Intraspecific Behavioral Variation Mediates Insect Prey Survival via Direct and Indirect Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System and Animal Collection
2.2. Experimental Design
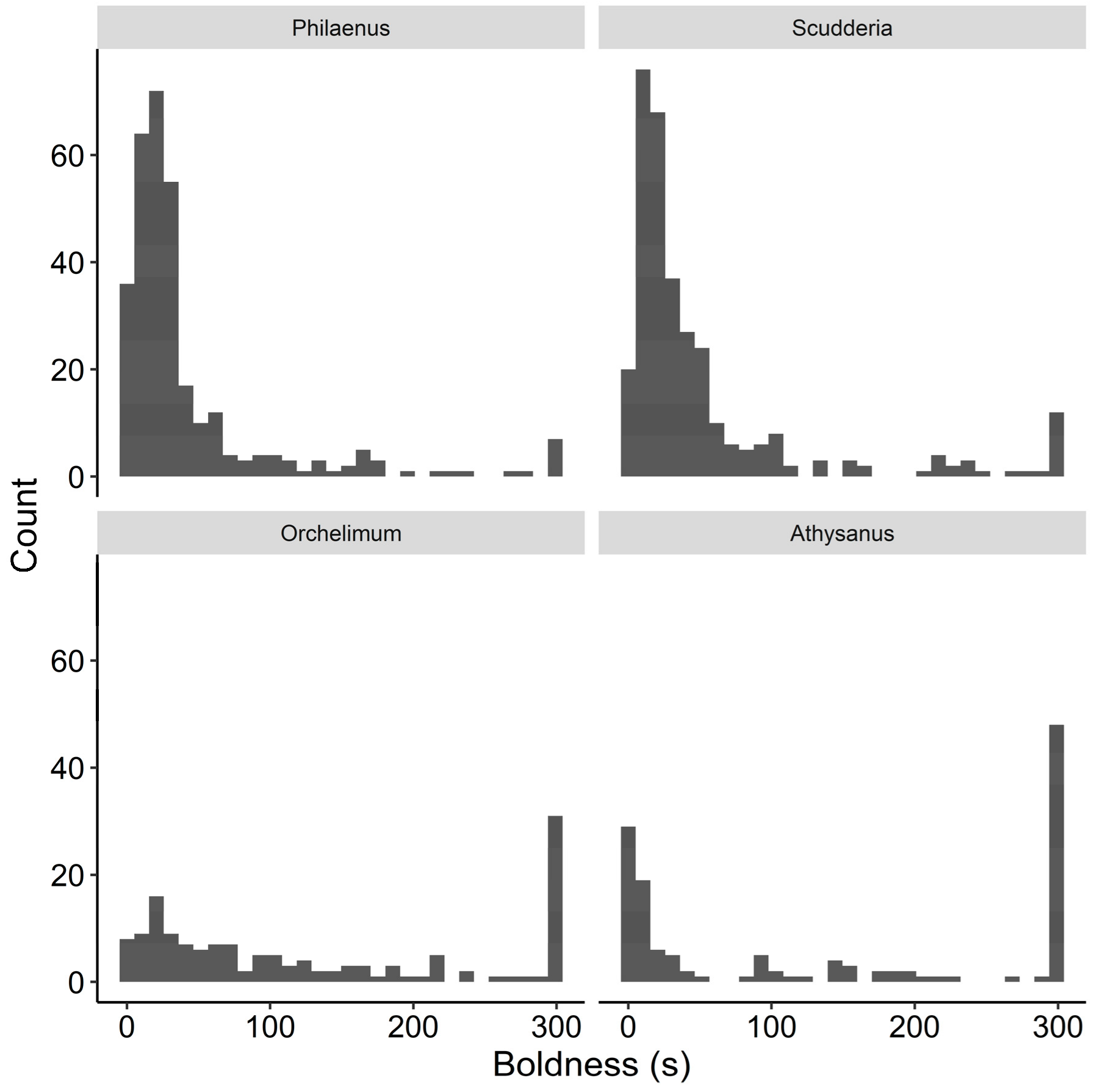
2.3. Prey Behavioral Assay
2.4. Mesocosm Design and Assembly
2.5. Analysis
3. Results
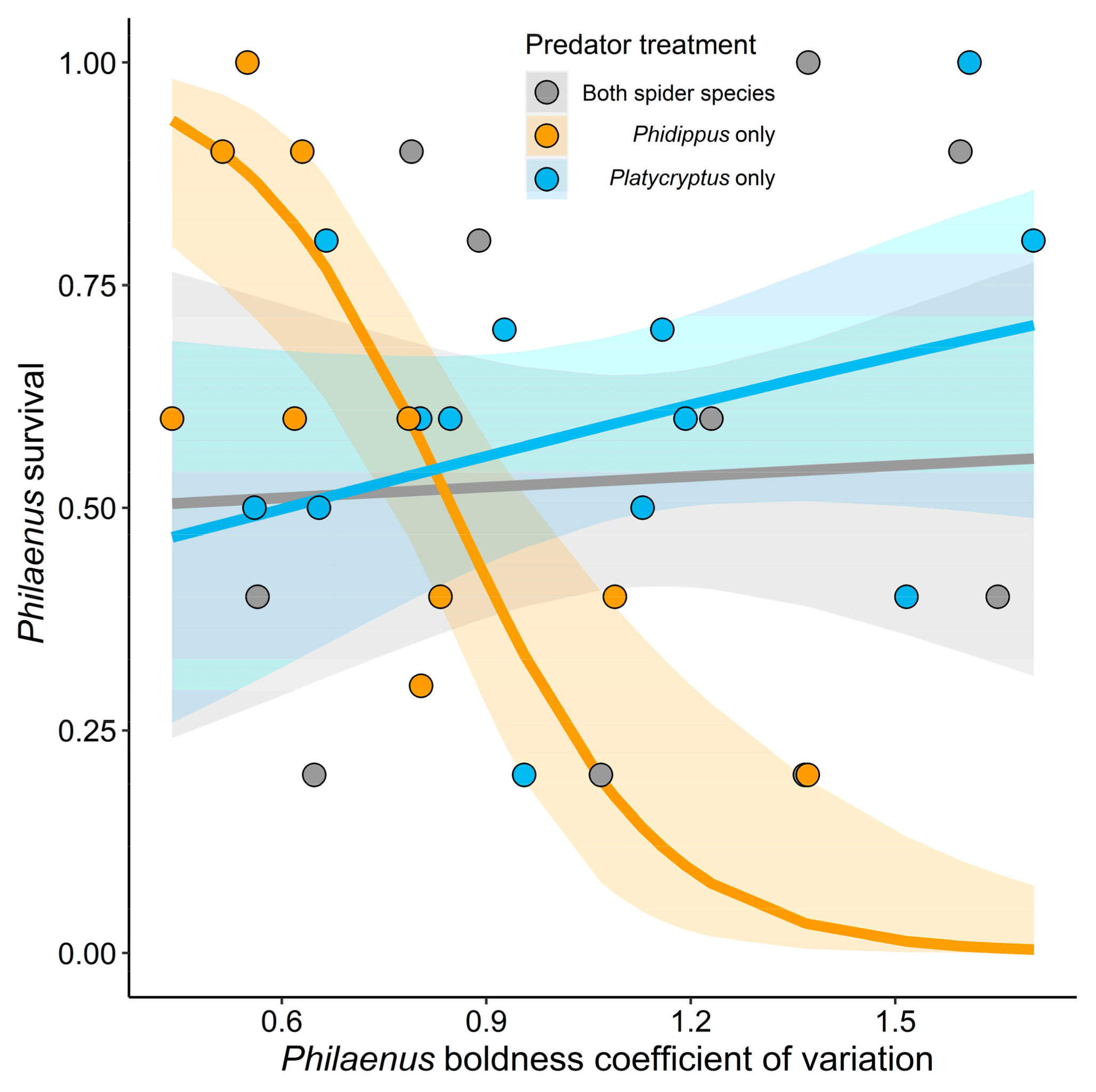
3.1. Philaenus Survival
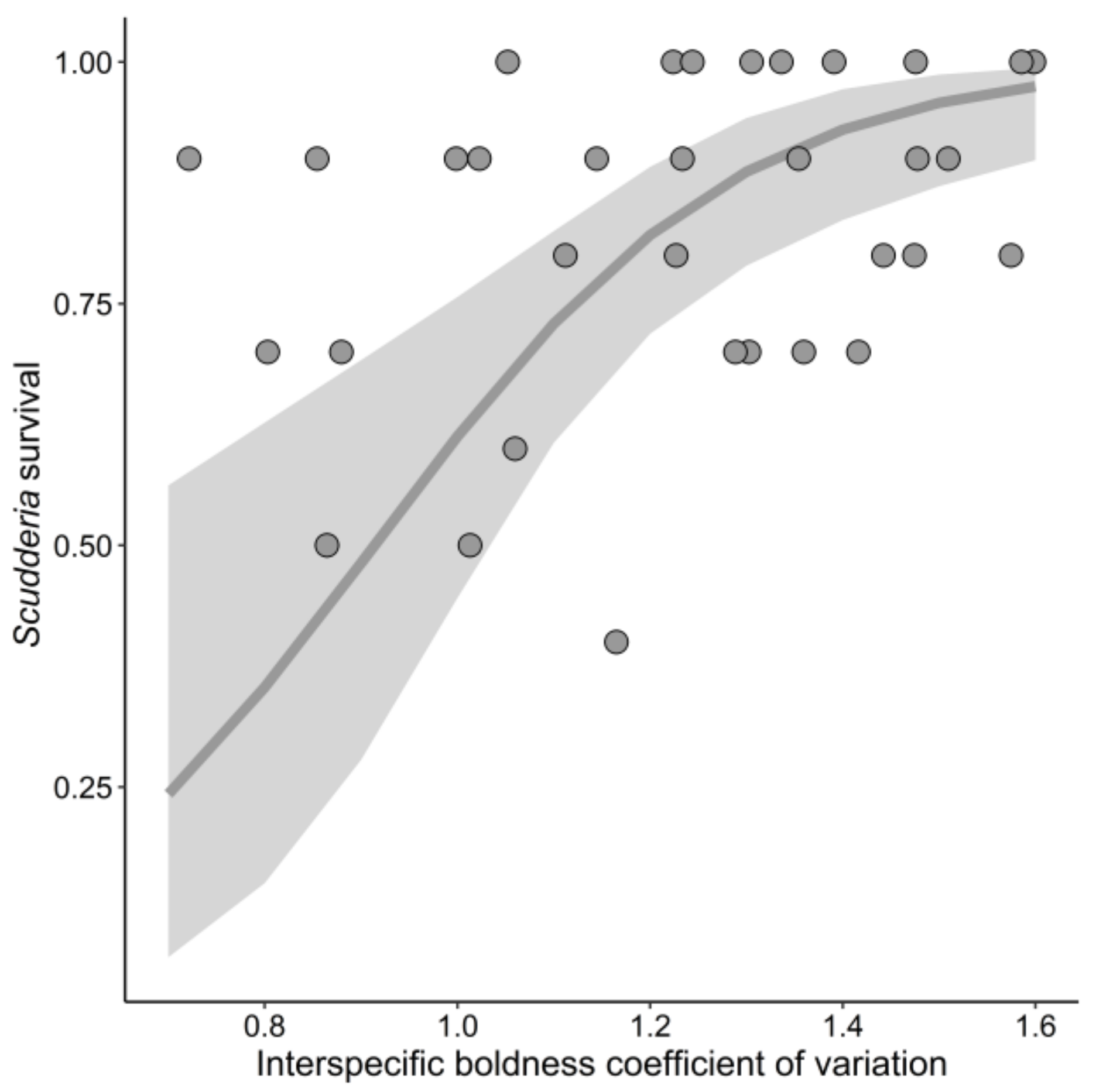
3.2. Scudderia Survival
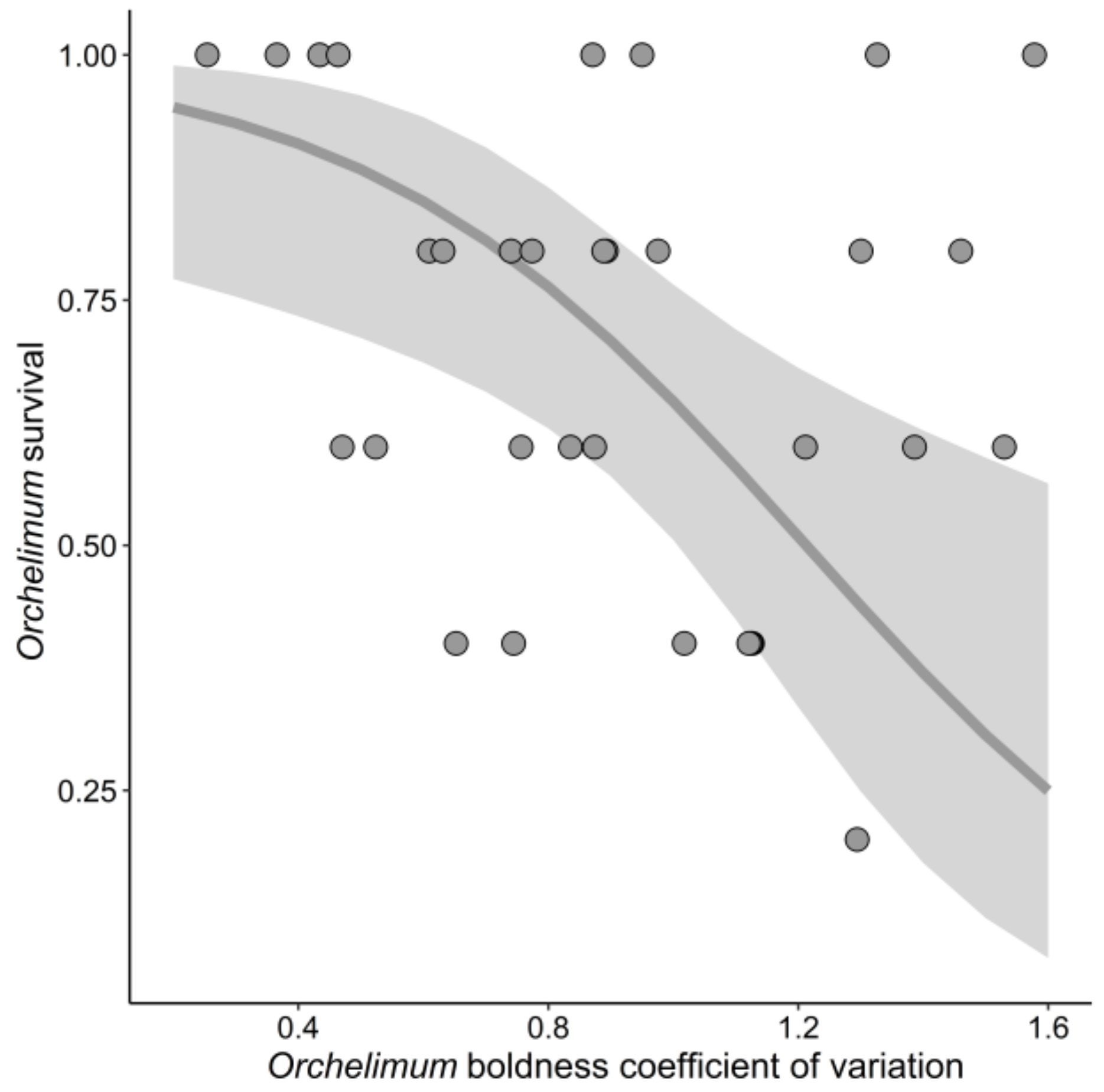
3.3. Orchelimum Survival
3.4. Athysanus survival
4. Discussion
4.1. Differences across Prey Species
4.2. Indirect Effect Mediated by a Shared Predator
4.3. Conclusion and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reed, W.L.; Janzen, F.J. Natural selection by avian predators on size and colour of a freshwater snail (Pomacea flagellata). Biol. J. Linn. Soc. 1999, 67, 331–342. [Google Scholar] [CrossRef]
- Strobbe, F.; McPeek, M.A.; Block, M.D.; Stoks, R. Survival selection imposed by predation on a physiological trait underlying escape speed. Funct. Ecol. 2010, 24, 1306–1312. [Google Scholar] [CrossRef]
- Moiron, M.; Laskowski, K.L.; Niemelä, P.T. Individual differences in behaviour explain variation in survival: A meta-analysis. Ecol. Lett. 2020, 23, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Holmes, T.H.; McCormick, M.I. Influence of prey body characteristics and performance on predator selection. Oecologia 2009, 159, 401–413. [Google Scholar] [CrossRef]
- Keiser, C.N.; Ingley, S.J.; Toscano, B.J.; Scharf, I.; Pruitt, J.N. Habitat complexity dampens selection on prey activity level. Ethology 2018, 124, 25–32. [Google Scholar] [CrossRef]
- Pretorius, J.D.; Lichtenstein, J.L.L.; Eliason, E.J.; Stier, A.C.; Pruitt, J.N. Predator-induced selection on urchin activity level depends on urchin body size. Ethology 2019, 125, 716–723. [Google Scholar] [CrossRef]
