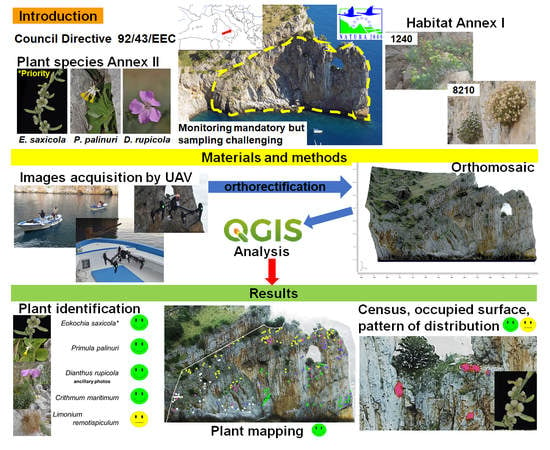
Monitoring of Plant Species and Communities on Coastal Cliffs: Is the Use of Unmanned Aerial Vehicles Suitable?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Target Species
2.3. UAV Aerial Survey: Planning and Execution
2.4. Plant Species Identification, Mapping, and Biometry of the Main Target Species
2.5. GIS Spatial Analysis
2.6. Statistical Analysis
- statistical population: totality of the individuals living on the analysed cliff and identified by all botanists;
- sampling unit: one individual;
- sample: a collection of individuals randomly selected within the sampling universe;
- variable: the area of each polygon;
- measurement: a single value of area.
- Among groups (botanists), it is the variance among polygons measured by different botanists due to a potential difference in measuring polygons.
- Among subgroups within groups, where a subgroup is a sample of randomly selected polygons measured by the same botanist; it is the variance between plants measured by the same data collector.
- Within subgroups. It is the variance within each plant polygon (error; between measurements of each polygon).
3. Results
3.1. Orthomosaic: Geometric Accuracy and Resolving Power
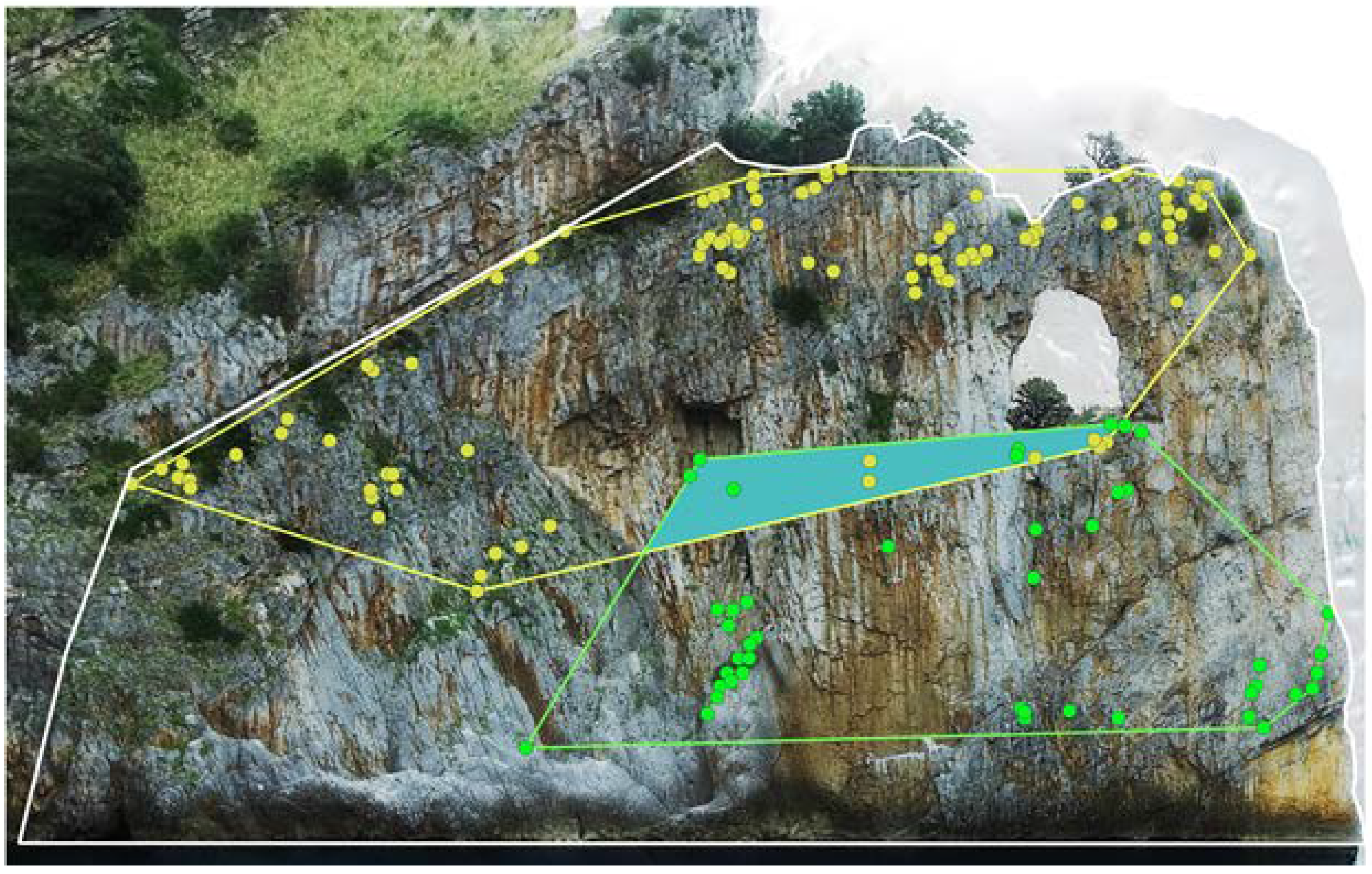
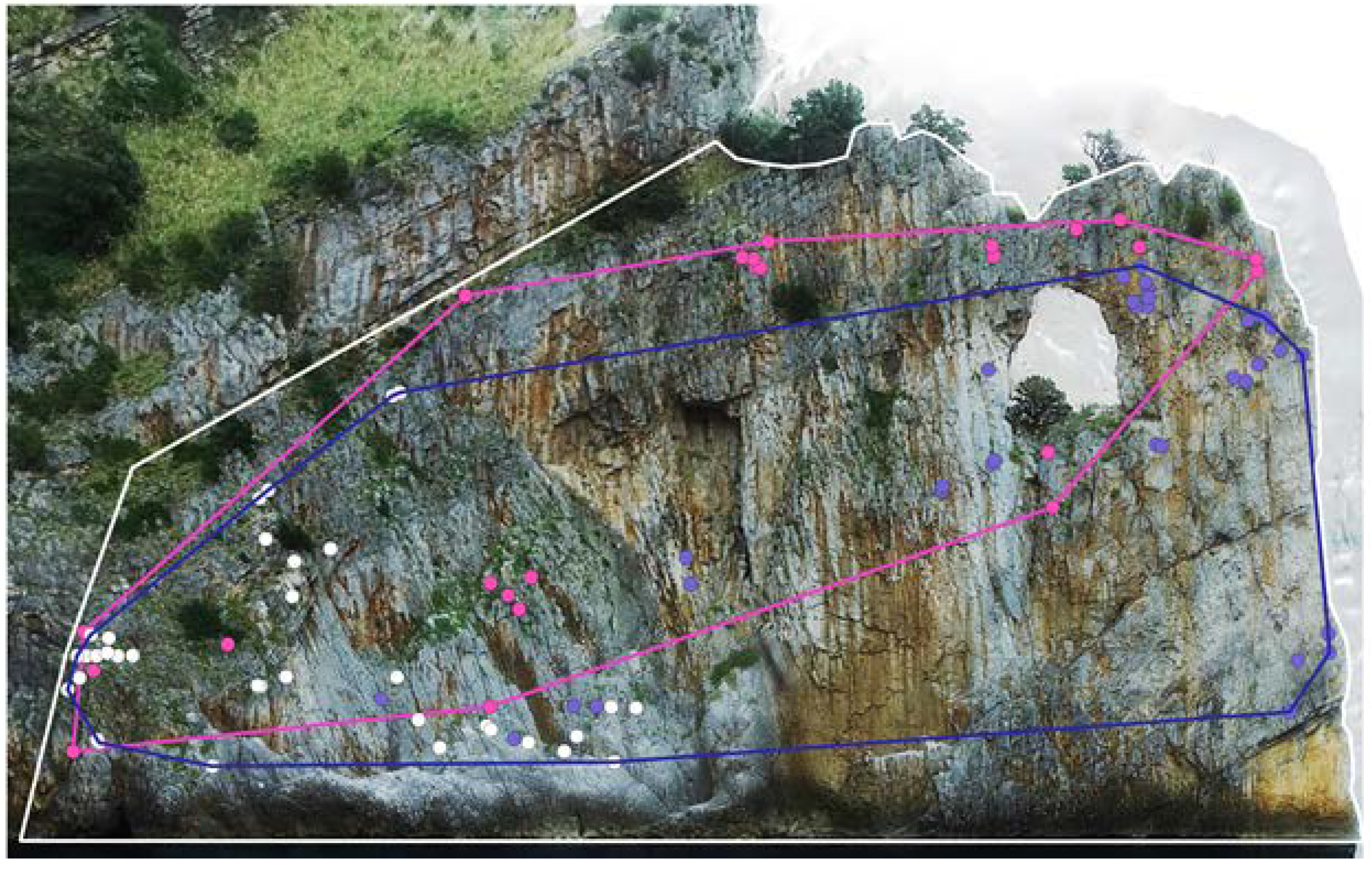
3.2. Plant Species Identification
