Coral Restoration Effectiveness: Multiregional Snapshots of the Long-Term Responses of Coral Assemblages to Restoration
Abstract
1. Introduction
2. Materials and Methods
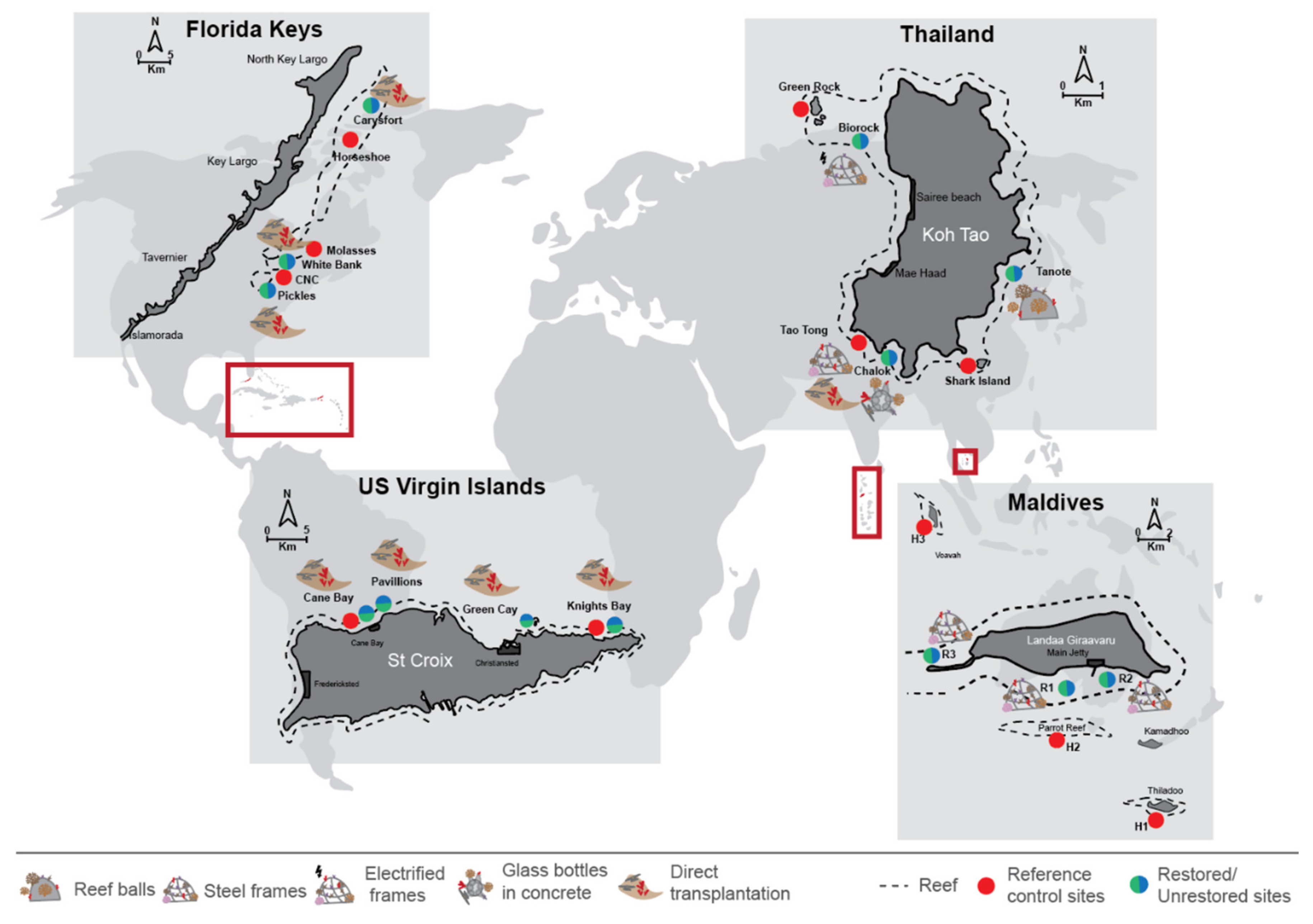
2.1. Study Sites
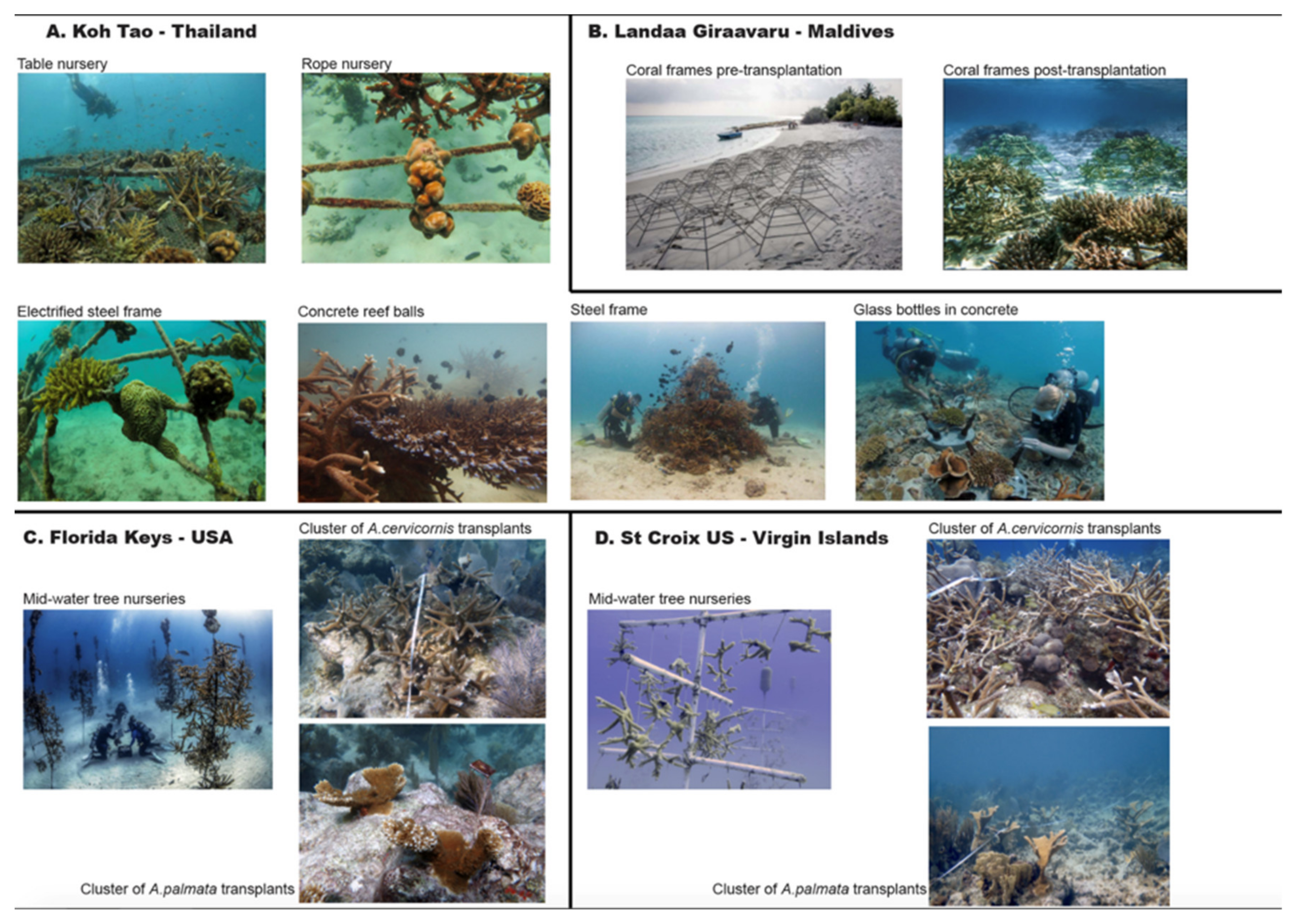
2.1.1. Box 1. Coral Restoration in Koh Tao, Thailand
2.1.2. Box 2. Coral Restoration in Landaa Giraavaru, Maldives
2.1.3. Box 3. Coral Restoration in the Florida Keys, USA
2.1.4. Box 4. Coral Restoration in St Croix, US Virgin Islands
2.2. Measuring ‘Snap Shots’ of Coral Assemblages’ Response to Restoration
2.2.1. Benthic Cover and Structural Complexity
2.2.2. Coral Health, Generic Richness, and Juvenile Recruitment
2.3. Data Analysis
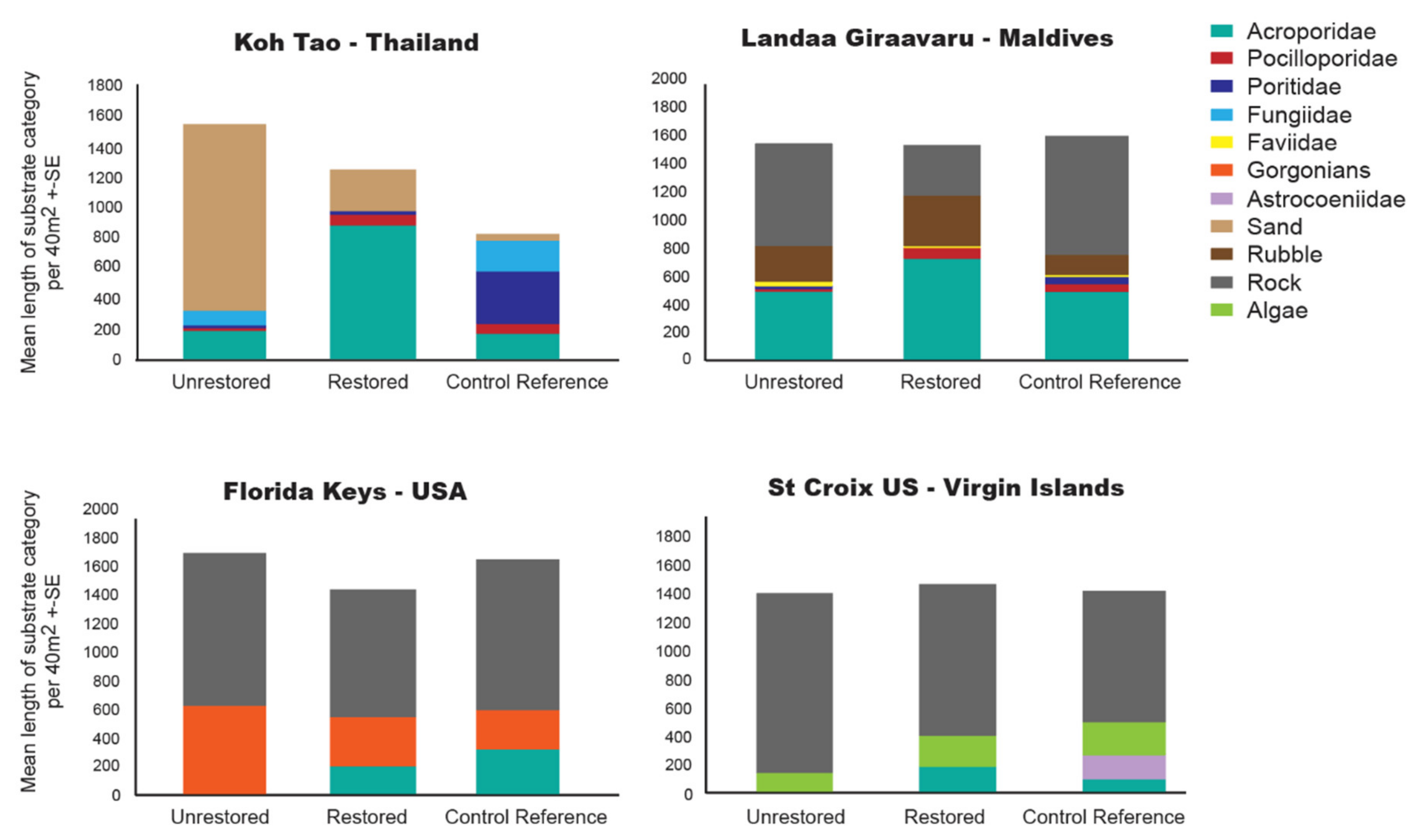
2.3.1. Benthic Cover
2.3.2. Structural Complexity
2.3.3. Coral Generic Richness Abundance of Juvenile Corals
2.3.4. Coral Health
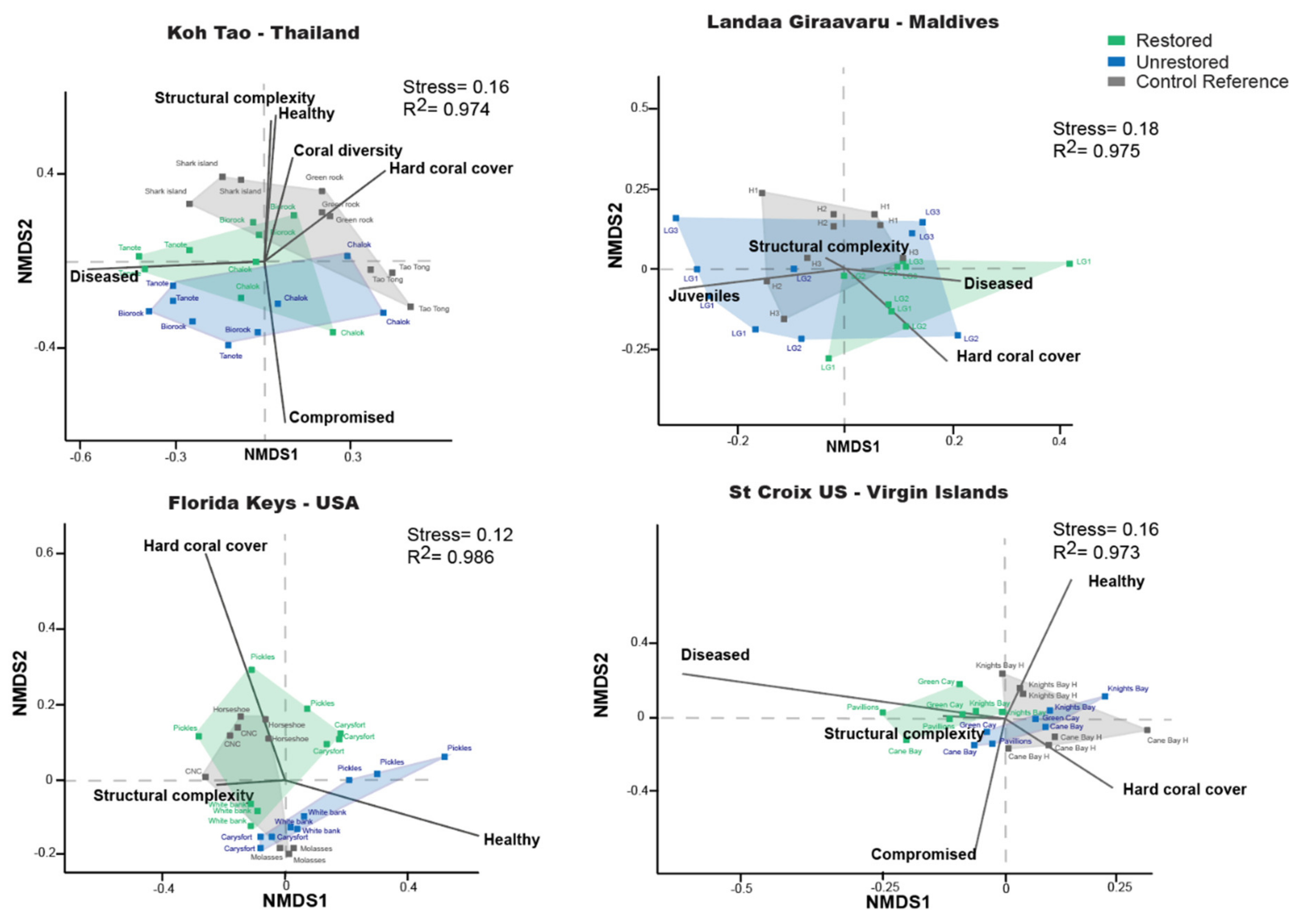
2.3.5. Coral Assemblages
3. Results
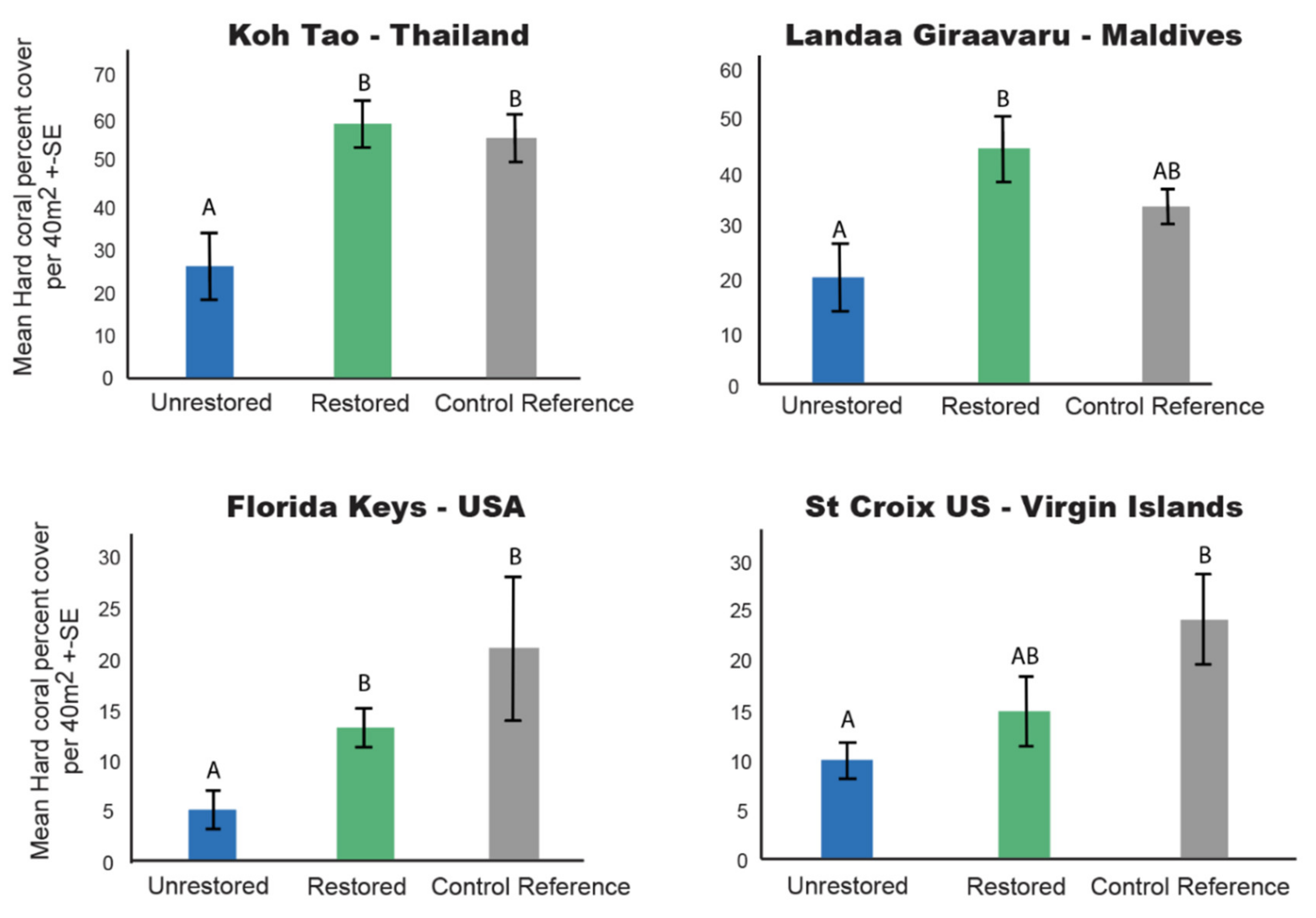
3.1. Hard Coral Cover
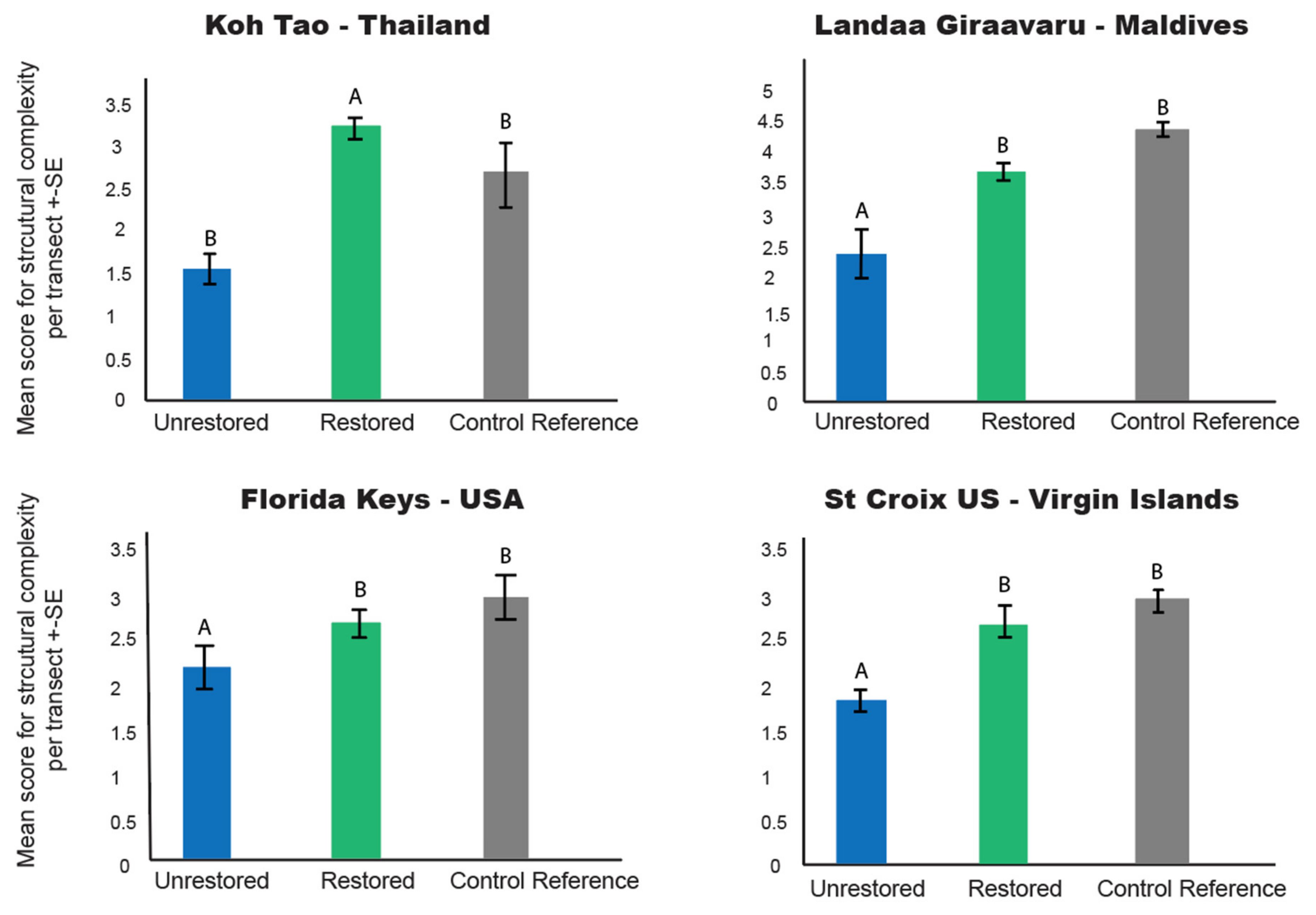
3.2. Structural Complexity
