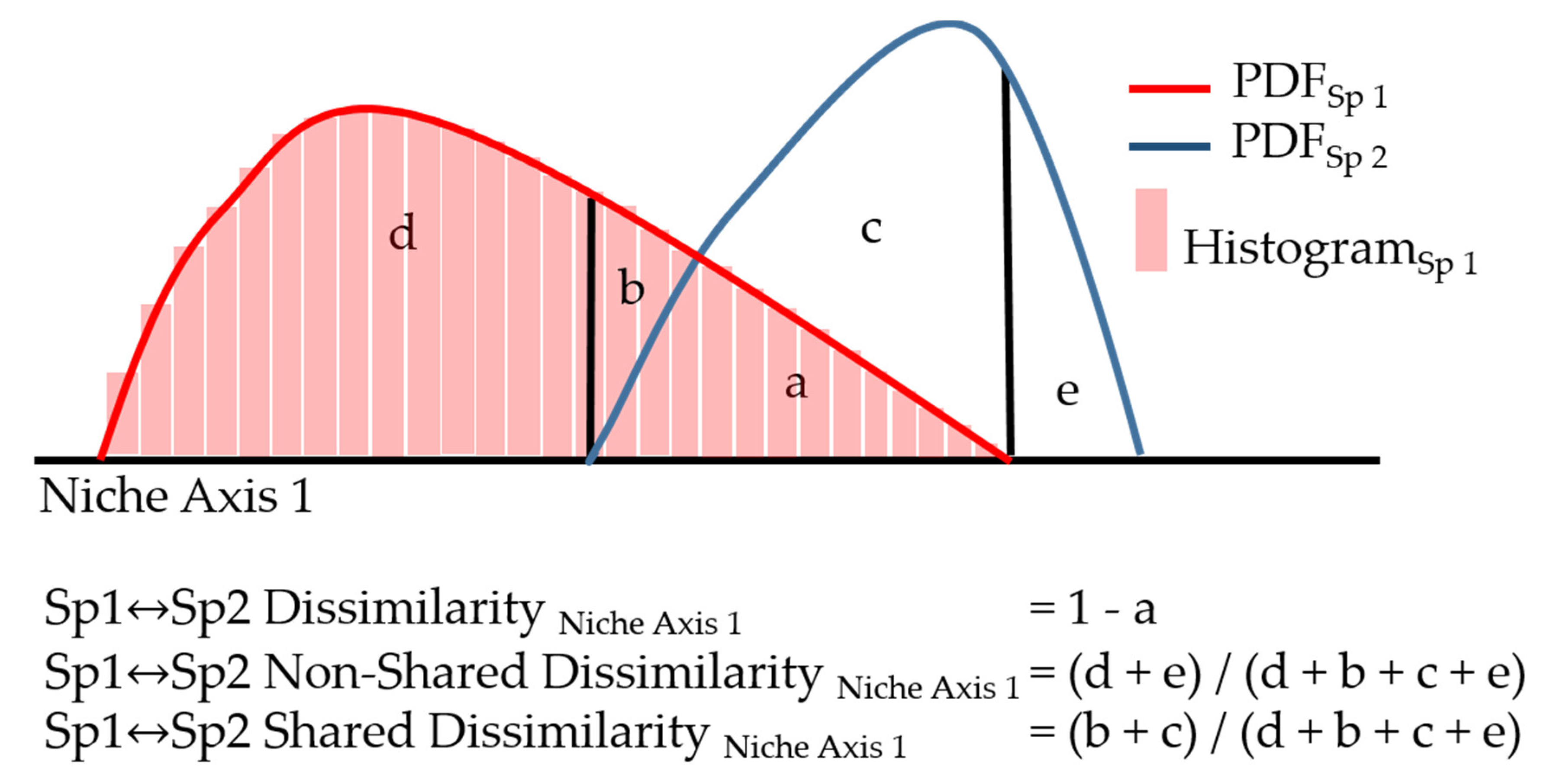
2.3. Data Analysis
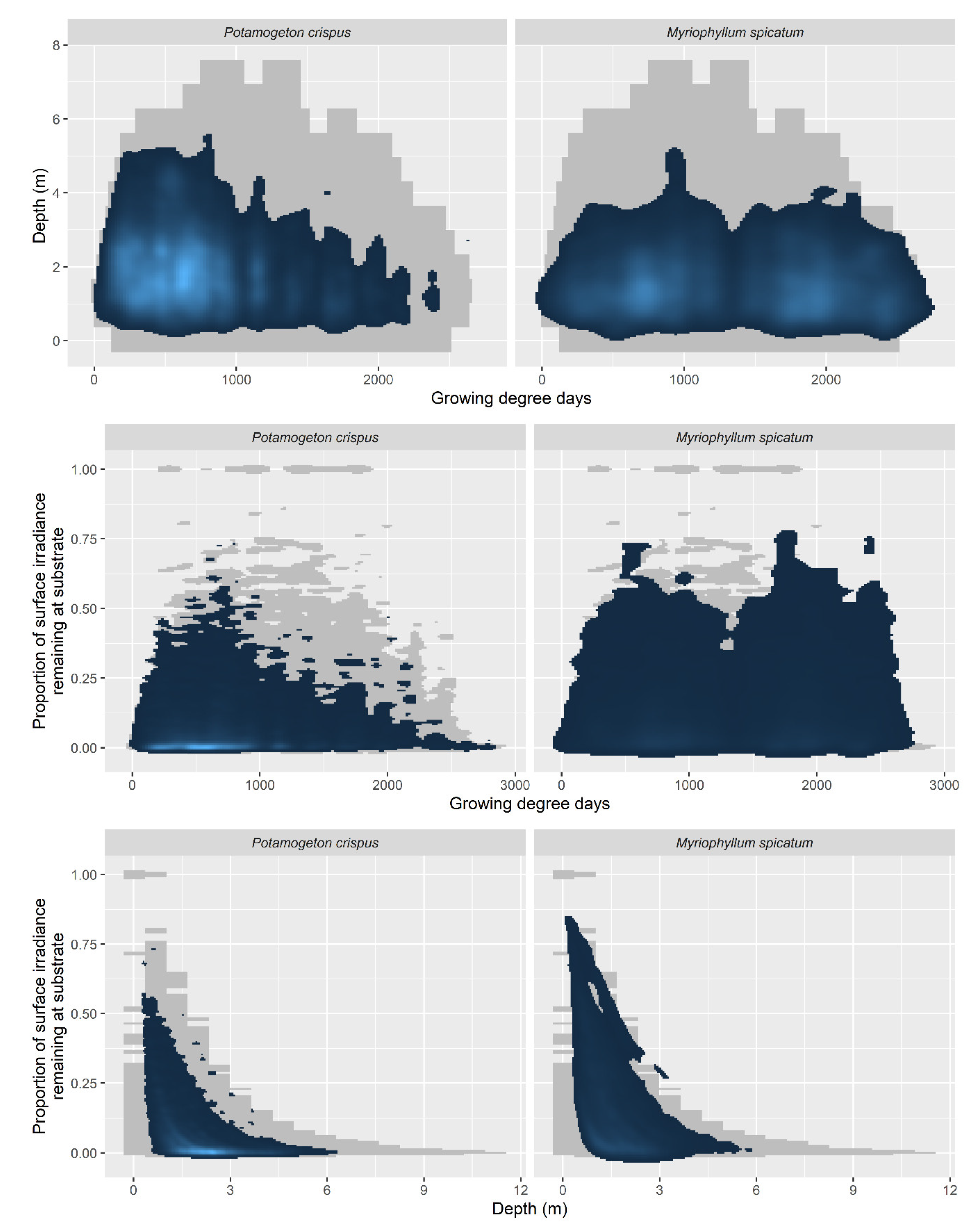
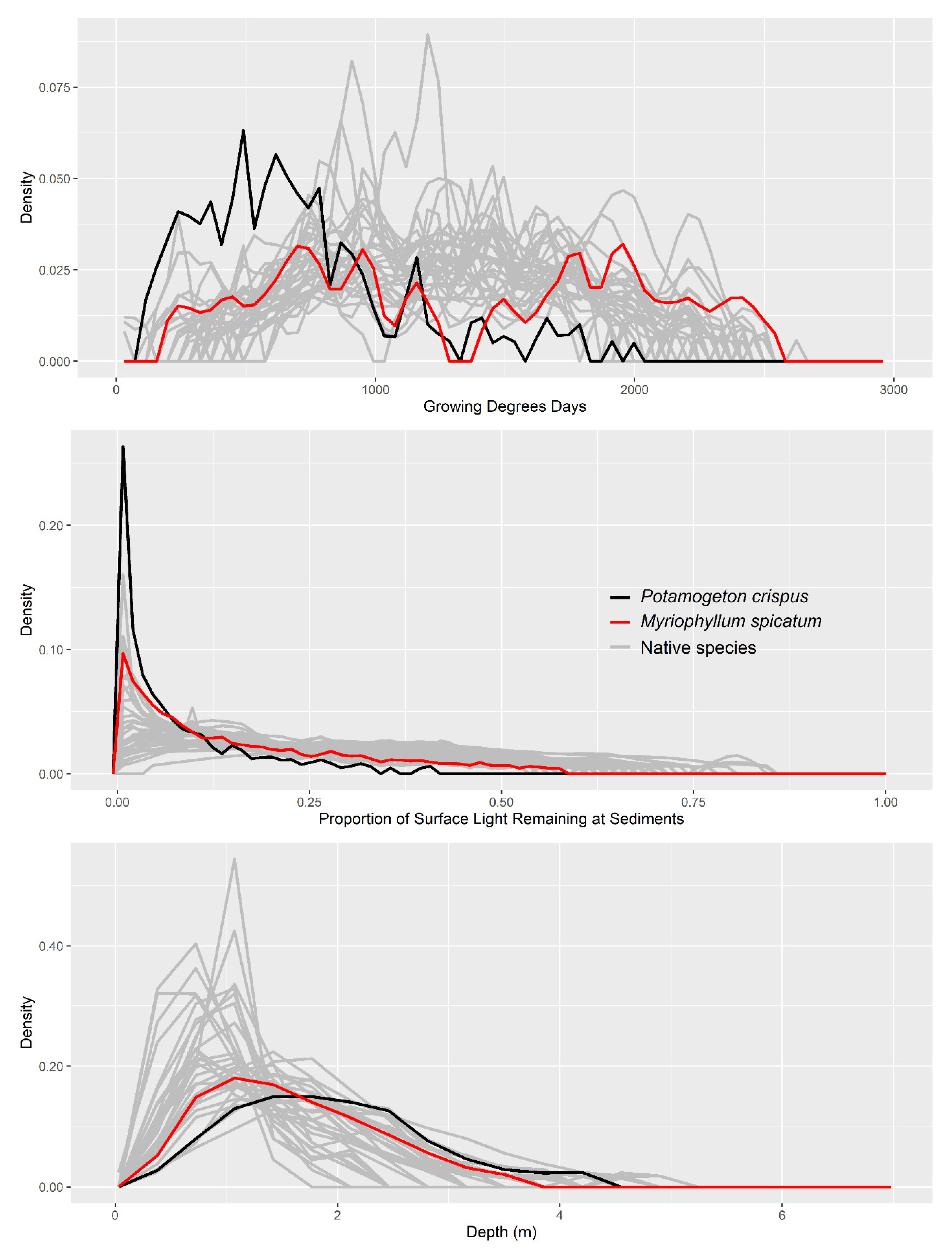
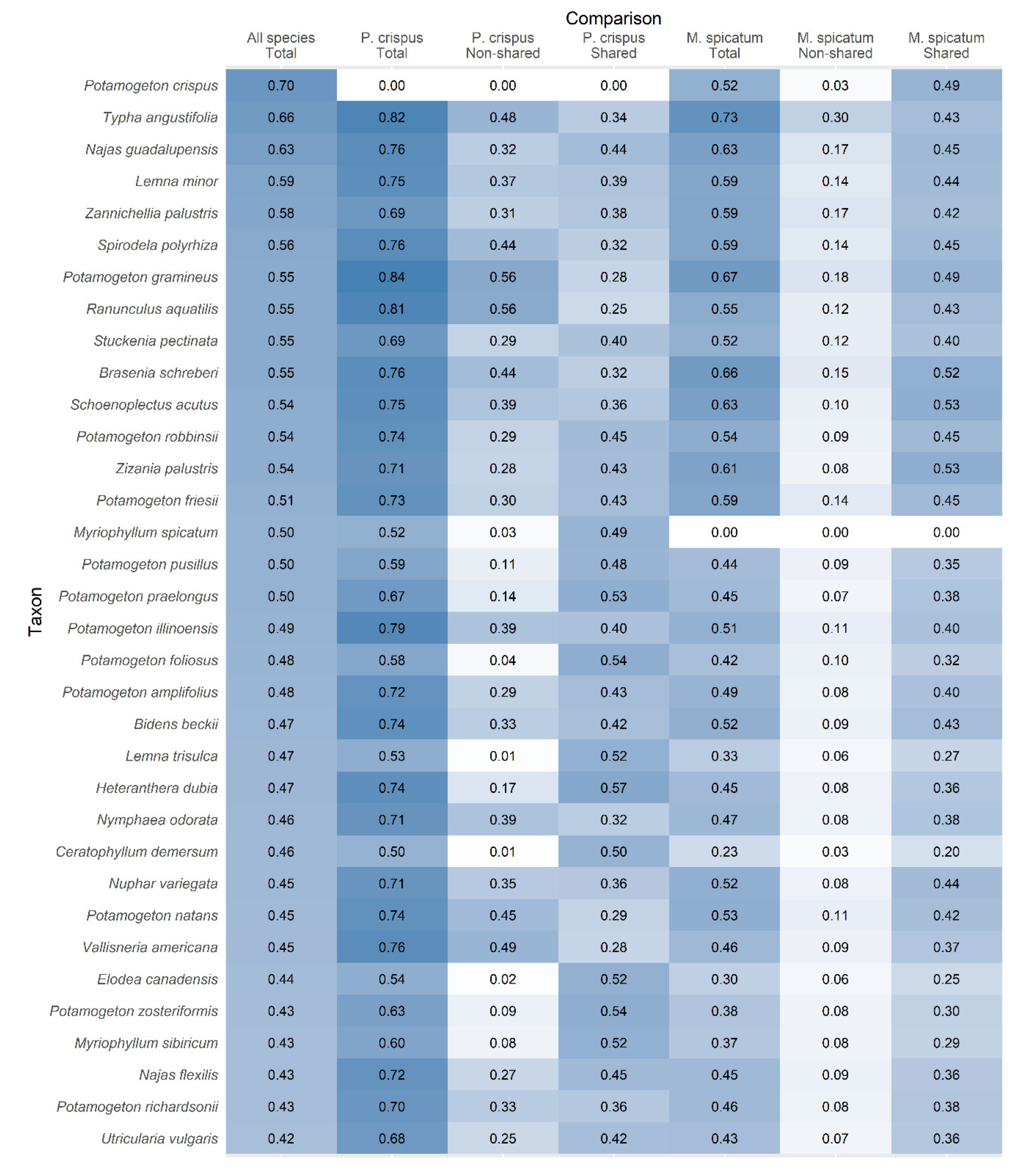
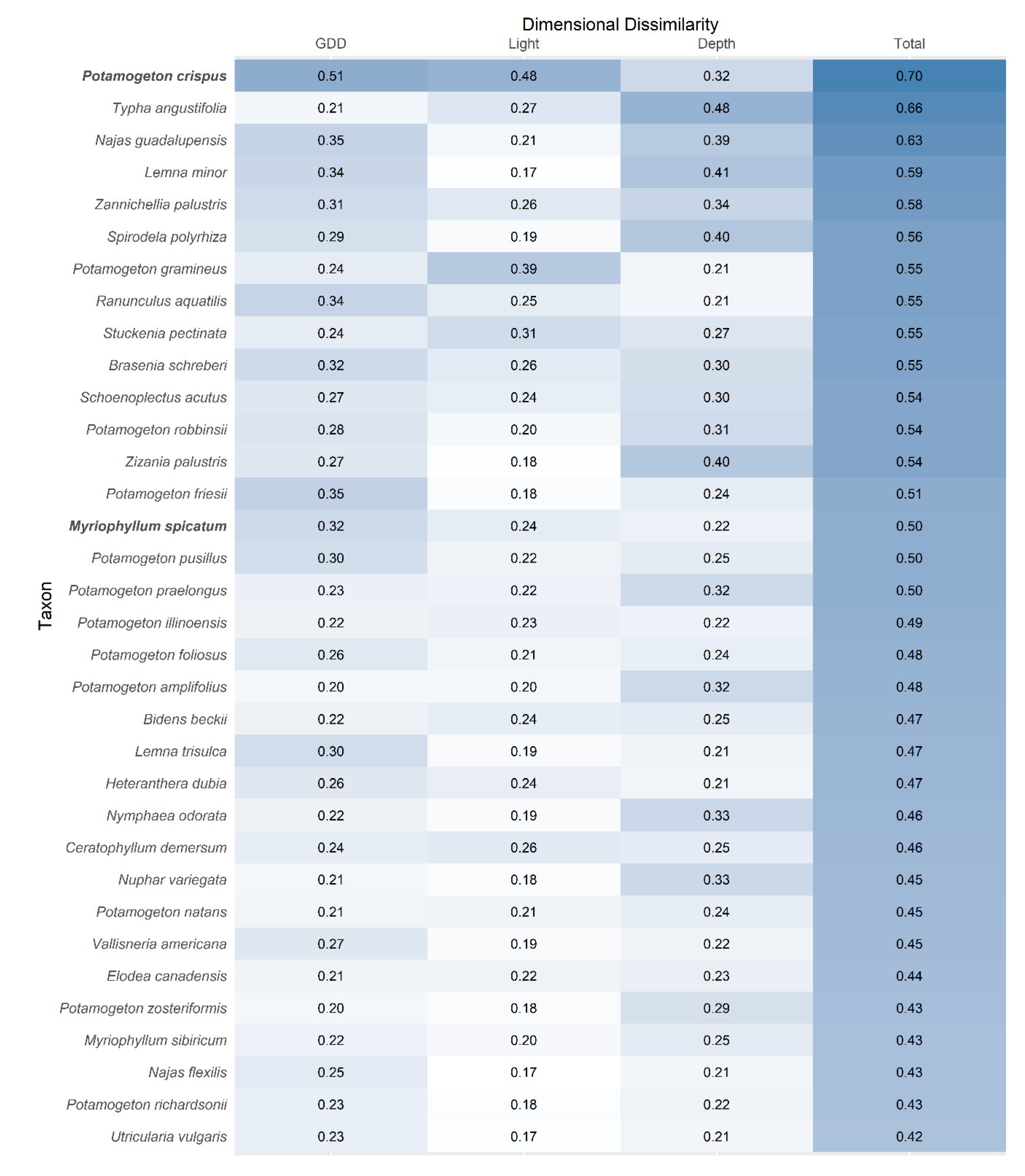
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Courchamp, F.; Fournier, A.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Jeschke, J.M.; Russell, J.C. Invasion biology: Specific problems and possible solutions. Trends Ecol. Evol. 2017, 32, 13–22. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Bradley, B.A.; Laginhas, B.B.; Whitlock, R.; Allen, J.M.; Bates, A.E.; Bernatchez, G.; Diez, J.M.; Early, R.; Lenoir, J.; Vilà, M.; et al. Disentangling the abundance-impact relationship for invasive species. Proc. Natl. Acad. Sci. USA 2019, 116, 9919–9924. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.T.; Flores-Moreno, H.; Bonser, S.P.; Warton, D.I.; Helm, A.; Warman, L.; Eldridge, D.J.; Jurado, E.; Hemmings, F.A.; Reich, P.B.; et al. Invasions: The trail behind, the path ahead, and a test of a disturbing idea. J. Ecol. 2012, 100, 116–127. [Google Scholar] [CrossRef]
- Boltovskoy, D.; Sylvester, F.; Paolucci, E.M. Invasive species denialism: Sorting out facts, beliefs, and definitions. Ecol. Evol. 2018, 8, 11190–11198. [Google Scholar] [CrossRef]
- Schirmel, J.; Bundschuh, M.; Entling, M.H.; Kowarik, I.; Buchholz, S. Impacts of invasive plants on resident animals across ecosystems, taxa, and feeding types: A global assessment. Glob. Chang. Biol. 2016, 22, 594–603. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Harpole, W.S.; Reichman, O.J.; Tilman, D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc. Natl. Acad. Sci. USA 2003, 100, 13384–13389. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.P.; Dibble, E.D. Ecological mechanisms of invasion success in aquatic macrophytes. Hydrobiologia 2015, 746, 23–37. [Google Scholar] [CrossRef]
- Levine, J.M.; Vilà, M.; D’Antonio, C.M.; Dukes, J.S.; Grigulis, K.; Lavorel, S. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. 2003, 270, 775–781. [Google Scholar] [CrossRef]