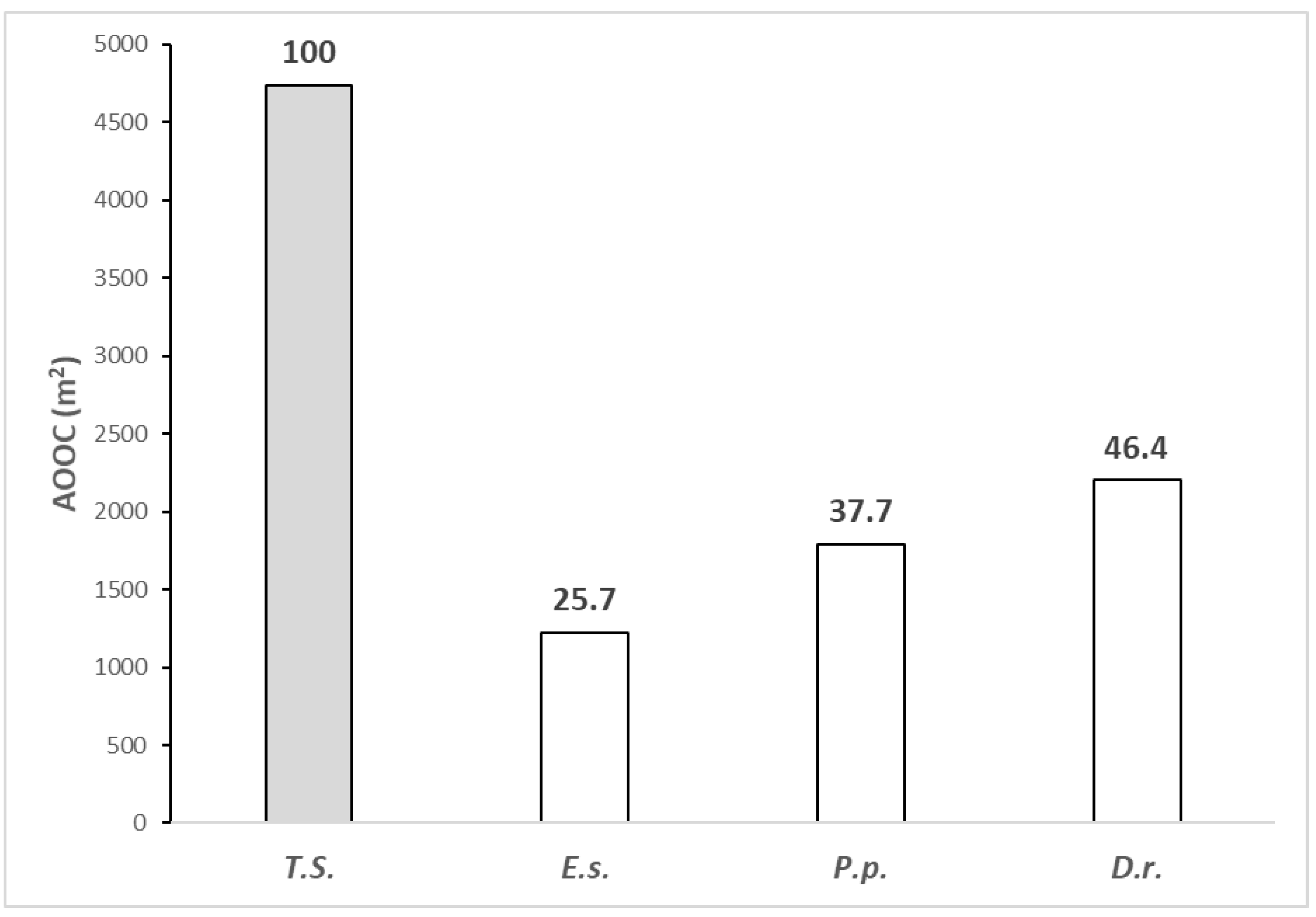
3.3. Analysis of the Plant Species Distribution
3.4. Analysis of Plant Community Distribution
3.5. Biometry of Eokochia Saxicola
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bartlett, R.M.; Matthes-Sears, U.; Larson, D.W. Organization of the Niagara Escarpment cliff community. II. Characterization of the physical environment. Can. J. Botany 1990, 68, 1931–1941. [Google Scholar] [CrossRef]
- Davis, P.H. Cliff Vegetation in the Eastern Mediterranean. J. Ecol. 1951, 39, 63–93. [Google Scholar] [CrossRef]
- Larson, D.W.; Matthes, U.; Kelly, P.E. Cliff Ecology: Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Kuntz, K.L.; Larson, D.W. Microtopographic control of vascular plant, bryophyte and lichen communities on cliff faces. Plant Ecol. 2006, 185, 239–253. [Google Scholar] [CrossRef]
- Wilson, B.J.; Cullen, C. Coastal cliff vegetation of the Catlins region Otago, South Island, New Zealand. N. Z. J. Bot. 1986, 24, 567–574. [Google Scholar] [CrossRef]
- Mifsud, S. Distribution of some rare or endemic chasmophytic and rupestral species growing along the coastal cliffs of the Maltese Islands. Webbia 2013, 68, 35–50. [Google Scholar] [CrossRef]
- Soriano, P.; Estrelles, E.; Bianchelli, M.; Galiè, M.; Biondi, E. Conservation aspects for chasmophytic species: Phenological behavior and seed strategies of the Central Apennine threatened endemism Moehringia papulosa Bertol. Plant Biosyst. 2012, 146, 143–152. [Google Scholar] [CrossRef]