- Paine, R.T. Food Web Complexity and Species Diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Huey, R.B.; Pianka, E.R. Ecological consequences of foraging mode. Ecology 1981, 62, 991–999. [Google Scholar] [CrossRef]
- Ritger, A.L.; Fountain, C.T.; Bourne, K.; Martín-Fernández, J.A.; Pierotti, M.E.R. Diet choice in a generalist predator, the invasive lionfish (Pterois volitans/miles). J. Exp. Mar. Biol. Ecol. 2020, 524, 151311. [Google Scholar] [CrossRef]
- Shultz, S.; Noë, R.; McGraw, W.S.; Dunbar, R.I.M. A community–level evaluation of the impact of prey behavioural and ecological characteristics on predator diet composition. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 725–732. [Google Scholar] [CrossRef]
- Dibble, C.J.; Rudolf, V.H.W. Phenotype-environment matching predicts both positive and negative effects of intraspecific variation. Am. Nat. 2019, 194, 47–58. [Google Scholar] [CrossRef]
- Lichtenstein, J.L.L.; Daniel, K.A.; Wong, J.B.; Wright, C.M.; Doering, G.N.; Costa-Pereira, R.; Pruitt, J.N. Habitat structure changes the relationships between predator behavior, prey behavior, and prey survival rates. Oecologia 2019, 190, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Modlmeier, A.P.; Keiser, C.N.; Watters, J.V.; Sih, A.; Pruitt, J.N. The keystone individual concept: An ecological and evolutionary overview. Anim. Behav. 2014, 89, 53–62. [Google Scholar] [CrossRef]
- Start, D. Individual and population differences shape species interactions and natural selection. Am. Nat. 2019, 194, 183–193. [Google Scholar] [CrossRef] [PubMed]
- McGhee, K.E.; Pintor, L.M.; Bell, A.M. Reciprocal behavioral plasticity and behavioral types during predator-prey interactions. Am. Nat. 2013, 182, 704–717. [Google Scholar] [CrossRef]