- Blumenthal, D.M.; Hufbauer, R.A. Increased plant size in exotic populations: A common-garden test with 14 invasive species. Ecology 2007, 88, 2758–2765. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- Hillerislambers, J.; Adler, P.B.; Harpole, W.S.; Levine, J.M.; Mayfield, M.M. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 227–248. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Divíšek, J.; Chytrý, M.; Beckage, B.; Gotelli, N.J.; Lososová, Z.; Pyšek, P.; Richardson, D.M.; Molofsky, J. Similarity of introduced plant species to native ones facilitates naturalization, but differences enhance invasion success. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Zwerschke, N.; van Rein, H.; Harrod, C.; Reddin, C.; Emmerson, M.C.; Roberts, D.; O’Connor, N.E. Competition between co-occurring invasive and native consumers switches between habitats. Funct. Ecol. 2018, 32, 2717–2729. [Google Scholar] [CrossRef]
- Escoriza, D.; Ruhí, A. Functional distance to recipient communities may favour invasiveness: Insights from two invasive frogs. Divers. Distrib. 2016, 22, 519–533. [Google Scholar] [CrossRef]
- Fournier, A.; Penone, C.; Pennino, M.G.; Courchamp, F. Predicting future invaders and future invasions. Proc. Natl. Acad. Sci. USA 2019, 116, 7905–7910. [Google Scholar] [CrossRef]
- Mack, R.N. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Darwin, C.R. On the Origin of Species by Means of Natural Selection, or Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Patten, B.C.; Auble, G.T. System theory of the ecological niche. Am. Nat. 1981, 117, 893–922. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Springer: Boston, MA, USA, 1958. [Google Scholar]
- Carpenter, S.R.; Mooney, H.A.; Agard, J.; Capistrano, D.; DeFries, R.S.; Diaz, S.; Dietz, T.; Duraiappah, A.K.; Oteng-Yeboah, A.; Pereira, H.M.; et al. Science for managing ecosystem services: Beyond the Millennium Ecosystem Assessment. Proc. Natl. Acad. Sci. USA 2009, 106, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, J. The niche-relationships of the California Thrasher. Auk 1917, 34, 427–433. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Hussner, A.; Stiers, I.; Verhofstad, M.J.J.M.; Bakker, E.S.; Grutters, B.M.C.; Haury, J.; van Valkenburg, J.L.C.H.; Brundu, G.; Newman, J.; Clayton, J.S.; et al. Management and control methods of invasive alien aquatic plants: A review. Aquat. Bot. 2017, 136, 112–137. [Google Scholar] [CrossRef]