- Sciandrello, S.; Guarino, R.; Minissale, P.; Spampinato, G. The endemic vascular flora of Peloritani Mountains (NE Sicily): Plant functional traits and phytogeographical relationships in the most isolated and fragmentary microplate of the Alpine orogeny. Plant Biosyst. 2014, 149, 838–854. [Google Scholar] [CrossRef]
- Van der Maarel, E.; van der Maarel-Versluys, M. Distribution and conservation status of littoral vascular plant species along the European coasts. J. Coast. Conserv. 1996, 2, 73–92. [Google Scholar] [CrossRef]
- Cooper, A. Plant species coexistence in cliff habitats. J. Biogeogr. 1997, 24, 483–494. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge Univ. Press: Cambridge, UK, 1988. [Google Scholar]
- Wardle, P. Vegetation of New Zealand; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Henle, K.; Bauch, B.; Auliya, M.; Külvik, M.; Pe‘er, G.; Schmeller, D.S.; Framstad, E. Priorities for biodiversity monitoring in Europe: A review of supranational policies and a novel scheme for integrative prioritization. Ecol. Indic. 2013, 33, 5–18. [Google Scholar] [CrossRef]
- DG Environment. Reporting under Article 17 of the Habitats Directive: Explanatory Notes and Guidelines for the Period 2013–2018; Brussels, Belgium, 2017; Available online: http://cdr.eionet.europa.eu/help/habitats_art17/index_html (accessed on 11 September 2019).