- Palkovacs, E.P.; Post, D.M. Eco-evolutionary interactions between predators and prey: Can predator-induced changes to prey communities feed back to shape predator foraging traits? Evol. Ecol. Res. 2008, 10, 699–720. [Google Scholar]
- Brose, U. Body-mass constraints on foraging behaviour determine population and food-web dynamics. Funct. Ecol. 2010, 24, 28–34. [Google Scholar] [CrossRef]
- Costa-Pereira, R.; Araújo, M.S.; Olivier, R.; Da, S.; Souza, F.L.; Rudolf, V.H.W. Prey limitation drives variation in allometric scaling of predator-prey interactions. Am. Nat. 2018, 192, E139–E149. [Google Scholar] [CrossRef]
- Boukal, D. Trait-and size-based descriptions of trophic links in freshwater food webs: Current status and perspectives. J. Limnol. 2014, 73, 171–185. [Google Scholar] [CrossRef]
- Gibert, J.P.; Brassil, C.E. Individual phenotypic variation reduces interaction strengths in a consumer–resource system. Ecol. Evol. 2014, 4, 3703–3713. [Google Scholar] [CrossRef]
- Brousseau, P.-M.; Gravel, D.; Handa, I.T. Trait matching and phylogeny as predictors of predator–prey interactions involving ground beetles. Funct. Ecol. 2018, 32, 192–202. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Pfennig, D.W. Patterns and power of phenotypic selection in nature. BioScience 2007, 57, 561–572. [Google Scholar] [CrossRef]
- Huang, C.; Sih, A. Experimental studies on behaviorally mediated, indirect interactions through a shared predator. Ecology 1990, 71, 1515–1522. [Google Scholar] [CrossRef]
- Toscano, B.J.; Fodrie, F.J.; Madsen, S.L.; Powers, S.P. Multiple prey effects: Agonistic behaviors between prey species enhances consumption by their shared predator. J. Exp. Mar. Biol. Ecol. 2010, 385, 59–65. [Google Scholar] [CrossRef]
- Dingemanse, N.J.; Kazem, A.J.N.; Réale, D.; Wright, J. Behavioural reaction norms: Animal personality meets individual plasticity. Trends Ecol. Evol. 2010, 25, 81–89. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Amarasekare, P.; Araújo, M.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef]