- Hussner, A.; Van De Weyer, K.; Gross, E.M.; Hilt, S. Comments on increasing number and abundance of non-indigenous aquatic macrophyte species in Germany. Weed Res. 2010, 50, 519–526. [Google Scholar] [CrossRef]
- Capers, R.; Selsky, R.; Bugbee, G.; White, J. Aquatic plant community invasibility and scale-dependent patterns in native and invasive species richness. Ecology 2007, 88, 3135–3143. [Google Scholar] [CrossRef]
- Muthukrishnan, R.; Hansel-Welch, N.; Larkin, D.J. Environmental filtering and competitive exclusion drive biodiversity-invasibility relationships in shallow lake plant communities. J. Ecol. 2018, 106, 2058–2070. [Google Scholar] [CrossRef]
- Schultz, R.; Dibble, E. Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: The role of invasive plant traits. Hydrobiologia 2012, 684, 1–14. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Hiatt, D.; Serbesoff-King, K.; Lieurance, D.; Gordon, D.R.; Flory, S.L. Allocation of invasive plant management expenditures for conservation: Lessons from Florida, USA. Conserv. Sci. Pract. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Hussner, A.; Nehring, S.; Hilt, S. From first reports to successful control: A plea for improved management of alien aquatic plant species in Germany. Hydrobiologia 2014, 737, 321–331. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Cronk, J.K.; Fennessy, M.S. Wetland Plants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Wetzel, R.G. Limnology; W.B. Saunders Company: Philidelphia, PA, USA, 1975. [Google Scholar]
- Hudon, C.; Lalonde, S.; Gagnon, P. Ranking the effects of site exposure, plant growth form, water depth, and transparency on aquatic plant biomass. Can. J. Fish. Aquat. Sci. 2000, 57, 31–42. [Google Scholar] [CrossRef]
