Abstract
Potamogeton crispus (curlyleaf pondweed) and Myriophyllum spicatum (Eurasian watermilfoil) are widely thought to competitively displace native macrophytes in North America. However, their perceived competitive superiority has not been comprehensively evaluated. Coexistence theory suggests that invader displacement of native species through competitive exclusion is most likely where high niche overlap results in competition for limiting resources. Thus, evaluation of niche similarity can serve as a starting point for predicting the likelihood of invaders having direct competitive impacts on resident species. Across two environmental gradients structuring macrophyte communities—water depth and light availability—both P. crispus and M. spicatum are thought to occupy broad niches. For a third dimension, phenology, the annual growth cycle of M. spicatum is typical of other species, whereas the winter-ephemeral phenology of P. crispus may impart greater niche differentiation and thus lower risk of native species being competitively excluded. Using an unprecedented dataset comprising 3404 plant surveys from Minnesota collected using a common protocol, we modeled niches of 34 species using a probabilistic niche framework. Across each niche dimension, P. crispus had lower overlap with native species than did M. spicatum; this was driven in particular by its distinct phenology. These results suggest that patterns of dominance seen in P. crispus and M. spicatum have likely arisen through different mechanisms, and that direct competition with native species is less likely for P. crispus than M. spicatum. This research highlights the utility of fine-scale, abundance-based niche models for predicting invader impacts.
1. Introduction
Invasive species are among the greatest threats to biodiversity worldwide [1,2]. Across taxonomic groups and trophic levels, invaders have consistent negative effects on native communities’ species richness, evenness, and other measures of diversity [3]. Despite these clear overall patterns, important gaps remain in our ability to predict how particular invaders will interact with particular native species. The strength and directionality of individual invader-native interactions, and the attendant impacts of invasions on communities as a whole, can be difficult to assess and are often poorly understood [1,4]. Variation in impacts of invasive species arises through multiple mechanisms by which invasive species interact with native species and alter invaded communities [4,5,6]. These mechanisms range from altering disturbance regimes [7] to better tolerating diseases [8] to directly competing for limiting resources [9]. Direct competition for limiting resources can cause competitive displacement of native species—one of multiple mechanisms that can be detrimental to native communities and give rise to a pattern of invader dominance [10,11].
According to contemporary coexistence theory, coexistence of species within a community is dependent on a balance between relative fitness (competitive) differences, which drive the most-fit species towards dominance, and stabilizing niche differences, which allow weaker competitors to avoid direct competition [12]. The likelihood that an invader will directly compete with a native species is a function of the degree to which the species’ environmental requirements (i.e., niche) overlap. When co-occurring species occupy very similar niches, the superior competitor should displace the inferior competitor, preventing stable coexistence [13]. Conversely, where differences in the niches occupied are sufficiently large, coexistence is possible [14]. Thus, the relation of an invader’s niche to those of resident species is increasingly recognized as a key predictor of community impacts [15,16]. Predicting contexts where invasive species will have the greatest ecological impacts is vital for prioritizing limited resources for invasive species management. Niche-based approaches to address this challenge have a long history in ecology and are an active area of current research [15,16,17,18,19,20,21,22,23,24].
In freshwater systems, invasive species are among the strongest drivers of change [25], and invasive aquatic plants cause significant ecological impacts [26,27]. Freshwater lakes are highly susceptible to plant invasions [28,29], and losses of diversity and ecosystem functioning associated with these invasions are of great concern to natural resource managers and other stakeholders [26,30]. Tremendous effort is allocated to preventing and mitigating potential impacts of invasive aquatic plants; better understanding of impacts could be used to improve effort allocation [31,32,33]. The abiotic (i.e., environmental) dimensions that structure freshwater plant communities are relatively well studied [34], but improved knowledge of the precise niches occupied by particular species, and the implications for invader-native interactions, are critical for predicting the effects of, and responding to, invasions. Water depth, light availability, and phenology are primary abiotic factors that define the niches of aquatic vegetation [35,36,37]. Water depth is one of the strongest gradients in aquatic systems, with plant species spanning from tolerating seasonally saturated soils to completing their entire life cycles underwater [35]. Additionally, depth gradients capture other important gradients such as nutrient availability [38], sediment resuspension, and hydraulic forces [39]. Light is a critical determinant of all plant growth that is particularly constraining in aquatic environments, where photosynthetically active radiation rapidly decays with water depth and transparency [35,40]. The seasonality of temperate freshwater systems is a strong driver of species distribution and annual growth patterns, and phenological niche differentiation, or use of distinct seasonal or temporal growth windows, influences community structure [36,41,42].
Two of the most widespread and problematic invasive aquatic plant species in temperate North America are Myriophyllum spicatum L. (Haloragaceae; Eurasian watermilfoil) and Potamogeton crispus L. (Potamogetonaceae; curlyleaf pondweed). Both of these species receive significant attention from resource managers, yet their niche overlap and interactions with native plant species—and thus their potential competitive impacts—may be quite different. Myriophyllum spicatum has broad tolerances of water depth [43,44,45] and light availability [45,46], as well as phenology typical of most temperate macrophytes [45,47]. Potamogeton crispus also occupies relatively broad water-depth and light gradients [48,49]; however, the phenology of P. crispus is distinct compared to other aquatic macrophytes in North American temperate lakes [49,50,51,52]. In these systems, P. crispus behaves as a winter annual, maintaining a life cycle in which it reaches peak biomass early in the summer, then senesces to dormant propagules until fall [48,51]. The unique phenological niche of P. crispus, and associated low temporal overlap with native species, may make it less likely to drive native species declines than M. spicatum.
To assess the potential for these two invasive species to competitively exclude native macrophytes, and to test the hypothesis that P. crispus would have lower niche overlap with native species than M. spicatum, we applied a recently introduced approach, probabilistic niche modeling [53,54], to a novel dataset. The dataset consisted of plant occurrence and environmental data for three key niche axes (depth, light, and phenology). Occurrence data were obtained from a variety of lake managers across Minnesota, USA, all implementing a unified survey methodology to collect data on plant occurrences at the local scale (i.e., within-lake distributions). To date, these data have been disparate in their storage and analysis—we retrieved and united these disconnected data to develop an extensive dataset covering a wide geographic range and time period. We incorporated these data into the probabilistic niche modeling framework, which allows robust evaluation of niche differentiation among species. This approach generated fine-scale niche models for Minnesota macrophytes incorporating both species occupancy along each niche axis and abundance within occupied segments of the niche (Figure 1). The use of a novel dataset that unites disparate but consistently collected local-scale plant data enables niche models to be built based on neighborhood-level species occurrences and fine-scale environmental variation, i.e., within-lake differences in habitat conditions. The probabilistic nature of this approach is made possible by inclusion of abundance data (how often was a species observed in particular conditions relative to all other conditions) and results in niche models that predict likelihood of occurrence along each modeled niche axis, as opposed to only defining niche boundaries [55]. Finally, the probabilistic niche modeling framework enabled attribution of niche differences to either differences in the niche space occupied (“non-shared niche differences”) or differences in the abundances of two species in co-occupied niche space (“shared niche differences”) (Figure 1). This extensive dataset and use of probabilistic niche models allowed (1) development of within-lake habitat models (as opposed to coarse regional models based on climate or other large-scale variables), (2) incorporation of abundance along niche axes to account for differential distribution within fundamental niches, and (3) discrimination of niche differentiation as both differences in niche boundaries and differences in relative abundances within shared niche space.