- Toscano, B.J.; Gownaris, N.J.; Heerhartz, S.M.; Monaco, C.J. Personality, foraging behavior and specialization: Integrating behavioral and food web ecology at the individual level. Oecologia 2016, 182, 55–69. [Google Scholar] [CrossRef]
- Modlmeier, A.P.; Keiser, C.N.; Wright, C.M.; Lichtenstein, J.L.; Pruitt, J.N. Integrating animal personality into insect population and community ecology. Curr. Opin. Insect Sci. 2015, 9, 77–85. [Google Scholar] [CrossRef]
- Hoefler, C.D.; Chen, A.; Jakob, E.M. The potential of a jumping spider, Phidippus clarus, as a biocontrol agent. J. Econ. Entomol. 2006, 99, 432–436. [Google Scholar] [CrossRef]
- Beckerman, A.P.; Uriarte, M.; Schmitz, O.J. Experimental evidence for a behavior-mediated trophic cascade in a terrestrial food chain. Proc. Natl. Acad. Sci. USA 1997, 94, 10735–10738. [Google Scholar] [CrossRef]
- Chase, J.M. Abiotic controls of trophic cascades in a simple grassland food chain. Oikos 1996, 77, 495–506. [Google Scholar] [CrossRef]
- Rothley, K.D.; Schmitz, O.J.; Cohon, J.L. Foraging to balance conflicting demands: Novel insights from grasshoppers under predation risk. Behav. Ecol. 1997, 8, 551–559. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, C.; Biro, P.A. On the validity of a single (boldness) assay in personality research. Ethology 2013, 119, 937–947. [Google Scholar] [CrossRef]
- Brown, C.; Braithwaite, V.A. Size matters: A test of boldness in eight populations of the poeciliid Brachyraphis episcopi. Anim. Behav. 2004, 68, 1325–1329. [Google Scholar] [CrossRef]
- Hedrick, A.V.; Kortet, R. Sex differences in the repeatability of boldness over metamorphosis. Behav. Ecol. Sociobiol. 2012, 66, 407–412. [Google Scholar] [CrossRef]