- Fenu, G.; Bacchetta, G.; Giacanelli, V.; Gargano, D.; Montagnani, C.; Orsenigo, S.; Cogoni, D.; Rossi, G.; Conti, F.; Santangelo, A.; et al. Conserving plant diversity in Europe: Outcomes, criticisms and perspectives of the Habitats Directive application in Italy. Biodivers Conserv. 2017, 26, 309. [Google Scholar] [CrossRef]
- Barron, S.J.; Delaney, A.; Perrin, P.M.; Martin, J.R.; O’Neill, F.H. National Survey and Assessment of the Conservation Status of Irish Sea Cliffs; Irish Wildlife Manuals; National Parks and Wildlife Service; Department of the Environment, Heritage and Local Government: Dublin, Ireland, 2011.
- Clark, P.; Hessel, A. The effects of rock climbing on cliff-face vegetation. Appl. Veg. Sci. 2015, 18, 705–715. [Google Scholar] [CrossRef]
- Farris, M.A. The effects of rock climbing on the vegetation of three Minnesota cliff systems. Can. J. Botany 1998, 76, 1981–1990. [Google Scholar]
- Goñi, D.; Garcia, M.B.; Guzman, D. Métodos para el censo y seguimiento de plantas rupicolas amenazadas. Pirineos 2006, 161, 33–58. [Google Scholar] [CrossRef]
- Kuntz, K.L.; Larson, D.W. Influences of microhabitat constraints and rock-climbing disturbance on cliff-face vegetation communities. Conserv. Biol. 2006, 20, 821–832. [Google Scholar] [CrossRef]