- Gillefalk, M.; Herzog, C.; Hilt, S. Phosphorus availability and growth of benthic primary producers in littoral lake sediments: Are differences linked to induced bank filtration? Water 2019, 11, 1111. [Google Scholar] [CrossRef]
- Hofmann, H.; Lorke, A.; Peeters, F. Wave-induced variability of the underwater light climate in the littoral zone. Verh. lnt. Verein. Limnol. 2008, 30, 627–632. [Google Scholar] [CrossRef][Green Version]
- Chambers, P.A. Light and nutrients in the control of aquatic plant community structure. II. In situ observations. J. Ecol. 1987, 75, 621–628. [Google Scholar] [CrossRef]
- Godoy, O.; Levine, J.M. Phenology effects on invasion success: Insights from coupling field experiments to coexistence theory. Ecology 2014, 95, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Torso, K.; Scofield, B.D.; Chess, D.W. Variations in aquatic macrophyte phenology across three temperate lakes in the Coeur d’Alene Basin. Aquat. Bot. 2020, 162, 103209. [Google Scholar] [CrossRef]
- Aiken, S.G.; Newroth, R.; Wiles, I. The biology of Canadian weeds. Myriophyllum spicatum L. Can. J. Plant Sci. 1979, 59, 201–215. [Google Scholar] [CrossRef]
- Eiswerth, M.; Donaldson, S.; Johnson, W. Potential environmental impacts and economic damages of Eurasian watermilfoil (Myriophyllum spicatum) in western Nevada and northeastern California. Weed Technol. 2000, 14, 511–518. [Google Scholar] [CrossRef]
- Barko, J.W.; Smart, M.R. Comparative Influences of Light and Temperature on the Growth and Metabolism of Selected Submersed Freshwater Macrophytes. Ecol. Monogr. 1981, 51, 219–236. [Google Scholar] [CrossRef]
- Smith, C.S.; Barko, J.W. Ecology of Eurasian watermilfoil. J. Aquat. Plant Manag. 1990, 28, 55–64. [Google Scholar]
- Titus, J.E.; Adams, M.S. Coexistence and the comparative light relations of the submersed macrophytes Myriophyllum spicatum L. and Vallisneria americana Michx. Oecologia 1979, 40, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Bolduan, B.R.; Van Eeckhout, G.C.; Quade, H.W.; Gannon, J.E. Potamogeton crispus—The other invader. Lake Reserv. Manag. 1994, 10, 113–125. [Google Scholar] [CrossRef]