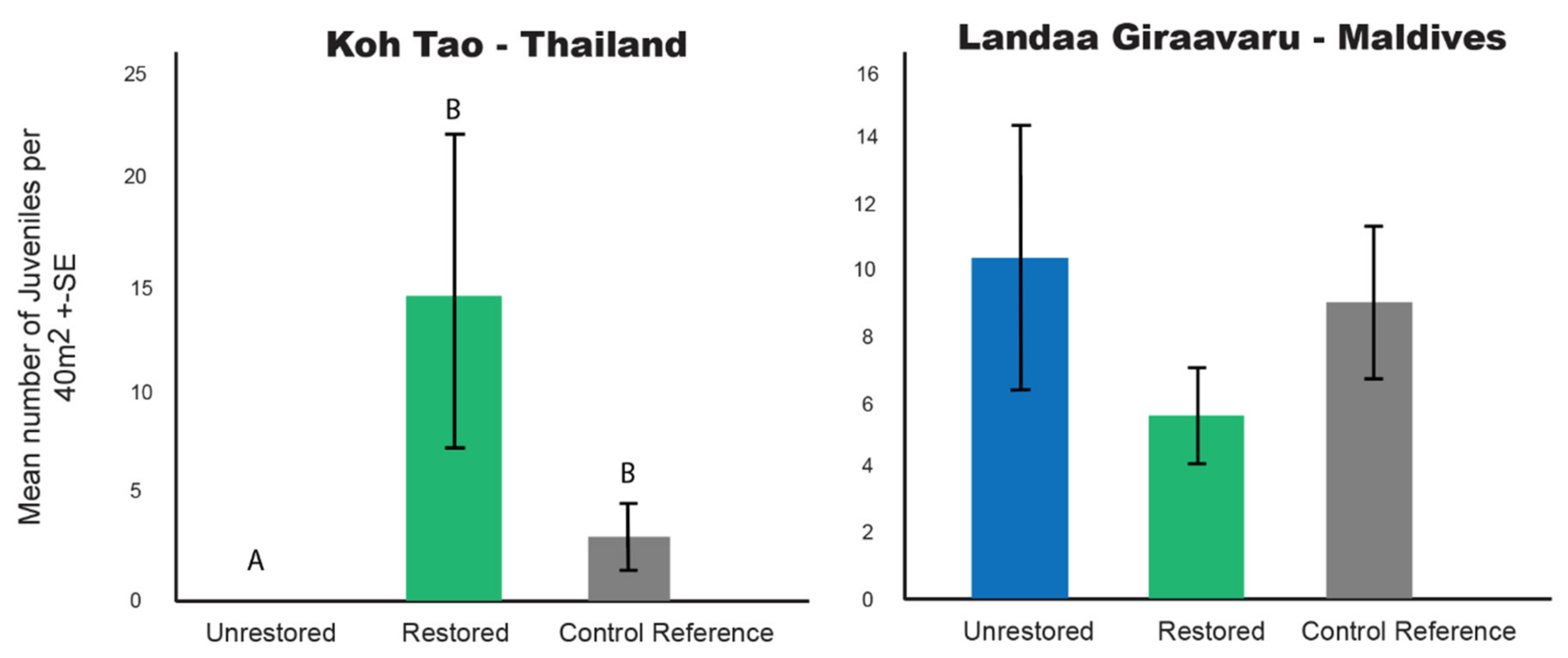
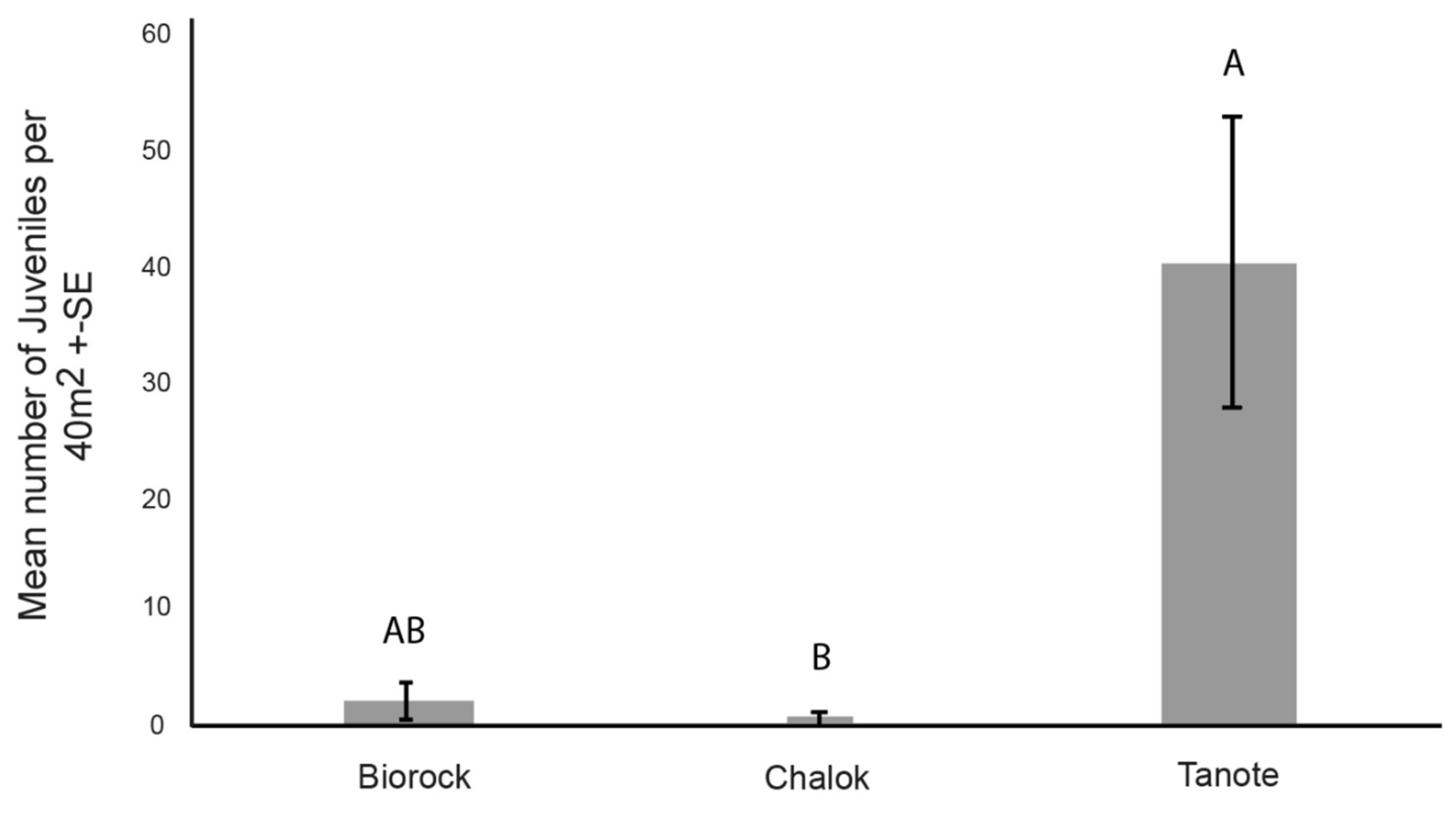
3.3. Number of Coral Juveniles
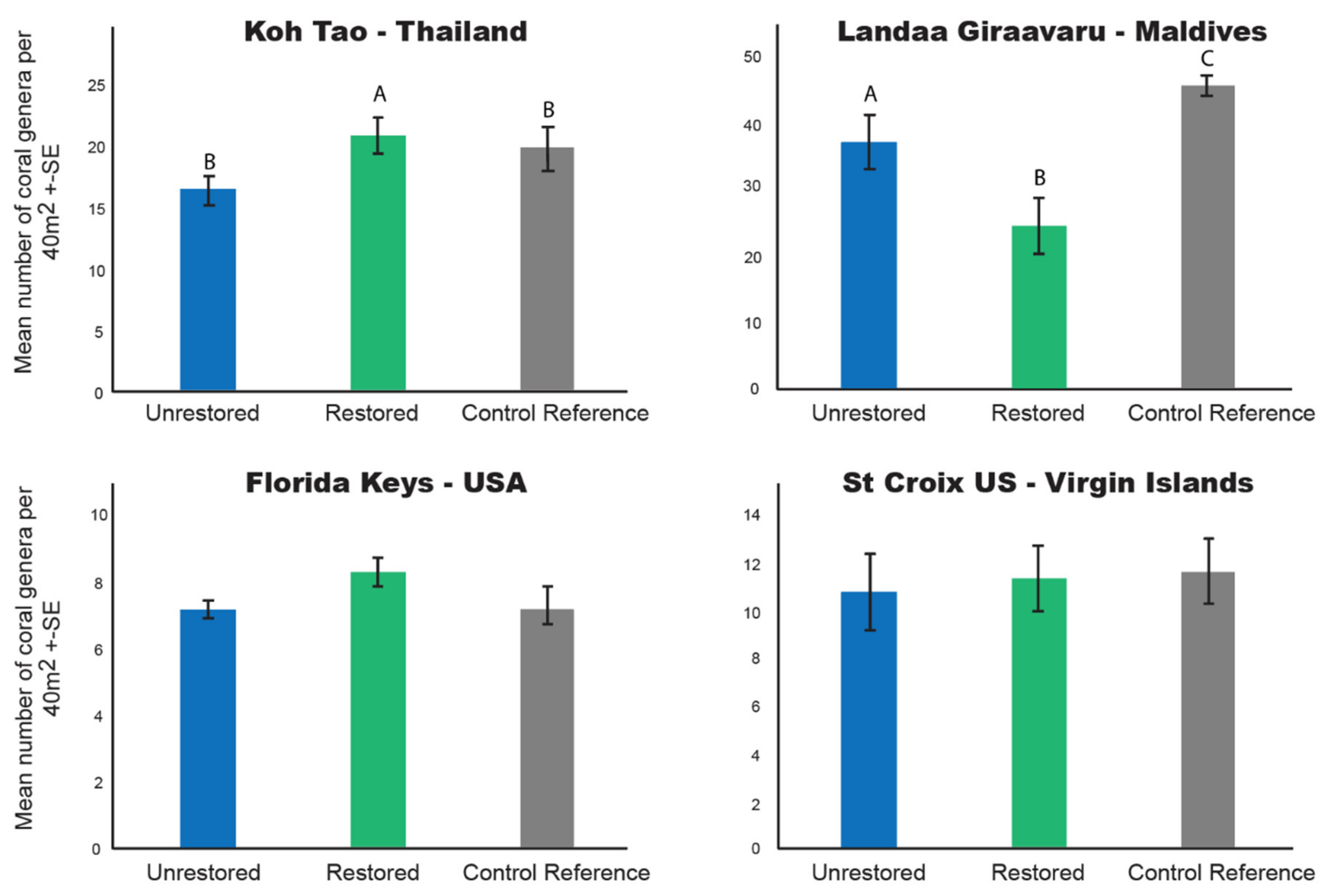
3.4. Coral Generic Richness
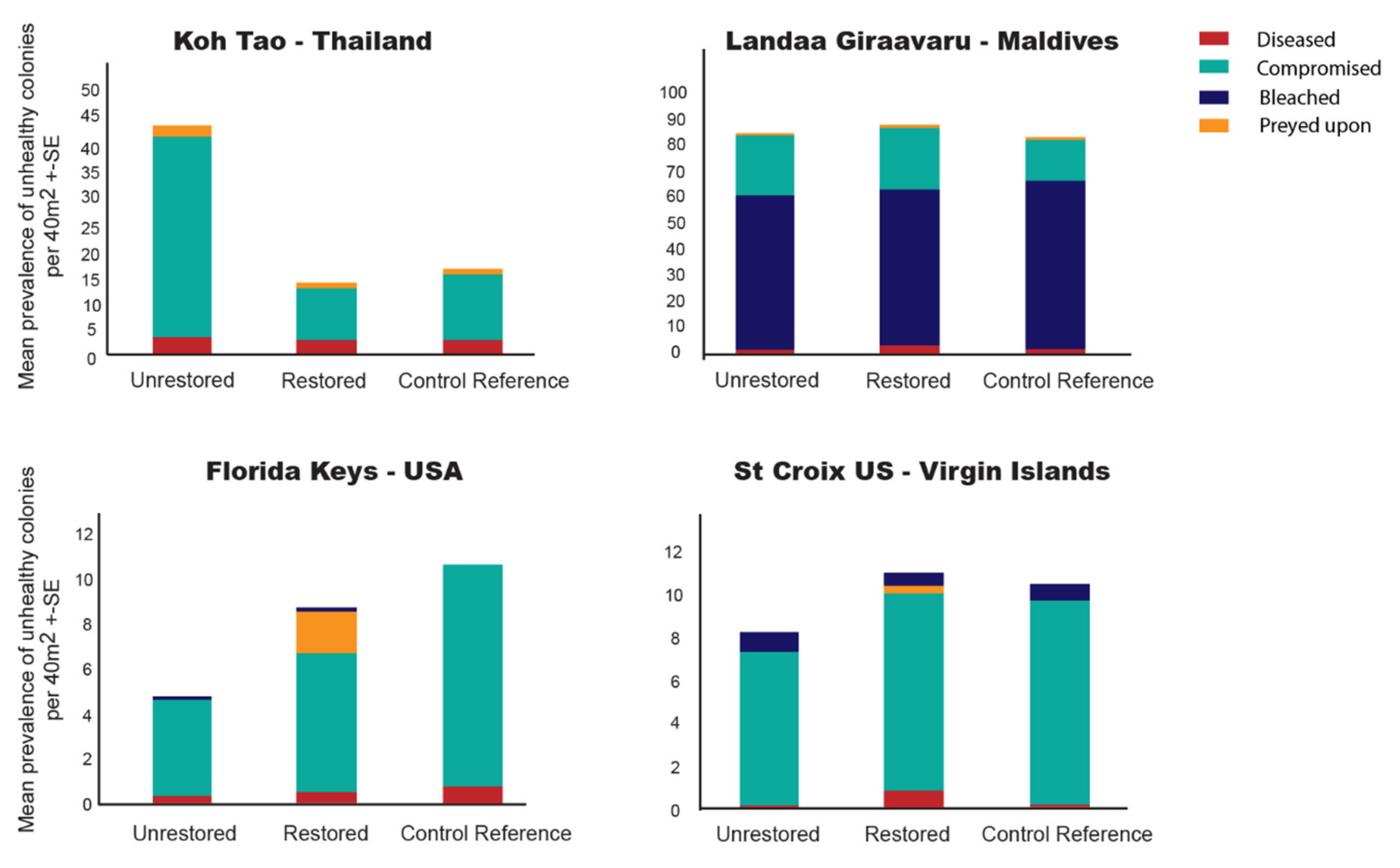
3.5. Coral Health
3.6. Composition of the Coral Assemblages
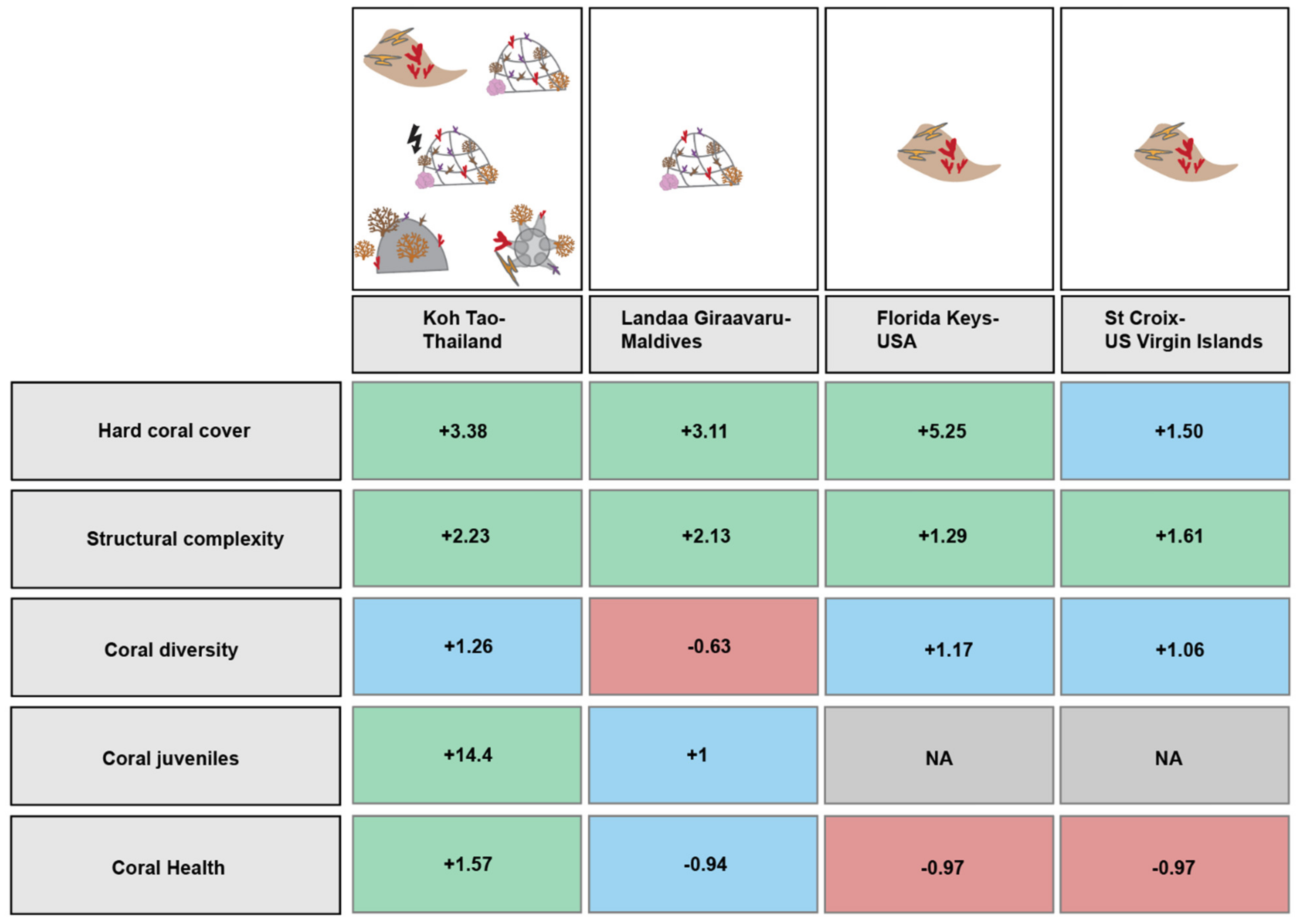
3.7. Summary and Links with Restoration Designs
4. Discussion
4.1. Restoration Increases Coral Cover and Structural Complexity
4.2. Other Coral-Based Indicators of Reef Resilience Vary among Restoration Programs
4.3. Coral Restoration Influences the Composition of the Benthic Community
5. Limitations and Further Research
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-term region-wide declines in Caribbean corals. Science 2003, 301, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Alvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Belwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.; Kerry, J.T.; Lough, J.; Baird, A.H.; Baums, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.Y.; Willis, B.L.; Beeden, R.; Birtles, A. The need for broader ecological and socio-economic tools to evaluate the effectiveness of coral restoration programs. Restor. Ecol. 2017, 25, 877–883. [Google Scholar] [CrossRef]