- Lorite, J.; Serrano, F.; Lorenzo, A.; Cañadas, E.M.; Ballesteros, M.; Peñas, J. Rock climbing alters plant species composition, cover, and richness in Mediterranean limestone cliffs. PLoS ONE 2017, 12, e0182414. [Google Scholar] [CrossRef]
- March-Salas, M.; Moreno-Moya, M.; Palomar, G.; Tejero-Ibarra, P.; Haeuser, E.; Pertierra, L.R. An innovative vegetation survey design in Mediterranean cliffs shows evidence of higher tolerance of specialized rock plants to rock climbing activity. Appl. Veg. Sci. 2018, 21, 289–297. [Google Scholar] [CrossRef]
- Nuzzo, V.A. Effects of rock climbing on cliff goldenrod (Solidago sciaphila Steele) in northwest Illinois. Am. Midl. Nat. 1995, 33, 229–241. [Google Scholar] [CrossRef]
- Gigante, D.; Attorre, F.; Venanzoni, R.; Acosta, A.T.R.; Agrillo, E.; Aleffi, M.; Alessi, N.; Allegrezza, M.; Angelini, P.; Angiolini, C.; et al. A methodological protocol for Annex I Habitats monitoring: The contribution of Vegetation science. Plant Sociol. 2016, 53, 77–87. [Google Scholar]
- Ercole, S.; Giacanelli, V.; Bacchetta, G.; Fenu, G.; Genovesi, P. Manuali per Il Monitoraggio di Specie e Habitat di Interesse Comunitario (Direttiva 92/43/CEE) in Italia: Specie Vegetali; ISPRA, Serie Manuali e Linee Guida, 140/2016; Istituto Superiore Per la Ricerca Ambientale: Rome, Italy, 2016.