- Tobiessen, P.; Snow, P.D. Temperature and light effects on the growth of Potamogeton crispus in Collins Lake, New York State. Can. J. Bot. 1984, 62, 2822–2826. [Google Scholar] [CrossRef]
- Chambers, P.A.; Spence, D.H.N.; Weeks, D.C. Photocontrol of turion formation by Potamogeton Crispus, L. in the laboratory and natural water. New Phytol. 1985, 99, 183–194. [Google Scholar] [CrossRef]
- Woolf, T.E.; Madsen, J. Seasonal biomass and carbohydrate allocation patterns in southern Minnesota curlyleaf pondweed populations. J. Aquat. Plant Manag. 2003, 41, 113–118. [Google Scholar]
- Adamec, L. Ecophysiological characteristics of turions of aquatic plants: A review. Aquat. Bot. 2018, 148, 64–77. [Google Scholar] [CrossRef]
- Carmona, C.P.; de Bello, F.; Mason, N.W.H.; Lepš, J. Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef]
- Blonder, B. Hypervolume concepts in niche- and trait-based ecology. Ecography (Cop.). 2018, 41, 1441–1455. [Google Scholar] [CrossRef]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. The n-dimensional hypervolume. Glob. Ecol. Biogeogr. 2014, 23, 595–609. [Google Scholar] [CrossRef]
- Perleberg, D.; Radomski, P.; Simon, S.; Carlson, K.; Knopik, J. Minnesota Lake Plant Survey Manual, for Use by MNDNR Fisheries Section and EWR Lake Habitat Program; Minnesota Department of Natural Resources: Brainerd, MN, USA, 2016. [Google Scholar]
- Johnson, J.A.; Jones, A.R.; Newman, R.M. Evaluation of lakewide, early season herbicide treatments for controlling invasive curlyleaf pondweed (Potamogeton crispus) in Minnesota lakes. Lake Reserv. Manag. 2012, 28, 346–363. [Google Scholar] [CrossRef]
- Jones, A.R.; Johnson, J.A.; Newman, R.M. Effects of repeated, early season, herbicide treatments of curlyleaf pondweed on native macrophyte assemblages in Minnesota lakes. Lake Reserv. Manag. 2012, 28, 364–374. [Google Scholar] [CrossRef]
- Verhoeven, M.R.; Larkin, D.J.; Newman, R.M. Constraining invader dominance: Effects of repeated herbicidal management and environmental factors on curlyleaf pondweed dynamics in 50 Minnesota lakes. Freshw. Biol. 2020, 1–14. [Google Scholar] [CrossRef]