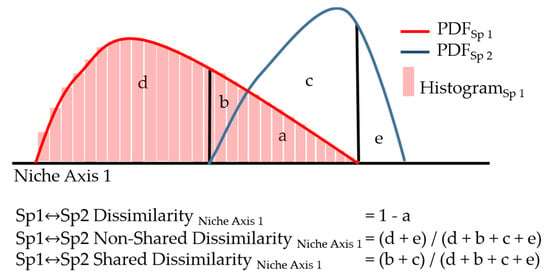
Figure 1.
Graphical depiction of niches modeled using probability density functions (PDFs) [53] for two hypothetical species along one niche axis. Each PDF is built from occurrence (histogram of occurrences shown for Sp 1 only) and associated environmental data and PDFs integrate to 1. Dissimilarity is estimated as follows: total dissimilarity = 1 − shared area (a), non-shared niche dissimilarity is the ratio of dissimilar PDF area in niche space occupied by only one of the two species (d, e) to all dissimilar PDF area (d + b + c + e), and shared dissimilarity is the ratio of dissimilar PDF area in niche space occupied by both species (b, c) to all dissimilar PDF area (d + b + c + e).
We used the probabilistic niche modeling framework to assess the potential for competition between the focal invasive species and native macrophyte species. We developed niche models for P. crispus, M. spicatum, and 32 native macrophyte species that were sufficiently represented in our dataset for modeling purposes. For each invader, we calculated its niche overlap with each native species across all three niche dimensions as a whole and for each individual dimension. We further distinguished niche differences between species that arose from differences in shared vs. non-shared niche space. Using these results, we tested the hypothesis that M. spicatum would have greater niche overlap with native species than P. crispus, suggesting greater potential for direct competitive displacement by M. spicatum.
2. Materials and Methods
2.1. Macrophyte Occurrence Data
In Minnesota, many organizations (state agencies, local units of government, consultants, and others) conduct point-intercept surveys of aquatic plant communities in lakes. These surveys are used for a wide variety of purposes, including baseline monitoring [56], long-term ecological trend monitoring (SLICE: https://www.dnr.state.mn.us/fisheries/slice/index.html), university research projects [57,58], and monitoring of management outcomes [59]. Recent work has illustrated the research opportunities these datasets provide, as they are cohesive surveys conducted using a common method over long time scales and wide geographic ranges [59,60,61,62]. We compiled plant occurrence and depth data from point-intercept surveys performed by agencies, researchers, and lake managers in the period 2000–2018. These data are representative of the geographic distribution of lakes in Minnesota (Figure 2).
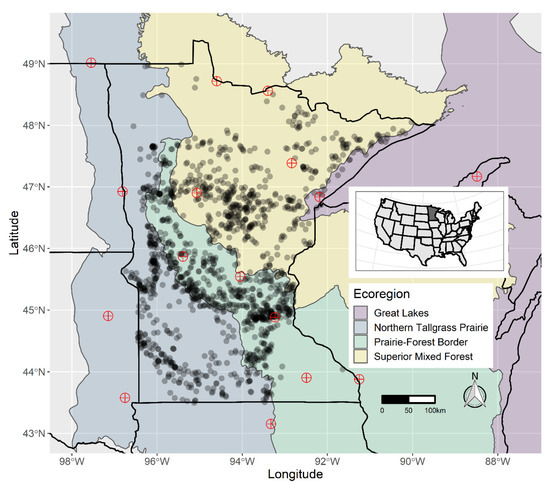
Figure 2.
Distribution of lakes with survey data included in this study (translucent black dots) and weather stations used for associated weather data (red circles with crosses) across major ecoregions of Minnesota, USA. Ecoregions represent areas with similar climatic and environmental conditions [63].
All data preparation, analysis, and visualization of results were conducted in R version 3.6.1 [64]. All code used for analyses and visualizations are included in Supplementary Material (Supplementary Code). Data used for analysis were collected via the point-intercept method, in which surveyors navigate to a predetermined grid of points in a lake and use a metal rake to sample macrophytes growing in that location. Use of point-intercept data ensured unbiased sample selection (point locations are pre-determined prior to surveys) and consistent methodology across surveys [65]. We analyzed data at the point scale, i.e., each record comprised one sampling point with associated survey information (date, surveyor, lake identifier), and the water depth and plant species recorded at the point. The average number of points sampled in point-intercept surveys was 104 (± 108, 1 SD; distribution shown in Supplementary Code). For our analysis, we treated each plant observation at each survey point as a data point (mean taxa observed per sample point 2.05 ± 1.62, 1 SD; Supplementary Code) and compiled associated data for each of these observations. Plant occurrences from each survey point were translated to scientific names from codes or common names using keys provided by the data contributors and referencing common names in regional identification guides [66,67,68,69]. We standardized species names based on the Taxonomic Name Resolution Service [70] using the “taxize” package in R 3.6.1 [64,71]. In total, we compiled 3404 surveys comprising 2514 lake-years of data (each lake × year combination comprises a lake-year) from 1526 Minnesota lakes surveyed at least once between 2000 and 2018, representing 353,148 total survey points, with a combined 564,038 macrophyte observations across 218 taxa (163 identified to species, 56 to higher taxa when surveyors were unable to resolve to species).
2.2. Environmental Data
Water depth data were collected by surveyors at each sampling point. We omitted sample points with no depth information, a depth of 0, or depths > 5 standard deviations above the mean (depth > 7 m, 0.4% of all occurrences). If units were not specified for depth (i.e., whether in feet or meters), we plotted the distribution of depths and plant occurrences observed in the survey and compared these to lake bathymetry and reasonable colonization depths of plants. For example, if a survey with unlabeled depths occurred in a lake with a maximum depth of 3 m, and unlabeled depths ranged from 0 to 8.5, we concluded that depth must have been recorded in feet.
Secchi depth data for each lake-year were collected from several sources and cleaned to ensure consistency. For most surveys, we used an empirical Secchi dataset compiled to develop predictive models of lake clarity for Minnesota (Kelsey Vitense, University of Minnesota, unpublished data). If a lake-year in our dataset did not have a corresponding value in this dataset, we used Secchi data collected by the surveyor if available. We only used Secchi measurements taken in summer (July, August, or September) to avoid measurements sensitive to large seasonal changes in water clarity [72]. Finally, summer Secchi data for a three-year window (the survey year, the year prior, and the year after) were used to calculate a mean Secchi value for each survey (lake-year) to minimize the effects of sampling error and variability in Secchi readings [73].
Three-year average Secchi depths for each survey and point-specific water depth were used to estimate light availability for each survey point [73,74,75,76], calculated as the proportion of surface irradiance remaining at the substrate (hereafter, light availability). Light at Secchi depth was assumed to be 10% of surface irradiance, which enabled us to use Secchi depth measurements to estimate a lake-level light decay constant, Kd [75]:
We then used the Lambert-Beer relationship to calculate the proportion of surface irradiance (light availability) remaining at the substrate for each plant observation’s associated depth to substrate [76] using the lake-wide Kd and assuming water clarity to be constant throughout each waterbody:
Secchi data were sufficient in coverage to calculate light availability for 82.9% of plant observations in our dataset.