- Harris, S.; Ramnarine, I.W.; Smith, H.G.; Pettersson, L.B. Picking personalities apart: Estimating the influence of predation, sex and body size on boldness in the guppy Poecilia reticulata. Oikos 2010, 119, 1711–1718. [Google Scholar] [CrossRef]
- Lichtenstein, J.L.L.; Wright, C.M.; McEwen, B.; Pinter-Wollman, N.; Pruitt, J.N. The multidimensional behavioural hypervolumes of two interacting species predict their space use and survival. Anim. Behav. 2017, 132, 129–136. [Google Scholar] [CrossRef]
- Bell, A.M.; Hankison, S.J.; Laskowski, K.L. The repeatability of behaviour: A meta-analysis. Anim. Behav. 2009, 77, 771–783. [Google Scholar] [CrossRef]
- Lichtenstein, J.L.L.; Rice, H.K.; Pruitt, J.N. Personality variation in two predator species does not impact prey species survival or plant damage in staged mesocosms. Behav. Ecol. Sociobiol. 2018, 72, 70. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Bovy, G.; Ellison, S.; Firth, D.; Friendly, M.; Gorjanc, G.; Graves, S. Package ‘car.’ Vienna R Found. Stat. Comput. 2012. [Google Scholar]
- Lüdecke, D. ggeffects: Tidy data frames of marginal effects from regression models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Payton, M.E. Contrasting diversity values: Statistical inferences based on overlapping confidence intervals. PLoS ONE 2013, 8, e56794. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M. The Theory of Ecological Communities; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar]
- Adriaensen, F.; Dhondt, A.A.; Dongen, S.V.; Lens, L.; Matthysen, E. Stabilizing selection on blue tit fledgling mass in the presence of sparrowhawks. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 1011–1016. [Google Scholar] [CrossRef]
- Burrows, M. Froghopper insects leap to new heights. Nature 2003, 424, 509-509. [Google Scholar] [CrossRef] [PubMed]