- Nault, M.; Mikulyuk, A.; Hauxwell, J.; Skogerboe, J.; Asplund, T.; Barton, M.; Wagner, K.; Hoyman, T.; Heath, E. Herbicide Treatments in Wisconsin Lakes: Building a Framework for Scientific Evaluation of Large-scale Herbicide Treatments in Wisconsin Lakes. NALMS Lake Line 2012, 32, 19–24. [Google Scholar]
- Kujawa, E.R.; Frater, P.; Mikulyuk, A.; Barton, M.; Nault, M.E.; Van Egeren, S.; Hauxwell, J. Lessons from a decade of lake management: Effects of herbicides on Eurasian watermilfoil and native plant communities. Ecosphere 2017, 8, e01718. [Google Scholar] [CrossRef]
- Frater, P.; Mikulyuk, A.; Barton, M.; Nault, M.; Wagner, K.; Hauxwell, J.; Kujawa, E. Relationships between water chemistry and herbicide efficacy of Eurasian watermilfoil management in Wisconsin lakes. Lake Reserv. Manag. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Anderson, M.; Clark, M.; Cornett, M.; Hall, K.; Olivero Sheldon, A.; Prince, J. Resilient Sites for Terrestrial Conservation in the Great Lakes and Tallgrass Prairie Region; The Nature Conservancy, Eastern Conservation Science and North America Region: Boston, MA, USA, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Madsen, J.D.; Wersal, R.M. A review of aquatic plant monitoring and assessment methods. J. Aquat. Plant Manag. 2017, 55, 1–12. [Google Scholar]
- Chadde, S.W. Wetland Plants of Minnesota: A Complete Guide to the Wetland and Aquatic Plants of the North Star State; Createspace Independent Publishing Platform: Lexington, KY, USA, 2012; ISBN 9781460940341. [Google Scholar]
- Crow, G.E.; Hellquist, C.B. Aquatic and Wetland Plants of Northeastern North America. Volum Pteridophytes, Gymnosperms and Angiosperms: Dicotyledons; University of Wisconsin Press: Madison, WI, USA, 2000. [Google Scholar]
- Crow, G.E.; Hellquist, C.B. Aquatic and Wetland Plants of Northeastern North America. Volume Angiosperms: Monocotyledons; University of Wisconsin Press: Madison, WI, USA, 2000. [Google Scholar]
- Skawinski, P.M. Aquatic Plants of the Upper Midwest; Paul Skawinski: Wausau, WI, USA, 2011; ISBN 978-1-4507-9247-9. [Google Scholar]