Kd = ln(10)/Secchi depth
Light availability = e^(−Kd × Depth to substrate)
We examined phenology using growing degree days (GDD), a measure of the heat energy received by a plant over a given time period (e.g., within a year) [77]. Growing degree days are a more robust way to assess phenology compared to time of year or day of the year, which do not account for geographic location or interannual variability in temperature [78,79]. Daily minimum and maximum temperatures were required to calculate GDD. For each lake, we identified the nearest weather station with comprehensive daily weather data using the “get_weather” function in the chillR package in R [80]. We used the same function to download daily weather data for each weather station from the Integrated Surface Dataset [81]. A total of 16 weather stations were used with a mean distance of 64 km (± 31 km, 1 SD) from associated lakes. Some stations were located outside of Minnesota, but these political boundaries are not associated with abrupt disjuncts in climate or environmental conditions, as for example if the borders were associated with abrupt changes in elevation (Figure 2). Using associated station data, we calculated GDD as,
where base temperature is temperature below which no plant growth is expected. We used 4 °C as the base temperature, following Boissezon et al. [82], and 1 March as the “biofix date”, i.e., the date on which GDD began accumulating each year. For some daily weather records, minimum and/or maximum temperatures were missing (<0.2% of all records). When this occurred, we substituted the previous day’s minimum and/or maximum temperature. We used the cumulative yearly GDD for each vegetation survey date as the GDD value associated with each survey point.
GDD = (maximum daily temperature + minimum daily temperature)/2 − base temperature,
Following the collection, cleaning, and merging of all datasets, we retained a total of 462,118 plant occurrences from 292,824 sample points for which we had complete depth, light, and phenology data.
2.3. Data Analysis
We constructed probabilistic niche models by fitting probability density functions (PDFs) to the occurrence of each species and axis of interest using a kernel density estimation (KDE) method [83,84,85]. These PDFs represent the realized niche occupied by that species based on the three niche dimensions we evaluated (depth, light, and phenology). The result is a model wherein any location within the realized niche of a species is represented by a probability of its occurrence at that location in three-dimensional niche space. We then compared niches between species to reveal where species are likely to compete for the same resources, i.e., where the species have overlapping niche requirements. We conducted niche modeling and dissimilarity evaluations among species using the “TPD” package in R [83].
To characterize the availability of potential niche space represented in our dataset, we first modeled the probabilistic niche of all survey points, i.e., generated a representation of the total “niche” occupied by all survey points. Probability distributions of sampling effort indicated sampling effort was non-uniform (Supplementary Code). Specifically, sampling was biased toward shallower sites and those with lower light availability, and survey efforts peaked mid-summer at 1500 GDD. Nonetheless, sampling effort was sufficient to provide good coverage of the multi-dimensional niche space across all three dimensions (Figure 3, Figures S1–S3). In addition, correlations among niche axes indicated no issues with collinearity for use in niche models (Light-GDD r = −0.01, Light-Depth r = −0.41, GDD-Depth r = −0.05; visualizations presented in Supplementary Code).
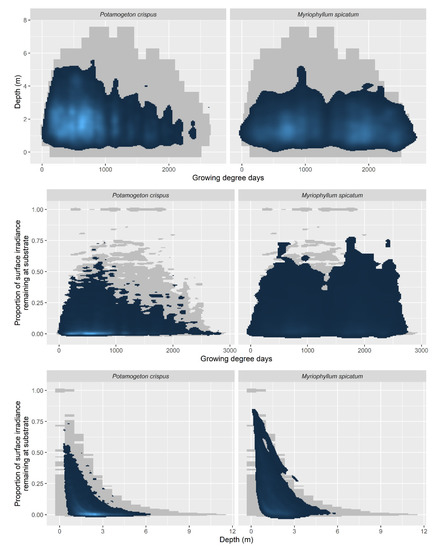
Figure 3.
Niche models of Potamogeton crispus and Myriophyllum spicatum across three environmental gradients: water depth, phenology (growing degree days), light availability (proportion of surface irradiance at substrate). Gray background depicts surveyed space in the niches, and blue cloud indicates probabilistic nature of niche model (light blue = high relative abundance; dark blue = low relative abundance).
We standardized niche data (centered data on the variable mean and scaled it to one standard deviation) to ensure equal weights among variables measured on different scales [54,85]. To ensure sufficient fit of PDFs, we limited our analysis to species with a minimum number of occurrences equal to en niche dimensions, or 1000 occurrences [85,86]. This restricted our dataset to 371,053 occurrences of 34 species used in the analysis.
For each species × niche dimension, we fit a PDF to the abundance data across that dimension using KDE, such that the final probability function for each dimension integrated to 1 (i.e., the area under the curve = 1; Figure 1). Differences between the probabilistic niche models produced for P. crispus and M. spicatum for each niche dimension were evaluated using a Kolmogorov-Smirnov test. We also constructed PDFs for each species across all three niche dimensions simultaneously, then estimated the dissimilarity of species’ niche models using the proportion of overlap in the volume (three niche dimensions) of the two species’ niche volumes [53]. In three-dimensional niche space, the total dissimilarity between two species is equivalent to 1 − the niche model PDF volume shared by the two species.
Dissimilarity among species was further decomposed into two elements: the dissimilarity of abundances within niche space shared between the two species and the dissimilarity measured as non-shared niche space occupied by only one of the two species (Figure 1) [53,83]. In this way, we were able to parse the relative importance of two different ways that niche space can be partitioned between species. Finally, we calculated the dissimilarity of each species from all other species (the total community) as the mean of all pairwise PDF dissimilarity values for that species. A two-proportion z-test was used to evaluate the hypothesis that the dissimilarity of P. crispus was more strongly driven by contributions of non-shared dissimilarity than that of M. spicatum. Species dissimilarity was further subdivided as the dissimilarity contributed individually by each of the three niche dimensions.
3. Results
For the 34 species evaluated, we produced PDFs for the three niche dimensions considered (Figure 3, Figures S1–S3). These distributions were constructed for multi-dimensional niche space (Figure 3) and each individual niche dimension (Figure 4). In both cases, there was substantial phenological niche separation for P. crispus, for which most occurrences were under low-GDD values (~750 GDD). Potamogeton crispus also had peaks in its abundance under low-light conditions and water depth of ~2.5 m (Figure 3). In comparison, M. spicatum showed broad phenology with two peaks, one near 900 GDD and the other at approximately 2000 GDD (Figure 4). Relative to P. crispus, there was lower occupancy under low-light conditions and broader occupancy across depths (Figure 3 and Figure 4).
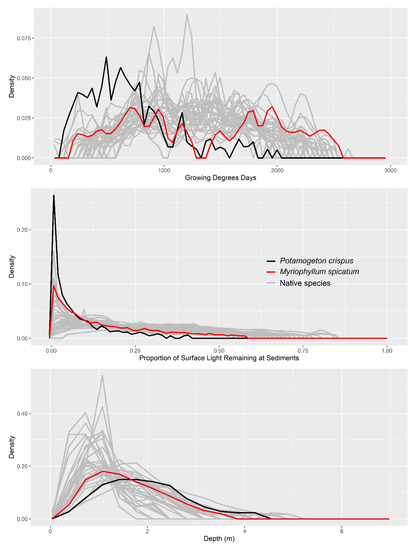
Figure 4.