- Schmera, D.; Rusterholz, H.P.; Baur, A.; Baur, B. Intensity-dependent impact of sport climbing on vascular plants and land snails on limestone cliffs. Biol. Conserv. 2018, 224, 63–70. [Google Scholar] [CrossRef]
- Alfaro-Saiz, E.; Granda, V.; Rodriguez, A.; Alonso-Redondo, R.; Garcìa-Gonzales, M.E. Optimal census method to estimate population sizes of species growing on rock walls: The case of mature Primula pedemontana. Glob. Ecol. Cons. 2019, 17, e00563. [Google Scholar] [CrossRef]
- Joint Nature Conservation Committee (JNCC). Common Standards Monitoring Guidance for Maritime Cliff and Slope Habitats; Joint Nature Conservation Committee: Peterborough, UK, 2004.
- Anderson, K.; Kevin, J.G. Lightweight unmanned aerial vehicles will revolutionize spatial ecology 2013. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Baena, S.; Boyd, D.S.; Moat, J. UAVs in pursuit of plant conservation-Real world experiences. Ecol. Inform. 2018, 47, 2–9. [Google Scholar] [CrossRef]
- Goncalves, J.; Henriques, R.; Alves, P.; Sousa-Silva, R.; Monteiro, A.T.; Lomba, A.; Marcos, B.; Honrado, J. Evaluating an unmanned aerial vehicle-based approach for assessing habitat extent and condition in fine-scale early successional mountain mosaics. Appl. Veg. Sci. 2016, 19, 132–146. [Google Scholar] [CrossRef]
- Nowack, M.; Dziób, K.; Bogawski, P. Unmanned Aerial Vehicles (UAVs) in environmental biology: A review. Eur. J. Ecol. 2018, 4, 56–74. [Google Scholar] [CrossRef]
- Tay, J.Y.L.; Erfmeier, A.; Kalwij, J.M. Reaching new heights: Can drones replace current methods to study plant population dynamics? Plant Ecol. 2018, 219, 1139–1150. [Google Scholar] [CrossRef]
- Woellner, R.; Wagner, T.C. Saving species, time and money: Application of unmanned aerial vehicles (UAVs) for monitoring of an endangered alpine river specialist in a small nature reserve. Biol. Conserv. 2019, 233, 162–175. [Google Scholar] [CrossRef]
- Rominger, K.; Meyer, S.E. Application of UAV-Based Methodology for Census of an Endangered Plant Species in a Fragile Habitat. Remote Sens. 2019, 11, 719. [Google Scholar] [CrossRef]
- Danzi, M.; Di Crescenzo, G.; Ramondini, M.; Santo, A. Use of unmanned aerial vehicles (UAVs) for photogrammetric surveys in rockfall instability studies. Rend. Online Soc. Geol. 2013, 24, 82–85. [Google Scholar]
- Mancini, F.; Castagnetti, C.; Rossi, P.; Dubbini, M.; Fazio, L.N.; Perrotti, M.; Lollino, P. An Integrated Procedure to Assess the Stability of Coastal Rocky Cliffs: From UAV Close-Range Photogrammetry to Geomechanical Finite Element Modeling. Remote Sens. 2017, 9, 1235. [Google Scholar] [CrossRef]
- Franke, K.W.; Lingwall, B.N.; Zimmaro, P.; Kayen, R.E.; Tommasi, P.; Chiabrando, F.; Santo, A. A Phased reconnaissance approach to documenting landslides following the 2016 Central Italy Earthquakes A Phased reconnaissance approach to documenting landslides following the 2016 Central Italy Earthquakes. Earthq. Spectra 2018, 34, 1693–1719. [Google Scholar] [CrossRef]