- Boyle, B.; Hopkins, N.; Lu, Z.; Raygoza Garay, J.A.; Mozzherin, D.; Rees, T.; Matasci, N.; Narro, M.L.; Piel, W.H.; Mckay, S.J.; et al. The taxonomic name resolution service: An online tool for automated standardization of plant names. BMC Bioinf. 2013, 14, 16. [Google Scholar] [CrossRef]
- Chamberlain, S.A.; Szöcs, E. Taxize: Taxonomic search and retrieval in R. F1000 Res. 2013, 2, 1–30. [Google Scholar] [CrossRef]
- Stadelmann, T.H.; Brezonik, P.L.; Kloiber, S. Seasonal patterns of chlorophyll a and secchi disk transparency in lakes of East-central Minnesota: Implications for design of ground—And satellite-based monitoring programs. Lake Reserv. Manag. 2001, 17, 299–314. [Google Scholar] [CrossRef]
- Preisendorfer, R.W. Eyeball optics of natural waters: Secchi disk science. Limnol. Oceanogr. 1986, 31, 909–926. [Google Scholar] [CrossRef]
- Steele, E.A.; Neuhausser, S. Comparison of methods for measuring visual water clarity. J. North Am. Benthol. Soc. 2002, 21, 326–335. [Google Scholar] [CrossRef]
- Megard, R.O.; Settles, J.C.; Boyer, H.A.; Combs, W.S.J. Light, Secchi disks, and trophic states. Limnol. Oceanogr. 1980, 25, 373–377. [Google Scholar] [CrossRef]
- Van Duin, E.H.S.; Blom, G.; Los, F.J.; Maffione, R.; Zimmerman, R.; Cerco, C.F.; Dortch, M.; Best, E.P.H. Modeling underwater light climate in relation to sedimentation, resuspension, water quality and autotrophic growth. Hydrobiologia 2001, 444, 25–42. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Cross, H.Z.; Zuber, M.S. Prediction of flowering dates in maize based on different methods of estimating thermal units. Agron. J. 1972, 64, 351–355. [Google Scholar] [CrossRef]
- Russelle, M.P.; Wilhelm, W.W.; Olson, R.A.; Power, J.F. Growth analysis based on degree days. Crop Sci. 1984, 24, 28–32. [Google Scholar] [CrossRef]
- Luedeling, E.; Kunz, A.; Blanke, M.M. Identification of chilling and heat requirements of cherry trees-a statistical approach. Int. J. Biometeorol. 2013, 57, 679–689. [Google Scholar] [CrossRef]
- NOAA. National Centers for Environmental Information Global Surface Hourly [2001–2018]; NOAA: Washington, DC, USA, 2018.