- Boström-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Cecarelli, D.; Cook, N.; Ferse, S.C.A.; Hancock, B.; Harisson, P.; Hein, M.Y.; Shaver, E.; et al. Coral restoration- a systematic review of methods, successes, failures, and future directions. PLoS ONE 2020, 15, e0226631. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Edwards, A.J. Coral transplantation as an aid to reef rehabilitation: Evaluation of a case study in the Maldives Islands. Coral Reefs 1995, 14, 201–213. [Google Scholar] [CrossRef]
- Rinkevich, B. What do we know about Eilat (Red Sea) reef degradation? A critical examination of the published literature. J. Exp. Mar. Biol. Ecol. 2005, 327, 183–200. [Google Scholar] [CrossRef]
- McLeod, E.; Anthony, K.R.N.; Mumby, P.J.; Maynard, J.A.; Beeden, R.; Graham, N.A.J.; Heron, S.F.; Hoegh-Guldberg, O.; Jupiter, S.; MacGowan, P.; et al. The future of resilience-based management in coral reef ecosystems. J. Environ. Manag. 2019, 233, 291–311. [Google Scholar] [CrossRef]
- Young, C.N.; Schopmeyer, S.A.; Lirman, D. A review of reef restoration and Coral propagation using the threatened genus Acropora in the Caribbean and western Atlantic. Bull. Mar. Sci. 2012, 88, 1075–1098. [Google Scholar] [CrossRef]
- Schopmeyer, S.A.; Lirman, D.; Bartels, E.; Gilliam, D.S.; Goergen, E.A.; Griffin, S.P.; Johnson, M.E.; Lustic, C.; Maxwell, K.; Walter, C.S. Regional restoration benchmarks for Acropora cervicornis. Coral Reefs 2017, 36, 1047–1057. [Google Scholar] [CrossRef]
- Maynard, J.A.; Marshall, P.A.; McLeod, E.; Gabbiy, A.; Van Hooidonk, K.; Planes, S.; Williams, G.J.; Raymundo, L.; Beeden, R.; Tameland, J. Coral Reef Resilience for Decision Support; UN Environment: Nairobi, Kenya, 2017. [Google Scholar]
- Hodgson, D.; McDonald, J.L.; Hosken, D.J. What do you mean “resilient”? Trends Ecol. Evol. 2015, 30, 503–506. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Donner, S.D.; Maynard, J.A.; MacNeil, A.; Graham, N.A.J.; Maina, J.; Baker, A.C.; Baker, A.C.; Alemu, A.I.; Beger, M.; et al. Prioritizing key resilience indicators to support coral reef management in a changing climate. PLoS ONE 2012, 7, e42884. [Google Scholar] [CrossRef] [PubMed]