- Bonsall, M.B.; Hassell, M.P. Apparent competition structures ecological assemblages. Nature 1997, 388, 371–373. [Google Scholar] [CrossRef]
- Holt, R.D.; Kotler, B.P. Short-term apparent competition. Am. Nat. 1987, 130, 412–430. [Google Scholar] [CrossRef]
- Dall, S.R.X.; Bell, A.M.; Bolnick, D.I.; Ratnieks, F.L.W. An evolutionary ecology of individual differences. Ecol. Lett. 2012, 15, 1189–1198. [Google Scholar] [CrossRef]
- Costa-Pereira, R.; Rudolf, V.H.W.; Souza, F.L.; Araújo, M.S. Drivers of individual niche variation in coexisting species. J. Anim. Ecol. 2018, 87, 1452–1464. [Google Scholar] [CrossRef]
| Term | χ2 | d.f. | P | |
|---|---|---|---|---|
| Philaenus survival model | Predator treatment | 0.19 | 2 | 0.91 |
| Philaenus average boldness | 2.01 | 1 | 0.16 | |
| Philaenus boldness CV | 0.13 | 1 | 0.72 | |
| Community average boldness | 0.84 | 1 | 0.36 | |
| Community boldness CV | 0.92 | 1 | 0.34 | |
| Predator treatment × Philaenus average boldness | 2.88 | 2 | 0.24 | |
| Predator treatment × Philaenus boldness CV | 10.55 | 2 | 0.01 | |
| Predator treatment × Community average boldness | 4.47 | 2 | 0.11 | |
| Predator treatment × Community boldness CV | 3.39 | 2 | 0.18 | |
| Scudderia survival model | Predator treatment | 0.98 | 2 | 0.61 |
| Scudderia average boldness | 1.01 | 1 | 0.31 | |
| Scudderia boldness CV | 0.25 | 1 | 0.62 | |
| Community average boldness | 4.38 | 1 | 0.04 | |
| Community boldness CV | 11.28 | 1 | <0.001 | |
| Predator treatment × Scudderia average boldness | 0.80 | 2 | 0.67 | |
| Predator treatment × Scudderia boldness CV | 0.23 | 2 | 0.89 | |
| Predator treatment × Community average boldness | 4.99 | 2 | 0.08 | |
| Predator treatment × Community boldness CV | 4.13 | 2 | 0.13 | |
| Orchelimum survival model | Predator treatment | 3.87 | 2 | 0.14 |
| Orchelimum average boldness | 0.31 | 1 | 0.58 | |
| Orchelimum boldness CV | 4.86 | 1 | 0.03 | |