Abundance-based niches for 32 native macrophyte species and two invasive species (Potamogeton crispus and Myriophyllum spicatum). Niche models are probability density functions fit by kernel density estimation such that each species’ niche integrates to 1.
On average, P. crispus was the most dissimilar to all species of any species evaluated, whereas M. spicatum was much more similar to other plant species (Figure 5). The dissimilarity of P. crispus was driven by a lower use of niche space shared with native species than M. spicatum. This difference in niche use was reflected in the strong relative contribution of non-shared niche space (Figure 5), indicating that a difference in absolute niche boundaries, rather than relative abundance within shared niche space, drove the dissimilarity of P. crispus. Whereas the total dissimilarity of P. crispus to native species was strongly influenced by non-shared niche space, the dissimilarity of M. spicatum was primarily driven by differences in shared niche space. In comparisons with 25 of 32 native species, non-shared niche dissimilarity was greater for P. crispus than it was for M. spicatum. In addition, when compared to each native species, the relative contribution of non-shared niche dissimilarity for P. crispus exceeded that of shared niche dissimilarity 72% of the time (24 of 33 cases) compared to 0% for M. spicatum (0 of 33 cases); this difference was significant (two proportion z-test with continuity correction, p < 0.001).
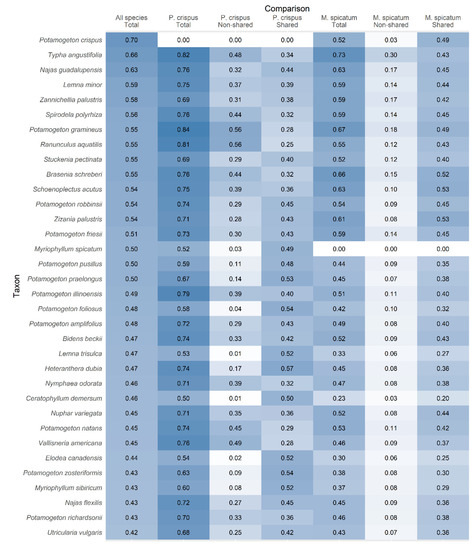
Figure 5.
Probabilistic niche dissimilarity between Potamogeton crispus, Myriophyllum spicatum, and 32 native species. Species are sorted according to their total dissimilarity to other species (mean of each species’ 33 pairwise comparisons). Shading corresponds with numeric values to facilitate visual comparisons.
The distribution of P. crispus and M. spicatum differed across all three niche dimensions evaluated. For P. crispus, niche differentiation was greatest in phenology (Figure 4 and Figure 6); P. crispus exhibited earlier annual growth (mean = 747 GDD) than M. spicatum (mean = 1351 GDD), and native species as a whole (mean = 1254 GDD). Potamogeton crispus also differed more from other species than did M. spicatum in terms of depth and light (Figure 4 and Figure 6). Overall, P. crispus exhibited earlier phenology and occupied locations that were deeper and had lower light availability than M. spicatum (Figure 3 and Figure 4, Kolmogorov-Smirnov tests: p < 0.001 for all three dimensions, D values = 0.41, 0.16, and 0.31 for GDD, depth, and light availability, respectively).
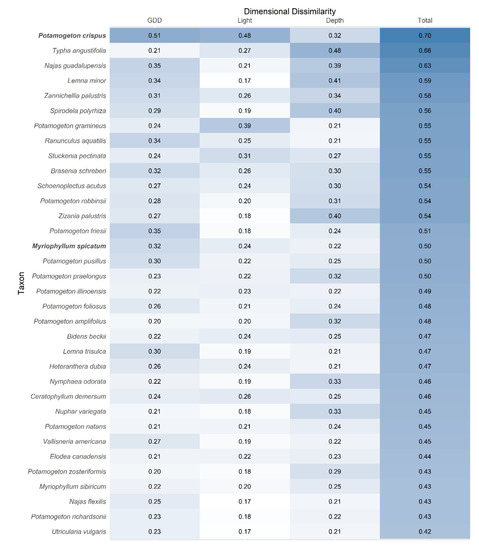
Figure 6.
Comparison of the dimensional dissimilarity of all species evaluated. The focal invasive species are denoted with boldface font. Species are sorted according to their total dissimilarity to other species (mean of each species’ 33 pairwise comparisons). Shading corresponds with numeric values to facilitate visual comparisons.
4. Discussion
Niche differentiation of P. crispus was greater than that of M. spicatum, suggesting that P. crispus may be less likely to directly displace native species through competition for limiting resources—thus, previously observed detrimental impacts on native assemblages for each of these species have likely arisen through differing mechanisms. Niche models exhibited three main patterns in support of this conclusion: (1) a lower overall similarity of the P. crispus niche to those of native macrophyte species, (2) a greater contribution of non-shared niche space (vs. shared niche space) to the overall dissimilarity of P. crispus than was observed for M. spicatum, and (3) less similarity between P. crispus and other macrophytes within each individual niche axis, with the phenological component making the greatest contribution to niche dissimilarity.
The expectation that invasive species’ competitive superiority will lead to the exclusion of co-occurring native species and give rise to invader dominance is often the basis of concerns regarding non-native plant invasions [7,11,87]. By comparing habitat-use strategies of invasive species to resident species of invaded communities, we assessed the potential risk of displacement of native species through competitive interactions. This study provides evidence for large differences in niche overlap with native species for two well-established invasive species that are the subject of intensive management efforts, and indicates that their impacts on native plant communities are likely to differ in magnitude with respect to direct competition and/or arise through different mechanisms [9,88].
Competition for limiting resources can lead to loss of native species following the addition of a competitively superior invader to a community [16], but thoroughly evaluating the extent to which resource competition drives impacts of invasive species to native communities requires experimental manipulations [11,89,90] rather than the observational data used in this study. Further, there are numerous indirect, non-competitive mechanisms by which invasive species can affect native organisms (e.g., [91]). For example, community impacts of P. crispus could arise through increased turbidity of lakes driven by its mid-summer senescence and release of biomass-contained nutrients that stimulate algal productivity [9,92,93]. With the niche-based approach we used, it would not be possible to predict such—potentially substantial—invader impacts. Our approach using observational data cannot address alternative, non-competitive mechanisms of interactions between invasive and native species, rather it provides an estimation of whether direct competition for limited niche space per se is likely to drive displacement of particular native species by particular invasive species. Thus, our findings should not be interpreted as meaning that P. crispus is not detrimental to native macrophytes or is necessarily less detrimental than M. spicatum.
Nonetheless, the distinctiveness of the niche occupied by P. crispus suggests that its potential for direct competitive displacement of native species is lower than that of M. spicatum [14]. Despite this, it is critical to note that both species have succeeded in establishing in North America and both can exhibit dominance of lake plant communities [48,94]. Our results suggest that P. crispus may achieve high abundance through occupancy of a relatively vacant niche [12,86], whereas M. spicatum reaches high abundances under conditions favored by common native species, which may indicate competitive superiority [87] and greater likelihood of driving native species declines via competitive exclusion.