- Osborne, K.; Dolman, A.M.; Burgess, S.C.; Johns, K.A. Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995–2009). PLoS ONE 2011, 6, e17516. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Graham, N.A.J.; Jackson, J.B.C.; Mumby, P.J.; Steneck, R.S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 2010, 25, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Kuffner, I.B.; Toh, L.T. A geological perspective on the degradation and conservation of western Atlantic coral reefs. Conserv. Biol. 2016, 30, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.S.; Fitz, H.C.; Gilnack, M.; Beets, J.; Hardin, J. Scleractinian coral recruitment patterns at Salt River Submariine Caynon, St. Croix, United States Virgin Islands. Coral Reefs 1984, 3, 69–76. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Dulvy, N.K.; Gill, J.A.; Côté, I.M.; Watkinson, A.R. Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 3019–3025. [Google Scholar] [CrossRef]
- Hoogenboom, M.O.; Frank, G.E.; Chase, T.J.; Jurriaans, S.; Alvarez-Noreiga, M.; Peterson, K.; Critchell, K.; Berry, K.L.E.; Nicolet, K.J.; Ransby, B.; et al. Environmental Drivers of Variation in Bleaching Severity of Acropora Species during an Extreme Thermal Anomaly. Front. Mar. Sci. 2017, 4, 1–16. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Jennings, S.; MacNeil, M.A.; Mouillot, D.; Wilson, S.K. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 2015, 518, 1–17. [Google Scholar] [CrossRef]
- Shaver, E.C.; Silliman, B.R. Time to cash in on positive interactions for coral restoration. PeerJ 2017, 5, e3499. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Aide, T.M. Restoration success: How is it being measured? Restor. Ecol. 2005, 13, 569–577. [Google Scholar] [CrossRef]
- Johnson, M.E.; Lustic, C.; Bartels, E.; Baums, I.B.; Giliam, D.S.; Larson, E.A.; Lirman, D.; Miller, M.W.; Nedimeyer, K.; Schopmeyer, S. Caribbean Acropora Restoration Guide: Best-Practices for Propagation and Population Enhancement. 2011, pp. 1–64. Available online: https://nsuworks.nova.edu/occ_facreports/71 (accessed on 9 March 2020).