- Fazio, N.L.; Perrotti, M.; Andriani, G.F.; Mancini, F.; Rossi, P.; Castagnetti, C.; Lollino, P. A new methodological approach to assess the stability of discontinuous rocky cliffs using in-situ surveys supported by UAV-based techniques and 3-D finite element model: A case study. Engineering 2019, 260, 3. [Google Scholar] [CrossRef]
- Jaud, M.; Letortu, P.; Théry, C.; Grandjean, P.; Costa, S.; Maquaire, O.; Davidson, R.; Le Dantec, N. UAV survey of a coastal cliff face—Selection of the best imaging angle. Measurement 2019, 139, 10–20. [Google Scholar] [CrossRef]
- Strumia, S.; Croce, A.; Santangelo, A. New distributional data of the rare endemic species Eokochia saxicola (Guss.) Freitag and G. Kadereit (Chenopodiaceae): Effects on biogeography and conservation. Plant Biosyst. 2014, 149, 559–564. [Google Scholar] [CrossRef]
- Elzinga, C.L.; Salzer, D.W.; Willoughby, J.W. Measuring & Monitoring Plant Populations; Bureau of Land Management Papers, BLM Technical Reference 1730-1; Bureau of Land Management: Denver, Colorado, 1988.
- Aronne, G.; De Micco, V.; Santangelo, A.; Santangelo, N.; Santo, A.; Buonanno, M. Coastal vertical cliffs of the National Park of Cilento: Reservoirs of endemic species. In Proceedings of the 7th International Conference on Engineering Mechanics, Structures, Engineering Geology (EMESEG 14), Salerno, Italy, 3–5 June 2014. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Blasi, C.; Marignani, M.; Copiz, R.; Fipaldini, M.; Bonacquisti, S.; Del Vico, E.; Rosati, L.; Zavattero, L. Important plant areas in Italy: From data to mapping. Biol. Conserv. 2011, 144, 220–226. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C. Italian Interpretation Manual of the Habitats (92/43/EEC Directive). Ministero dell’Ambiente e della Tutela del Territorio e del Mare. 2009. Available online: http://vnr.unipg.it/habitat/ (accessed on 18 December 2019).
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Euro+Med (2006-): Euro+Med PlantBase—the Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 11 September 2019).
- Angelini, P.; Casella, L.; Grignetti, A.; Genovesi, P. Manuali per il Monitoraggio di Specie e Habitat di Interesse Comunitario (Direttiva 92/43/CEE) in Italia: Habitat; ISPRA, Serie Manuali e Linee Guida, 142/2016; Istituto Superiore Per la Ricerca Ambientale: Rome, Italy, 2016.
- Genovesi, P.; Angelini, P.; Bianchi, E.; Dupré, E.; Ercole, S.; Giacanelli, V.; Ronchi, F.; Stoch, F. Specie e Habitat di Interesse Comunitario in Italia: Distribuzione, Stato di Conservazione e Trend; ISPRA, Serie Rapporti, 194/2014; Istituto Superiore Per la Ricerca Ambientale: Rome, Italy, 2014.
- Rossi, G.; Montagnani, C.; Gargano, D.; Peruzzi, L.; Abeli, T.; Ravera, S.; Cogoni, A.; Fenu, G.; Magrini, S.; Gennai, M.; et al. Lista Rossa della Flora Italiana. 1. Policy Species e altre Specie Minacciate; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Stamperia Romana: Rome, Italy, 2013. [Google Scholar]
- Chappuis, E. Crithmum Maritimum. The IUCN Red List of Threatened Species. 2014. Available online: https://www.iucnredlist.org/ (accessed on 18 December 2019).