We also found that the dissimilarity of P. crispus to the native community was driven much more by occupancy of non-shared (novel) niche space than in the case of M. spicatum, for which there was greater occupancy of niche space well used by native species. These differences are important to consider in the context of the influence these invaders may have on native species [88,95] and suggest that the competitive threat to native species posed by P. crispus may be lower. However, because the phenological niche we evaluated (GDD) has a strong association with annual growth patterns, P. crispus may be dominating a phenological niche that is important for critical early life stages of native species that are underrepresented through rake-based surveys. Specifically, rake-based methods under sample low-growing or recently sprouted plants [96]. Yet, it is precisely during short-statured, early life stages when macrophyte germinants may be most susceptible to shading by P. crispus individuals that are further into their annual life cycle. Such a phenomenon would be missed by the sampling methods and phenological measures applied in this study. However, in the case of P. crispus, we found that the niche occupied by its early phenology was also highly dissimilar from native species in terms of depth. Because of this, we suspect that P. crispus is capitalizing on seasonal water clarity variations to grow at deeper locations than would be possible for plants with later phenology that encounter lower light availability [72]. In other words, P. crispus may be exploiting a deeper, earlier niche to grow in locations that have insufficient light availability by midsummer (due to high algal biomass in productive, nutrient-rich lakes in temperate regions) [97]. This suggests that P. crispus may have a minimal competitive effect on an early or critical phase of native growth because it is not only temporally separated from native species but also spatially separated.
Interestingly, for both P. crispus and M. spicatum, phenological differentiation was the greatest contributor to niche dissimilarity, though it was exhibited in different ways. In P. crispus, the strong effect of an early-season growth strategy was evident [51], whereas the phenological niche we observed for M. spicatum was highly bimodal. This bimodality is consistent with previous descriptions of M. spicatum phenology [98,99]. However, other studies have described high biomass throughout the growing season, accentuated by two summer peaks [46,98,99,100], whereas our analysis identified a larger apparent gap in its presence in surveys, despite representative sampling effort across GDD. This pattern of bimodality has previously been attributed to sloughing of plant parts between two flowering peaks [100], but the pattern observed in our results shows a lack of presence of M. spicatum in mid-season surveys (our dataset did not include biomass at occurrences). Future work could follow multiple populations through the growing season to evaluate whether observed bimodality arises from of a two-peaked phenology, a variation in the phenological pattern among lakes (populations tending to have either early- or late-season phenology), or a product of both.
A variety of mechanisms may allow each species to compensate for stressors along each of these three axes. For example, M. spicatum exhibits no significant photosynthetic advantage for low-light conditions, but instead tolerates low-light conditions through the ability to elongate and form a canopy at the water surface where light is sufficient for growth [101,102]. For P. crispus, an inverted reproductive cycle and the ability to grow under low temperatures enable it to persist in deeper and more eutrophic waters at times of year when algal biomass is lower and light availability is sufficient for photosynthesis [51,52,72,103]. In short, P. crispus can capitalize on early phenology to grow in locations that have insufficient light later in the growing season. In ecosystems with strong seasonality, as in northern temperate lakes, the influence of temperature on community structure can occur through differences in occupancy of different phenological niches [41,42]. The role of phenology in invasions remains an area of active research, especially as it relates to competition avoidance in early stages of invasion [104].
Even with this dataset, which is unusually large in comparison to other macrophyte studies, there are limitations to the retrospective niche modeling approach using observational data. For example, data demands of the PDF niche model used here meant that only 34 of 163 recorded species had sufficient data to develop three-dimensional niche models. Had we considered a fourth niche dimension (e.g., soluble nutrient concentrations), modeling would have required 10,000 occurrences per species and limited our analysis to just 12 species. In addition, the scale of the dataset meant that measurements of some environmental data were not available. For example, water chemistry data have been collected in a subset of the lakes analyzed, but the need for complete data records for our analysis meant that these data were not useable for our study. We believe that our use of weather station data to calculate annual GDD is reasonable, but lake-level water temperatures would be more accurate—these data, however, are not available. As a consequence, the models we have derived may be shifted with respect to the actual thermal regime of a lake at any given time (i.e., average air temperature will generally increase faster than lagging water temperatures throughout the year), but because of the comparative nature of this study, all modeled niches are subjected to the same direction and magnitude of this potential bias. It is important to note that we have not captured every factor that influences macrophyte niches in this study. For some cases, such as free-floating plants (which have no physiological reason to be constrained by water depth, yet show a strong depth pattern in their niche occupancy; Figures S1 and S3), observed patterns likely reflect the effects of an unobserved covarying factor, such as wave-induced surface movement. In addition, nutrient loading has been associated with dominance of invasive species [8]. Light is often, but not always driven by nutrient concentrations in Minnesota lakes [105], thus future work should explicitly explore the mechanism by which nutrient enrichment promotes invader dominance in these systems. We acknowledge these limitations and suggest that each be addressed or expanded upon in the future.
It is rarely feasible to evaluate the full niche of an invasive species in its invaded range, where it is unlikely to have yet reached the full extent of its potential spread. Moreover, it is difficult to infer whether co-occurrences with native species translate to antagonistic interactions [106]. However, we can make preliminary inferences regarding interactions between native and invasive species when there is suitable information to characterize the niche of an invading species, as enabled by the large state-wide dataset we used here. By evaluating multiple niches across many species, we were able to draw inferences about the extent to which species may be competing or avoiding competition. Experiments to directly quantify the strength of competitive interactions between these invaders and resident plant communities should subsequently test our finding that P. crispus exhibits less competitive interaction with native species than M. spicatum.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/12/4/162/s1, Figures S1–S3: Visualizations of niches for all species analyzed. Code S1: Statistical software code (R) for conducting the primary data cleaning, analysis, and visualizations presented in this manuscript and supplementary figures.