| Community average boldness | 0.47 | 1 | 0.49 | |
| Community boldness CV | 1.42 | 1 | 0.23 | |
| Predator treatment × Orchelimum average boldness | 0.50 | 2 | 0.78 | |
| Predator treatment × Orchelimum boldness CV | 0.90 | 2 | 0.64 | |
| Predator treatment × Community average boldness | 5.41 | 2 | 0.07 | |
| Predator treatment × Community boldness CV | 2.30 | 2 | 0.32 | |
| Athysanus survival model | Predator treatment | 1.79 | 2 | 0.41 |
| Athysanus average boldness | 0.41 | 1 | 0.52 | |
| Athysanus boldness CV | 0.00 | 1 | 0.97 | |
| Community average boldness | 0.54 | 1 | 0.46 | |
| Community boldness CV | 0.19 | 1 | 0.66 | |
| Predator treatment × Athysanus average boldness | 3.10 | 2 | 0.21 | |
| Predator treatment × Athysanus boldness CV | 2.35 | 2 | 0.31 | |
| Predator treatment × Community average boldness | 2.30 | 2 | 0.32 | |
| Predator treatment × Community boldness CV | 0.04 | 2 | 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, B.J.; Lichtenstein, J.L.L.; Costa-Pereira, R. Intraspecific Behavioral Variation Mediates Insect Prey Survival via Direct and Indirect Effects. Diversity 2020, 12, 152. https://doi.org/10.3390/d12040152
Toscano BJ, Lichtenstein JLL, Costa-Pereira R. Intraspecific Behavioral Variation Mediates Insect Prey Survival via Direct and Indirect Effects. Diversity. 2020; 12(4):152. https://doi.org/10.3390/d12040152
Chicago/Turabian StyleToscano, Benjamin J., James L. L. Lichtenstein, and Raul Costa-Pereira. 2020. "Intraspecific Behavioral Variation Mediates Insect Prey Survival via Direct and Indirect Effects" Diversity 12, no. 4: 152. https://doi.org/10.3390/d12040152
APA StyleToscano, B. J., Lichtenstein, J. L. L., & Costa-Pereira, R. (2020). Intraspecific Behavioral Variation Mediates Insect Prey Survival via Direct and Indirect Effects. Diversity, 12(4), 152. https://doi.org/10.3390/d12040152