- Boissezon, A.; Auderset Joye, D.; Garcia, T. Temporal and spatial changes in population structure of the freshwater macroalga Nitellopsis obtusa (Desv.) J.Groves. Bot. Lett. 2018, 165, 103–114. [Google Scholar] [CrossRef]
- Carmona, C.P.; Bello, F.; Mason, N.W.H.; Lepš, J. Trait probability density (TPD): Measuring functional diversity across scales based on TPD with R. Ecology 2019, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. Using n-dimensional hypervolumes for species distribution modelling: A response to Qiao et al. (). Glob. Ecol. Biogeogr. 2017, 26, 1071–1075. [Google Scholar] [CrossRef]
- Blonder, B.; Morrow, C.B.; Maitner, B.; Harris, D.J.; Lamanna, C.; Violle, C.; Enquist, B.J.; Kerkhoff, A.J. New approaches for delineating n -dimensional hypervolumes. Methods Ecol. Evol. 2018, 9, 305–319. [Google Scholar] [CrossRef]
- Blonder, B. Do hypervolumes have holes? Am. Nat. 2016, 187, E93–E105. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. CRC. Crit. Rev. Plant Sci. 2004, 23, 431–452. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Gilbert, B.; Levine, J.M. Plant invasions and the niche. J. Ecol. 2009, 97, 609–615. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Agrawal, A.A.; Bever, J.D.; Gilbert, G.S.; Hufbauer, R.A.; Klironomos, J.N.; Maron, J.L.; Morris, W.F.; Parker, I.M.; Power, A.G.; et al. Biotic interactions and plant invasions. Ecol. Lett. 2006, 9, 726–740. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Borer, E.T.; Boucher, V.L.; Burton, R.S.; Cottingham, K.L.; Goldwasser, L.; Gram, W.K.; Kendall, B.E.; Micheli, F. Competition, seed limitation, disturbance, and reestablishment of California native annual forbs. Ecol. Appl. 2003, 13, 575–592. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Hilt, S.; Henschke, I.; Rücker, J.; Nixdorf, B. Can submerged macrophytes influence turbidity and trophic state in deep lakes? Suggestions from a case study. J. Environ. Qual. 2010, 39, 725. [Google Scholar] [CrossRef]
- James, W.F.; Dechamps, A.; Turyk, N.; Mcginley, P. Contribution of Potamogeton Crispus Decay to the Phosphorus Budget of McGinnis Lake, Wisconsin; U.S. Army Engineer Research and Development Center: Vicksburg, MS, USA, 2007. [Google Scholar]
- Boylen, C.W.; Eichler, L.W.; Madsen, J.D. Loss of native aquatic plant species in a community dominated by Eurasian watermilfoil. Hydrobiologia 1999, 415, 207–211. [Google Scholar] [CrossRef]
- Scheele, B.C.; Foster, C.N.; Banks, S.C.; Lindenmayer, D.B. Niche contractions in declining species: Mechanisms and consequences. Trends Ecol. Evol. 2017, 32, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Newman, M. A comparison of two methods for sampling biomass of aquatic plants. J. Aquat. Plant Manag. 2011, 49, 1–8. [Google Scholar]
- Marshall, C.T.; Peters, R.H. General patterns in the seasonal development of chlorophyll a for temperate lakes. Limnol. Oceanogr. 1989, 34, 856–867. [Google Scholar] [CrossRef]
- Titus, J.; Goldstein, R.A.; Adams, M.S.; Mankin, J.B.; O’neill, R.V.; Weiler, P.R., Jr.; Shugart, H.H.; Booth, R.S. A production model for Myriophyllum spicatum L. Ecology 1997, 56, 1129–1138. [Google Scholar] [CrossRef]
- Grace, J.B.; Wetzel, R.G. The production biology of Eurasian watermilfoil (Myriophyllum spicatum L.): A Review. J. Aquat. Plant. Manag. 1978, 16, 1–11. [Google Scholar]
- Adams, M.S.; McCracken, M.D. Seasonal Production of the Myriophyllum Component of the Littoral of Lake Wingra, Wisconsin. J. Ecol. 1974, 62, 457. [Google Scholar] [CrossRef]
- Galatowitsch, S.M.; Anderson, N.O.; Ascher, P.D. Invasiveness in wetland plants in temperate North America. Wetlands 1999, 19, 733–755. [Google Scholar] [CrossRef]
- Valley, R.D.; Bremigan, M.T. Effects of macrophyte bed architecture on largemouth bass foraging: Implications of exotic macrophyte invasions. Trans. Am. Fish. Soc. 2002, 131, 234–244. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, S.; Liang, W.; He, F.; Wu, Z. Effects of sediment anoxia and light on turion germination and early growth of Potamogeton crispus. Hydrobiologia 2009, 628, 111–119. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 1–21. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhoeven, M.R.; Glisson, W.J.; Larkin, D.J. Niche Models Differentiate Potential Impacts of Two Aquatic Invasive Plant Species on Native Macrophytes. Diversity 2020, 12, 162. https://doi.org/10.3390/d12040162
Verhoeven MR, Glisson WJ, Larkin DJ. Niche Models Differentiate Potential Impacts of Two Aquatic Invasive Plant Species on Native Macrophytes. Diversity. 2020; 12(4):162. https://doi.org/10.3390/d12040162
Chicago/Turabian StyleVerhoeven, Michael R., Wesley J. Glisson, and Daniel J. Larkin. 2020. "Niche Models Differentiate Potential Impacts of Two Aquatic Invasive Plant Species on Native Macrophytes" Diversity 12, no. 4: 162. https://doi.org/10.3390/d12040162
APA StyleVerhoeven, M. R., Glisson, W. J., & Larkin, D. J. (2020). Niche Models Differentiate Potential Impacts of Two Aquatic Invasive Plant Species on Native Macrophytes. Diversity, 12(4), 162. https://doi.org/10.3390/d12040162