Author Contributions
Conceptualization, M.R.V., W.J.G. and D.J.L.; methodology, M.R.V., W.J.G. and D.J.L.; formal analysis, M.R.V.; investigation, M.R.V.; data curation, M.R.V.; writing—original draft preparation, M.R.V.; writing—review and editing, M.R.V., W.J.G. and D.J.L.; visualization, M.R.V.; funding acquisition, M.R.V., W.J.G. and D.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Minnesota Environmental and Natural Resources Trust Fund as recommended by the Minnesota Aquatic Invasive Species Research Center (MAISRC) and the Legislative-Citizen Commission on Minnesota Resources. The APC was funded by MAISRC. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. CON-75851, project 00074041. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Acknowledgments
We thank Justin Townsend, Noah Berg, Carolyn Kalinowski, James Dickson, and Natalie Holmes for their help in inventorying plant survey data. We are very grateful to all of the surveyors who generously organized and shared their data with us. For their exceptional contributions of data, we are particularly indebted to James Johnson of Freshwater Scientific Services, Matt Berg of Endangered Resource Services, Meg Rattei of Barr Engineering, Eric Fieldseth of AIS Consulting Services, Steve McComas of Blue Water Science, Jill Sweet of the Minnhehaha Creek Watershed District, Cole Loewen of the Clearwater River Watershed District, Britta Belden of the Capitol Region Watershed District, the Minnesota Department of Natural Resources Invasive Species Program and Lake Ecology Unit, Andrea Prichard of Ramsey Conservation District, and Rob Brown of the Minneapolis Parks District. We thank Ray Newman for the invitation to contribute to this special issue and three anonymous reviewers for comments that substantially improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Courchamp, F.; Fournier, A.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Jeschke, J.M.; Russell, J.C. Invasion biology: Specific problems and possible solutions. Trends Ecol. Evol. 2017, 32, 13–22. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Bradley, B.A.; Laginhas, B.B.; Whitlock, R.; Allen, J.M.; Bates, A.E.; Bernatchez, G.; Diez, J.M.; Early, R.; Lenoir, J.; Vilà, M.; et al. Disentangling the abundance-impact relationship for invasive species. Proc. Natl. Acad. Sci. USA 2019, 116, 9919–9924. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.T.; Flores-Moreno, H.; Bonser, S.P.; Warton, D.I.; Helm, A.; Warman, L.; Eldridge, D.J.; Jurado, E.; Hemmings, F.A.; Reich, P.B.; et al. Invasions: The trail behind, the path ahead, and a test of a disturbing idea. J. Ecol. 2012, 100, 116–127. [Google Scholar] [CrossRef]
- Boltovskoy, D.; Sylvester, F.; Paolucci, E.M. Invasive species denialism: Sorting out facts, beliefs, and definitions. Ecol. Evol. 2018, 8, 11190–11198. [Google Scholar] [CrossRef]
- Schirmel, J.; Bundschuh, M.; Entling, M.H.; Kowarik, I.; Buchholz, S. Impacts of invasive plants on resident animals across ecosystems, taxa, and feeding types: A global assessment. Glob. Chang. Biol. 2016, 22, 594–603. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Harpole, W.S.; Reichman, O.J.; Tilman, D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc. Natl. Acad. Sci. USA 2003, 100, 13384–13389. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.P.; Dibble, E.D. Ecological mechanisms of invasion success in aquatic macrophytes. Hydrobiologia 2015, 746, 23–37. [Google Scholar] [CrossRef]
- Levine, J.M.; Vilà, M.; D’Antonio, C.M.; Dukes, J.S.; Grigulis, K.; Lavorel, S. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. 2003, 270, 775–781. [Google Scholar] [CrossRef]
- Blumenthal, D.M.; Hufbauer, R.A. Increased plant size in exotic populations: A common-garden test with 14 invasive species. Ecology 2007, 88, 2758–2765. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- Hillerislambers, J.; Adler, P.B.; Harpole, W.S.; Levine, J.M.; Mayfield, M.M. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 227–248. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Divíšek, J.; Chytrý, M.; Beckage, B.; Gotelli, N.J.; Lososová, Z.; Pyšek, P.; Richardson, D.M.; Molofsky, J. Similarity of introduced plant species to native ones facilitates naturalization, but differences enhance invasion success. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Zwerschke, N.; van Rein, H.; Harrod, C.; Reddin, C.; Emmerson, M.C.; Roberts, D.; O’Connor, N.E. Competition between co-occurring invasive and native consumers switches between habitats. Funct. Ecol. 2018, 32, 2717–2729. [Google Scholar] [CrossRef]
- Escoriza, D.; Ruhí, A. Functional distance to recipient communities may favour invasiveness: Insights from two invasive frogs. Divers. Distrib. 2016, 22, 519–533. [Google Scholar] [CrossRef]
- Fournier, A.; Penone, C.; Pennino, M.G.; Courchamp, F. Predicting future invaders and future invasions. Proc. Natl. Acad. Sci. USA 2019, 116, 7905–7910. [Google Scholar] [CrossRef]
- Mack, R.N. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Darwin, C.R. On the Origin of Species by Means of Natural Selection, or Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Patten, B.C.; Auble, G.T. System theory of the ecological niche. Am. Nat. 1981, 117, 893–922. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Springer: Boston, MA, USA, 1958. [Google Scholar]
- Carpenter, S.R.; Mooney, H.A.; Agard, J.; Capistrano, D.; DeFries, R.S.; Diaz, S.; Dietz, T.; Duraiappah, A.K.; Oteng-Yeboah, A.; Pereira, H.M.; et al. Science for managing ecosystem services: Beyond the Millennium Ecosystem Assessment. Proc. Natl. Acad. Sci. USA 2009, 106, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, J. The niche-relationships of the California Thrasher. Auk 1917, 34, 427–433. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Hussner, A.; Stiers, I.; Verhofstad, M.J.J.M.; Bakker, E.S.; Grutters, B.M.C.; Haury, J.; van Valkenburg, J.L.C.H.; Brundu, G.; Newman, J.; Clayton, J.S.; et al. Management and control methods of invasive alien aquatic plants: A review. Aquat. Bot. 2017, 136, 112–137. [Google Scholar] [CrossRef]
- Hussner, A.; Van De Weyer, K.; Gross, E.M.; Hilt, S. Comments on increasing number and abundance of non-indigenous aquatic macrophyte species in Germany. Weed Res. 2010, 50, 519–526. [Google Scholar] [CrossRef]
- Capers, R.; Selsky, R.; Bugbee, G.; White, J. Aquatic plant community invasibility and scale-dependent patterns in native and invasive species richness. Ecology 2007, 88, 3135–3143. [Google Scholar] [CrossRef]
- Muthukrishnan, R.; Hansel-Welch, N.; Larkin, D.J. Environmental filtering and competitive exclusion drive biodiversity-invasibility relationships in shallow lake plant communities. J. Ecol. 2018, 106, 2058–2070. [Google Scholar] [CrossRef]