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red Listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- US Dept. of Defence. Military Standard—Photographic Lenses (MIL-STD-150A). Section 5.1.1.7, Resolving Power Target. Available online: https://www.techstreet.com/standards/mil-mil-std-150a?product_id=1465242 (accessed on 19 December 2019).
- Getzin, S.; Wiegand, K.; Schöning, I. Assessing biodiversity in forests using very high-resolution images and unmanned aerial vehicles. Methods Ecol. Evol. 2012, 3, 397–404. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- Barone Lumaga, M.R.; Santangelo, A.; Strumia, S. Morpho-functional traits influencing the fitness of highly endangered Eokochia saxicola (Guss.) Freitag & G. Kadereit (Amaranthaceae). Flora 2015, 218, 11–17. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Occurrence of morphological and anatomical adaptative traits in young and adult plants of the rare Mediterranean cliff species Primula palinuri Petagna. Sci. World J. 2012, 9, 471814. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Morpho-anatomical traits for plant adaptation to drought. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Richards, J. Primula; Timber Press: Portland, OR, USA, 2003. [Google Scholar]
- Holzschuh, A. Does rock climbing threaten cliff biodiversity? A critical review. Biol. Conserv. 2016, 204, 153–162. [Google Scholar] [CrossRef]
- Buonanno, M.; Aronne, G.; Strumia, S.; Danzi, M.; Santo, A.; Santangelo, A. Cliff vegetation monitoring using close range photogrammetry and UAS: Technical issues and practical hints. In Proceedings of the Small Unmanned Aerial Systems for Environmental Research, 5th International Conference, Vila Real, Portugal, 28–30 June 2017. [Google Scholar]
- Getzin, S.; Nuske, R.S.; Wiegand, K. Using Unmanned Aerial Vehicles (UAV) to Quantify Spatial Gap Patterns in Forests. Remote Sens. 2014, 6, 6988–7004. [Google Scholar] [CrossRef]


| Source of Variation | SS | df | MS | F | Sig. |
|---|---|---|---|---|---|
| Among groups (botanists) | 0.25465 | 3 | 0.08488 | 3.848 | n.s. |
| Among subgroups within groups (among polygons measured by the same botanist) | 11.76080 | 36 | 0.32669 | 73.074 | *** |
| Within subgroups (error; between measurement of each polygon) | 0.71530 | 160 | 0.00447 | ||
| Total | 12.73076 | 199 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strumia, S.; Buonanno, M.; Aronne, G.; Santo, A.; Santangelo, A. Monitoring of Plant Species and Communities on Coastal Cliffs: Is the Use of Unmanned Aerial Vehicles Suitable? Diversity 2020, 12, 149. https://doi.org/10.3390/d12040149
Strumia S, Buonanno M, Aronne G, Santo A, Santangelo A. Monitoring of Plant Species and Communities on Coastal Cliffs: Is the Use of Unmanned Aerial Vehicles Suitable? Diversity. 2020; 12(4):149. https://doi.org/10.3390/d12040149
Chicago/Turabian StyleStrumia, Sandro, Maurizio Buonanno, Giovanna Aronne, Antonio Santo, and Annalisa Santangelo. 2020. "Monitoring of Plant Species and Communities on Coastal Cliffs: Is the Use of Unmanned Aerial Vehicles Suitable?" Diversity 12, no. 4: 149. https://doi.org/10.3390/d12040149
APA StyleStrumia, S., Buonanno, M., Aronne, G., Santo, A., & Santangelo, A. (2020). Monitoring of Plant Species and Communities on Coastal Cliffs: Is the Use of Unmanned Aerial Vehicles Suitable? Diversity, 12(4), 149. https://doi.org/10.3390/d12040149