- Wongthong, P.; Harvey, N. Integrated coastal management and sustainable tourism: A case study of the reef-base SCUBA dive industry from Thailand. Ocean. Coast. Manag. 2014, 95, 138–146. [Google Scholar] [CrossRef]
- Weterings, R. A GIS-based assessment to the threats to the natural environment on Koh Tao, Thailand. Kasetsart. J. Nat. Sci. 2011, 45, 743–755. [Google Scholar]
- Szuster, B.W.; Dietrich, J. Small island tourism development plan implementation: The case of Koh Tao, Thailand. Environ. Asia 2014, 7, 124–132. [Google Scholar]
- Nichols, R. Effectiveness of Artificial Reefs as Alternative Dive Sites to Reduce Diving Pressure on Natural Reefs, a Case Study of Koh Tao, Thailand. Bachelor’s Thesis, Conservation Biology, University of Cumbria, Cumbria, UK, 2013. [Google Scholar]
- Romeo, L. Tracing Anthropogenic Nutrient Inputs Using δ15N Levels in Algae Tissue Koh Tao, Thailand. Master’s Thesis, MAS Marine Biodiversity and Conservation, CMBC, Scripps Institute of Oceanography, UCSD, La Jolla, CA, USA, 2014. [Google Scholar]
- Cheevaporn, V.; Menasveta, P. Water pollution and habitat degradation in the Gulf of Thailand. Mar. Pollut. Bull. 2003, 47, 43–51. [Google Scholar] [CrossRef]
- Lamb, J.B.; True, J.D.; Piromvaragorn, S.; Willis, B.L. Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biol. Conserv. 2014, 178, 88–96. [Google Scholar] [CrossRef]
- Hein, M.Y.; Lamb, J.B.; Scott, C.; Willis, B.L. Assessing baseline levels of coral health in a newly established marine protected area in a global scuba diving hotspot. Mar. Environ. Res. 2014, 103, 56–65. [Google Scholar] [CrossRef]
- Scott, C.M.; Mehrotra, R.; Hein, M.Y.; Moerland, M.S.; Hoeksema, B.W. Population dynamics of corallivores (Drupella and Acanthaster) on coral reefs of Koh Tao, a diving destination in the gulf of Thailand. Raffles. Bull. Zool. 2017, 65, 68–79. [Google Scholar]
- Phongsuwan, N.; Chankong, A.; Yamarunpatthana, C.; Chansang, H.; Boonprakob, R.; Petchkumnerb, P.; Thongtham, N.; Paokantha, S.; Chanmethakul, T.; Panchauyapoom, P.; et al. Status and changing patterns on coral reefs in Thailand during the last two decades. Deep Sea Res. 2013, 96, 19–24. [Google Scholar] [CrossRef]
- McClanahan, T.R. Bleaching damage and recovery potential of Maldivian coral reefs. Mar. Poll. Bull. 2000, 40, 587–597. [Google Scholar] [CrossRef]
- Jaleel, A. The status of the coral reefs and the management approaches: The case of the Maldives. Ocean. Coast. Manag. 2013, 82, 104–118. [Google Scholar] [CrossRef]
- Donahue, S.; Acosta, A.; Akins, L.; Ault, J.; Bohnsack, J.; Boyer, J.; Callahan, M.; Causey, B.; Cox, C.; Delaney, J.; et al. The State of Coral Reef Ecosystems of the Florida Keys. In The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States; NOAA Technical Memorandum NOS NCCOS 73; Waddell, J.E., Clarke, A.M., Eds.; NOAA/NCCOS Center for Coastal Monitoring and Assessment’s Biogeography Team: Silver Spring, MD, USA, 2008; 569p, pp. 161–187, 569p. [Google Scholar]
- Ruzicka, R.R.; Colella, M.A.; Porter, J.W.; Morrison, J.M.; Kidney, J.A.; Brinkhuis, V.; Lunz, K.S.; Macaulay, K.A.; Bartlett, L.A.; Colee, J. Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Mar. Ecol. Prog. Ser. 2013, 489, 125–141. [Google Scholar] [CrossRef]
- Lirman, D.; Schopmeyer, S.; Manzello, D.; Gramer, L.J.; Precht, W.F.; Muller-Karger, F.; Banks, K.; Barnes, B.; Bartels, E.; Bourque, A.; et al. Severe 2010 cold-water event caused unprecedented mortality to corals of the Florida Reef Tract and reversed previous survivorship patterns. PLoS ONE 2011, 6, e23047. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Miller, M.W. Attributing mortality among drivers of population decline in Acropora palmata in the Florida Keys (USA). Coral Reefs 2012, 31, 369–382. [Google Scholar] [CrossRef]
- Miller, M.W.; Marmet, C.; Cameron, C.M.; Williams, D.E. Prevalence, consequences, and mitigation of fireworm predation on endangered staghorn coral. Mar. Ecol. Prog. Ser. 2014, 516, 187–194. [Google Scholar] [CrossRef]
- Johns, G.M.; Leeworthy, V.R.; Bell, F.W.; Bonn, M.A. Socio-Economic Study of Reefs in Southeast Florida—Final Report; Hazen and Sawyer Environmental Engineers & Scientists: Hollywood, FL, USA, 2001. [Google Scholar]
- Kruczynski, W.L.; McManus, F. Water quality concerns in the Florida Keys: Sources, effects, and solutions. In The Everglades, Florida Bay, and Coral Reefs in the Florida Keys: An Ecosystem Sourcebook; Porter, J.W., Porter, K.G., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 827–881. [Google Scholar]
- Bythell, J.C.; Hillis-Starr, Z.M.; Rogers, C.S. Local variability but landscape stability in coral reef communities following repeated hurricane impacts. Mar. Ecol. Prog. Ser. 2000, 204, 93–100. [Google Scholar] [CrossRef]
- Fisco, D.P. Post hurricane dynamics and status of coral reefs St. Croix, US Virgin Islands. Geography 2008, 23, 1098–1102. [Google Scholar]
- Schrack, E.C.; Brumbaugh, R.; Crisley, K.; Hancock, B. Restoration Works: Highlights from a Decade of Partnership between The Nature Conservancy and the National Oceanic and Atmospheric Adminitration’s Restoration Centre; The Nature Conservancy: Arlington, WV, USA, 2012. [Google Scholar]
- Canfield, R.H. Application of the line interception method in sampling range vegetation. J. For. 1941, 39, 388–394. [Google Scholar]
- Polunin, N.V.C.; Roberts, C.M. Greater biomass and value of target coral-reef fishes in two small Caribbean marine reserves. Mar. Ecol. Prog. Ser. 1993, 100, 167–176. [Google Scholar] [CrossRef]