- Schultz, R.; Dibble, E. Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: The role of invasive plant traits. Hydrobiologia 2012, 684, 1–14. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Hiatt, D.; Serbesoff-King, K.; Lieurance, D.; Gordon, D.R.; Flory, S.L. Allocation of invasive plant management expenditures for conservation: Lessons from Florida, USA. Conserv. Sci. Pract. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Hussner, A.; Nehring, S.; Hilt, S. From first reports to successful control: A plea for improved management of alien aquatic plant species in Germany. Hydrobiologia 2014, 737, 321–331. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Cronk, J.K.; Fennessy, M.S. Wetland Plants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Wetzel, R.G. Limnology; W.B. Saunders Company: Philidelphia, PA, USA, 1975. [Google Scholar]
- Hudon, C.; Lalonde, S.; Gagnon, P. Ranking the effects of site exposure, plant growth form, water depth, and transparency on aquatic plant biomass. Can. J. Fish. Aquat. Sci. 2000, 57, 31–42. [Google Scholar] [CrossRef]
- Gillefalk, M.; Herzog, C.; Hilt, S. Phosphorus availability and growth of benthic primary producers in littoral lake sediments: Are differences linked to induced bank filtration? Water 2019, 11, 1111. [Google Scholar] [CrossRef]
- Hofmann, H.; Lorke, A.; Peeters, F. Wave-induced variability of the underwater light climate in the littoral zone. Verh. lnt. Verein. Limnol. 2008, 30, 627–632. [Google Scholar] [CrossRef][Green Version]
- Chambers, P.A. Light and nutrients in the control of aquatic plant community structure. II. In situ observations. J. Ecol. 1987, 75, 621–628. [Google Scholar] [CrossRef]
- Godoy, O.; Levine, J.M. Phenology effects on invasion success: Insights from coupling field experiments to coexistence theory. Ecology 2014, 95, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Torso, K.; Scofield, B.D.; Chess, D.W. Variations in aquatic macrophyte phenology across three temperate lakes in the Coeur d’Alene Basin. Aquat. Bot. 2020, 162, 103209. [Google Scholar] [CrossRef]
- Aiken, S.G.; Newroth, R.; Wiles, I. The biology of Canadian weeds. Myriophyllum spicatum L. Can. J. Plant Sci. 1979, 59, 201–215. [Google Scholar] [CrossRef]
- Eiswerth, M.; Donaldson, S.; Johnson, W. Potential environmental impacts and economic damages of Eurasian watermilfoil (Myriophyllum spicatum) in western Nevada and northeastern California. Weed Technol. 2000, 14, 511–518. [Google Scholar] [CrossRef]
- Barko, J.W.; Smart, M.R. Comparative Influences of Light and Temperature on the Growth and Metabolism of Selected Submersed Freshwater Macrophytes. Ecol. Monogr. 1981, 51, 219–236. [Google Scholar] [CrossRef]
- Smith, C.S.; Barko, J.W. Ecology of Eurasian watermilfoil. J. Aquat. Plant Manag. 1990, 28, 55–64. [Google Scholar]
- Titus, J.E.; Adams, M.S. Coexistence and the comparative light relations of the submersed macrophytes Myriophyllum spicatum L. and Vallisneria americana Michx. Oecologia 1979, 40, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Bolduan, B.R.; Van Eeckhout, G.C.; Quade, H.W.; Gannon, J.E. Potamogeton crispus—The other invader. Lake Reserv. Manag. 1994, 10, 113–125. [Google Scholar] [CrossRef]
- Tobiessen, P.; Snow, P.D. Temperature and light effects on the growth of Potamogeton crispus in Collins Lake, New York State. Can. J. Bot. 1984, 62, 2822–2826. [Google Scholar] [CrossRef]
- Chambers, P.A.; Spence, D.H.N.; Weeks, D.C. Photocontrol of turion formation by Potamogeton Crispus, L. in the laboratory and natural water. New Phytol. 1985, 99, 183–194. [Google Scholar] [CrossRef]
- Woolf, T.E.; Madsen, J. Seasonal biomass and carbohydrate allocation patterns in southern Minnesota curlyleaf pondweed populations. J. Aquat. Plant Manag. 2003, 41, 113–118. [Google Scholar]
- Adamec, L. Ecophysiological characteristics of turions of aquatic plants: A review. Aquat. Bot. 2018, 148, 64–77. [Google Scholar] [CrossRef]
- Carmona, C.P.; de Bello, F.; Mason, N.W.H.; Lepš, J. Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef]
- Blonder, B. Hypervolume concepts in niche- and trait-based ecology. Ecography (Cop.). 2018, 41, 1441–1455. [Google Scholar] [CrossRef]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. The n-dimensional hypervolume. Glob. Ecol. Biogeogr. 2014, 23, 595–609. [Google Scholar] [CrossRef]
- Perleberg, D.; Radomski, P.; Simon, S.; Carlson, K.; Knopik, J. Minnesota Lake Plant Survey Manual, for Use by MNDNR Fisheries Section and EWR Lake Habitat Program; Minnesota Department of Natural Resources: Brainerd, MN, USA, 2016. [Google Scholar]
- Johnson, J.A.; Jones, A.R.; Newman, R.M. Evaluation of lakewide, early season herbicide treatments for controlling invasive curlyleaf pondweed (Potamogeton crispus) in Minnesota lakes. Lake Reserv. Manag. 2012, 28, 346–363. [Google Scholar] [CrossRef]
- Jones, A.R.; Johnson, J.A.; Newman, R.M. Effects of repeated, early season, herbicide treatments of curlyleaf pondweed on native macrophyte assemblages in Minnesota lakes. Lake Reserv. Manag. 2012, 28, 364–374. [Google Scholar] [CrossRef]
- Verhoeven, M.R.; Larkin, D.J.; Newman, R.M. Constraining invader dominance: Effects of repeated herbicidal management and environmental factors on curlyleaf pondweed dynamics in 50 Minnesota lakes. Freshw. Biol. 2020, 1–14. [Google Scholar] [CrossRef]
- Nault, M.; Mikulyuk, A.; Hauxwell, J.; Skogerboe, J.; Asplund, T.; Barton, M.; Wagner, K.; Hoyman, T.; Heath, E. Herbicide Treatments in Wisconsin Lakes: Building a Framework for Scientific Evaluation of Large-scale Herbicide Treatments in Wisconsin Lakes. NALMS Lake Line 2012, 32, 19–24. [Google Scholar]
- Kujawa, E.R.; Frater, P.; Mikulyuk, A.; Barton, M.; Nault, M.E.; Van Egeren, S.; Hauxwell, J. Lessons from a decade of lake management: Effects of herbicides on Eurasian watermilfoil and native plant communities. Ecosphere 2017, 8, e01718. [Google Scholar] [CrossRef]
- Frater, P.; Mikulyuk, A.; Barton, M.; Nault, M.; Wagner, K.; Hauxwell, J.; Kujawa, E. Relationships between water chemistry and herbicide efficacy of Eurasian watermilfoil management in Wisconsin lakes. Lake Reserv. Manag. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Anderson, M.; Clark, M.; Cornett, M.; Hall, K.; Olivero Sheldon, A.; Prince, J. Resilient Sites for Terrestrial Conservation in the Great Lakes and Tallgrass Prairie Region; The Nature Conservancy, Eastern Conservation Science and North America Region: Boston, MA, USA, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Madsen, J.D.; Wersal, R.M. A review of aquatic plant monitoring and assessment methods. J. Aquat. Plant Manag. 2017, 55, 1–12. [Google Scholar]
- Chadde, S.W. Wetland Plants of Minnesota: A Complete Guide to the Wetland and Aquatic Plants of the North Star State; Createspace Independent Publishing Platform: Lexington, KY, USA, 2012; ISBN 9781460940341. [Google Scholar]