- Beeden, R.; Willis, B.L.; Raymundo, L.J.; Page, C.A.; Weil, E. Underwater Cards for Assessing Coral Health on Indo-Pacific Reefs; Coral Reef Targeted Research and Capacity Building for Management Program, The University of Queensland: St. Lucia, Australia, 2008. [Google Scholar]
- Weil, E.; Hooten, A.J. Underwater Cards for Assessing Coral Health on Caribbean Reefs; CRTR Program Project Executing Agency, Centre for Marine Studies, Gerhmann Building, The University of Queensland: St. Lucia, Australia, 2008. [Google Scholar]
- Lamb, J.B.; Williamson, D.H.; Russ, G.R.; Willis, B.L. Protected areas mitigate diseases of reef-building corals by reducing damage from fishing. Ecology 2015, 96, 2555–2567. [Google Scholar] [CrossRef]
- Babcock, R.C.; Baird, A.H.; Piromvaragorn, S.; Thomson, D.P.; Willis, B.L. Identification of scleractinian coral recruits from Indo-Pacific reefs. Zool. Stud. 2003, 42, 211–212. [Google Scholar]
- Hoey, A.S.; Pratchett, M.S.; Cvitanovic, C. High macroalgal cover and low coral recruitment undermines the potential resilience of the world’s southernmost coral reef assemblages. PLoS ONE 2011, 6, e25824. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Lirman, D.; Thyberg, T.; Herlan, J.; Young-Lahiff, C.; Schopmeyer, S.; Huntington, B.; Santos, R.; Drury, C. Propagation of the threatened staghorn coral Acropora cervicornis: Methods to minimize the impacts of fragment collection and maximize production. Coral Reefs 2010, 29, 729–735. [Google Scholar] [CrossRef]
- Forrester, G.E.; Maynard, A.; Schofield, S.; Taylor, K. Evaluating causes of transplant stress in fragments of Acropora palmata or coral reef restoration. Bull. Mar. Sc. 2012, 88, 1099–1113. [Google Scholar] [CrossRef]
- Forrester, G.E.; Ferguson, M.A.; O’Connell-Rodwell, C.E.; Jarecki, L.L. Long-term survival and colony growth of Acropora palmata fragments transplanted by volunteers for restoration. Aquat. Conserv. 2014, 24, 81–91. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef]
- Hixon, M.A.; Beets, J.P. Shelter characterisitics and Caribbean fish assemblages: Experiments with artificial reefs. Bull. Mar. Sci. 1989, 44, 666–680. [Google Scholar]
- Ladd, M.C.; Burkepile, D.E.; Shantz, A.A. Near-term impacts of coral restoration on target species, coral reef community structure, and ecological processes. Rest. Ecol. 2019, 27, 1166–1176. [Google Scholar] [CrossRef]
- Edwards, A.J.; Clark, S. Coral transplantation: A useful management tool or misguided meddling? Mar. Pollut. Bull. 1998, 37, 474–487. [Google Scholar] [CrossRef]
- Miller, M.W.; Barimo, J. Assessment of juvenile coral populations at two reef restoration sites in the Florida Keys National Marine Sanctuary: Indicators of success? Bull. Mar. Sci. 2001, 69, 395–405. [Google Scholar]
- Connell, J.H.; Hughes, T.P.; Wallace, C.C. A 30-years study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol. Monogr. 1997, 67, 461–488. [Google Scholar] [CrossRef]
- Glassom, D.; Celliers, L.; Schleyer, M.H. Coral recruitment patterns at Sodwana Bay, South Africa. Coral Reefs 2006, 25, 485–492. [Google Scholar] [CrossRef]
- Hughes, T.P.; Tanner, J.E. Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 2000, 81, 2259–2263. [Google Scholar] [CrossRef]
- Van Woesik, R.; Ripple, K.; Miller, S.L. Macroalgae reduces survival of nursery-reared Acropora corals in the Florida reef tract. Rest. Ecol. 2018, 6, 563–569. [Google Scholar] [CrossRef]
- Aronson, R.B.; Precht, W.F. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 2001, 460, 25–38. [Google Scholar] [CrossRef]
- Johnston, L.; Miller, M.W. Negative indirect effects of neighbors on imperiled scleractinian corals. Coral Reefs 2014, 33, 1047–1056. [Google Scholar] [CrossRef]
- Miller, M.W.; Lohr, K.E.; Cameron, C.M.; Williams, D.E.; Peters, E.C. Disease dynamics and potential mitigation among restored and wild staghorn coral, Acropora cervicornis. PeerJ 2014, 2, e541. [Google Scholar] [CrossRef]
- National Marine Fisheries Service. Recovery Plan for Elkhorn (A. palmata) and Staghorn (A. cervicornis) Corals; Acropora Recovery Team for the National Marine Fisheries Service: Silver Spring, MD, USA, 2015. [Google Scholar]
- Ladd, M.C.; Miller, M.W.; Hunt, J.H.; Sharp, W.C.; Burkepile, D.E. Harnessing ecological processes to facilitate coral restoration. Front. Ecol. Environ. 2018, 16, 239–247. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hein, M.Y.; Beeden, R.; Birtles, A.; Gardiner, N.M.; Le Berre, T.; Levy, J.; Marshall, N.; Scott, C.M.; Terry, L.; Willis, B.L. Coral Restoration Effectiveness: Multiregional Snapshots of the Long-Term Responses of Coral Assemblages to Restoration. Diversity 2020, 12, 153. https://doi.org/10.3390/d12040153
Hein MY, Beeden R, Birtles A, Gardiner NM, Le Berre T, Levy J, Marshall N, Scott CM, Terry L, Willis BL. Coral Restoration Effectiveness: Multiregional Snapshots of the Long-Term Responses of Coral Assemblages to Restoration. Diversity. 2020; 12(4):153. https://doi.org/10.3390/d12040153
Chicago/Turabian StyleHein, Margaux Y., Roger Beeden, Alastair Birtles, Naomi M. Gardiner, Thomas Le Berre, Jessica Levy, Nadine Marshall, Chad M. Scott, Lisa Terry, and Bette L. Willis. 2020. "Coral Restoration Effectiveness: Multiregional Snapshots of the Long-Term Responses of Coral Assemblages to Restoration" Diversity 12, no. 4: 153. https://doi.org/10.3390/d12040153
APA StyleHein, M. Y., Beeden, R., Birtles, A., Gardiner, N. M., Le Berre, T., Levy, J., Marshall, N., Scott, C. M., Terry, L., & Willis, B. L. (2020). Coral Restoration Effectiveness: Multiregional Snapshots of the Long-Term Responses of Coral Assemblages to Restoration. Diversity, 12(4), 153. https://doi.org/10.3390/d12040153