- Crow, G.E.; Hellquist, C.B. Aquatic and Wetland Plants of Northeastern North America. Volum Pteridophytes, Gymnosperms and Angiosperms: Dicotyledons; University of Wisconsin Press: Madison, WI, USA, 2000. [Google Scholar]
- Crow, G.E.; Hellquist, C.B. Aquatic and Wetland Plants of Northeastern North America. Volume Angiosperms: Monocotyledons; University of Wisconsin Press: Madison, WI, USA, 2000. [Google Scholar]
- Skawinski, P.M. Aquatic Plants of the Upper Midwest; Paul Skawinski: Wausau, WI, USA, 2011; ISBN 978-1-4507-9247-9. [Google Scholar]
- Boyle, B.; Hopkins, N.; Lu, Z.; Raygoza Garay, J.A.; Mozzherin, D.; Rees, T.; Matasci, N.; Narro, M.L.; Piel, W.H.; Mckay, S.J.; et al. The taxonomic name resolution service: An online tool for automated standardization of plant names. BMC Bioinf. 2013, 14, 16. [Google Scholar] [CrossRef]
- Chamberlain, S.A.; Szöcs, E. Taxize: Taxonomic search and retrieval in R. F1000 Res. 2013, 2, 1–30. [Google Scholar] [CrossRef]
- Stadelmann, T.H.; Brezonik, P.L.; Kloiber, S. Seasonal patterns of chlorophyll a and secchi disk transparency in lakes of East-central Minnesota: Implications for design of ground—And satellite-based monitoring programs. Lake Reserv. Manag. 2001, 17, 299–314. [Google Scholar] [CrossRef]
- Preisendorfer, R.W. Eyeball optics of natural waters: Secchi disk science. Limnol. Oceanogr. 1986, 31, 909–926. [Google Scholar] [CrossRef]
- Steele, E.A.; Neuhausser, S. Comparison of methods for measuring visual water clarity. J. North Am. Benthol. Soc. 2002, 21, 326–335. [Google Scholar] [CrossRef]
- Megard, R.O.; Settles, J.C.; Boyer, H.A.; Combs, W.S.J. Light, Secchi disks, and trophic states. Limnol. Oceanogr. 1980, 25, 373–377. [Google Scholar] [CrossRef]
- Van Duin, E.H.S.; Blom, G.; Los, F.J.; Maffione, R.; Zimmerman, R.; Cerco, C.F.; Dortch, M.; Best, E.P.H. Modeling underwater light climate in relation to sedimentation, resuspension, water quality and autotrophic growth. Hydrobiologia 2001, 444, 25–42. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Cross, H.Z.; Zuber, M.S. Prediction of flowering dates in maize based on different methods of estimating thermal units. Agron. J. 1972, 64, 351–355. [Google Scholar] [CrossRef]
- Russelle, M.P.; Wilhelm, W.W.; Olson, R.A.; Power, J.F. Growth analysis based on degree days. Crop Sci. 1984, 24, 28–32. [Google Scholar] [CrossRef]
- Luedeling, E.; Kunz, A.; Blanke, M.M. Identification of chilling and heat requirements of cherry trees-a statistical approach. Int. J. Biometeorol. 2013, 57, 679–689. [Google Scholar] [CrossRef]
- NOAA. National Centers for Environmental Information Global Surface Hourly [2001–2018]; NOAA: Washington, DC, USA, 2018.
- Boissezon, A.; Auderset Joye, D.; Garcia, T. Temporal and spatial changes in population structure of the freshwater macroalga Nitellopsis obtusa (Desv.) J.Groves. Bot. Lett. 2018, 165, 103–114. [Google Scholar] [CrossRef]
- Carmona, C.P.; Bello, F.; Mason, N.W.H.; Lepš, J. Trait probability density (TPD): Measuring functional diversity across scales based on TPD with R. Ecology 2019, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. Using n-dimensional hypervolumes for species distribution modelling: A response to Qiao et al. (). Glob. Ecol. Biogeogr. 2017, 26, 1071–1075. [Google Scholar] [CrossRef]
- Blonder, B.; Morrow, C.B.; Maitner, B.; Harris, D.J.; Lamanna, C.; Violle, C.; Enquist, B.J.; Kerkhoff, A.J. New approaches for delineating n -dimensional hypervolumes. Methods Ecol. Evol. 2018, 9, 305–319. [Google Scholar] [CrossRef]
- Blonder, B. Do hypervolumes have holes? Am. Nat. 2016, 187, E93–E105. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. CRC. Crit. Rev. Plant Sci. 2004, 23, 431–452. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Gilbert, B.; Levine, J.M. Plant invasions and the niche. J. Ecol. 2009, 97, 609–615. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Agrawal, A.A.; Bever, J.D.; Gilbert, G.S.; Hufbauer, R.A.; Klironomos, J.N.; Maron, J.L.; Morris, W.F.; Parker, I.M.; Power, A.G.; et al. Biotic interactions and plant invasions. Ecol. Lett. 2006, 9, 726–740. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Borer, E.T.; Boucher, V.L.; Burton, R.S.; Cottingham, K.L.; Goldwasser, L.; Gram, W.K.; Kendall, B.E.; Micheli, F. Competition, seed limitation, disturbance, and reestablishment of California native annual forbs. Ecol. Appl. 2003, 13, 575–592. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Hilt, S.; Henschke, I.; Rücker, J.; Nixdorf, B. Can submerged macrophytes influence turbidity and trophic state in deep lakes? Suggestions from a case study. J. Environ. Qual. 2010, 39, 725. [Google Scholar] [CrossRef]
- James, W.F.; Dechamps, A.; Turyk, N.; Mcginley, P. Contribution of Potamogeton Crispus Decay to the Phosphorus Budget of McGinnis Lake, Wisconsin; U.S. Army Engineer Research and Development Center: Vicksburg, MS, USA, 2007. [Google Scholar]
- Boylen, C.W.; Eichler, L.W.; Madsen, J.D. Loss of native aquatic plant species in a community dominated by Eurasian watermilfoil. Hydrobiologia 1999, 415, 207–211. [Google Scholar] [CrossRef]
- Scheele, B.C.; Foster, C.N.; Banks, S.C.; Lindenmayer, D.B. Niche contractions in declining species: Mechanisms and consequences. Trends Ecol. Evol. 2017, 32, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Newman, M. A comparison of two methods for sampling biomass of aquatic plants. J. Aquat. Plant Manag. 2011, 49, 1–8. [Google Scholar]
- Marshall, C.T.; Peters, R.H. General patterns in the seasonal development of chlorophyll a for temperate lakes. Limnol. Oceanogr. 1989, 34, 856–867. [Google Scholar] [CrossRef]
- Titus, J.; Goldstein, R.A.; Adams, M.S.; Mankin, J.B.; O’neill, R.V.; Weiler, P.R., Jr.; Shugart, H.H.; Booth, R.S. A production model for Myriophyllum spicatum L. Ecology 1997, 56, 1129–1138. [Google Scholar] [CrossRef]
- Grace, J.B.; Wetzel, R.G. The production biology of Eurasian watermilfoil (Myriophyllum spicatum L.): A Review. J. Aquat. Plant. Manag. 1978, 16, 1–11. [Google Scholar]
- Adams, M.S.; McCracken, M.D. Seasonal Production of the Myriophyllum Component of the Littoral of Lake Wingra, Wisconsin. J. Ecol. 1974, 62, 457. [Google Scholar] [CrossRef]
- Galatowitsch, S.M.; Anderson, N.O.; Ascher, P.D. Invasiveness in wetland plants in temperate North America. Wetlands 1999, 19, 733–755. [Google Scholar] [CrossRef]
- Valley, R.D.; Bremigan, M.T. Effects of macrophyte bed architecture on largemouth bass foraging: Implications of exotic macrophyte invasions. Trans. Am. Fish. Soc. 2002, 131, 234–244. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, S.; Liang, W.; He, F.; Wu, Z. Effects of sediment anoxia and light on turion germination and early growth of Potamogeton crispus. Hydrobiologia 2009, 628, 111–119. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 1–21. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).