Functional Divergence Drives Invasibility of Plant Communities at the Edges of a Resource Availability Gradient
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Design
2.2. Measuring Functional and Mechanistic Traits
2.3. Functional Characterization of the Plant Communities
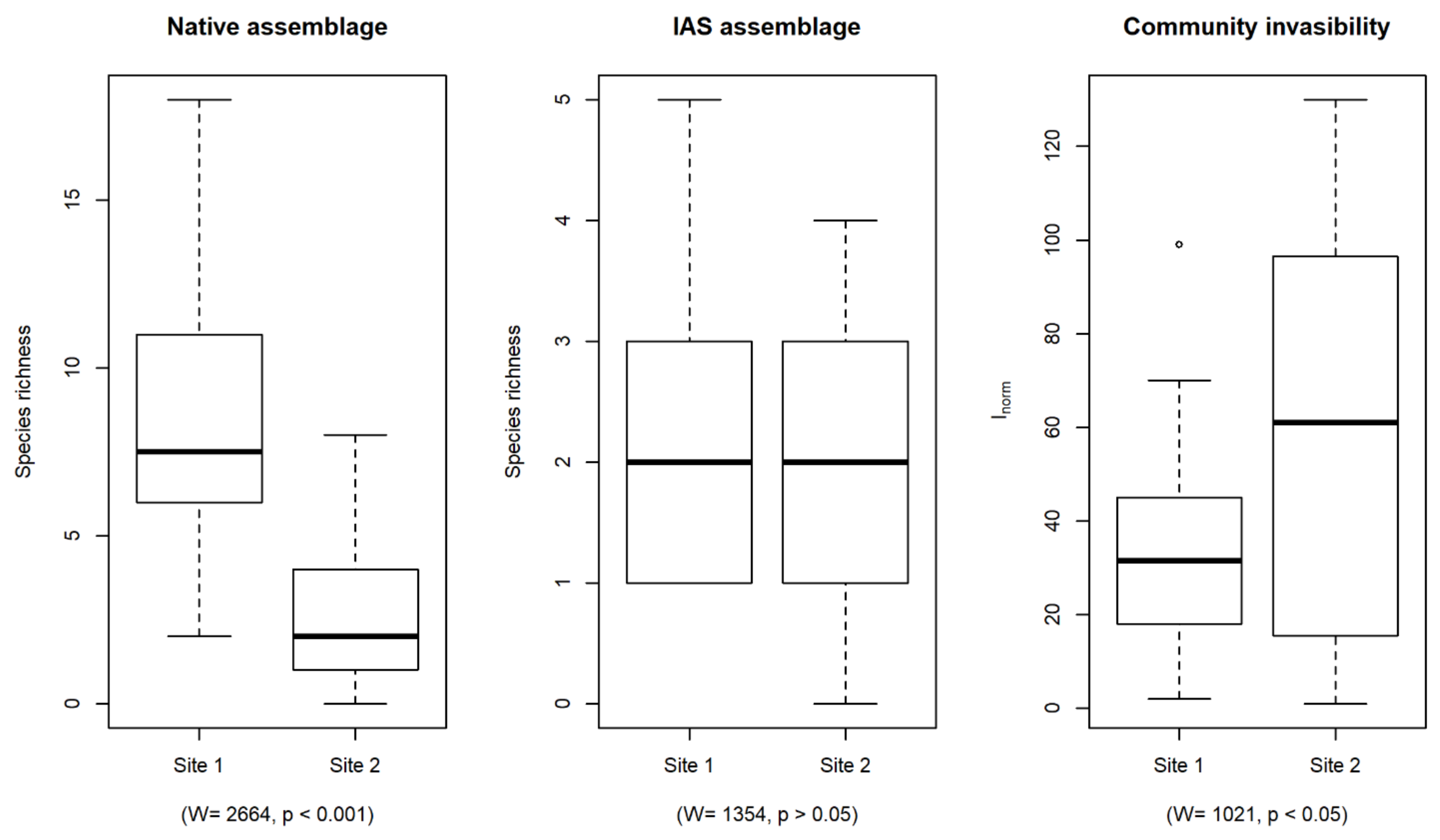
3. Results
4. Discussion
Functional Features of Native Species Pool Facilitating Community Invasibility
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lambertini, M.; Leape, J.; Marton-Lefèvre, J.; Mittermeier, R.A.; Rose, M.; Robinson, J.G.; Stuart, S.N.; Waldman, B.; Genovesi, P. Invasives: A major conservation threat. Science 2011, 333, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Van Klunen, M.; Ansong, M.; et al. Global rise in emerging alien species results from increased accessibility of new source pools. Proc. Nat. Acad. Sci. USA 2018, 2017, 19429. [Google Scholar] [CrossRef] [PubMed]
- Essl, F.; Dullinger, S.; Rabitsch, W.; Hulme, P.E.; Hülber, K.; Jarošík, V.; Kleinbauer, I.; Krausmann, F.; Kühn, I.; Nentwig, W.; et al. Socioeconomic legacy yields an invasion debt. Proc. Nat. Acad. Sci. USA 2011, 108, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Pyšek, P.; et al. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Richardson, D.M.; Midgley, G.F. Will Climate Change Promote Alien Plant Invasions? In Biological Invasions. Ecological Studies (Analysis and Synthesis); Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 193. [Google Scholar]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. A synthesis of plant invasion effects on biodiversity across spatial scales. Am. J. Bot. 2011, 98, 539–548. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef]
- Qian, H.; Guo, Q. Linking biotic homogenization to habitat type, invasiveness and growth form of naturalized alien plants in North America. Divers. Distrib. 2010, 16, 119–125. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Aronson, M.F.; Williams, N.S.; Celesti-Grapow, L.; Cilliers, S.; Clarkson, B.D.; Pyšek, P. Beta diversity of urban floras among European and non-European cities. Global Ecol. Biogeogr. 2014, 23, 769–779. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Plant invasions: Merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geog. Earth Environ. 2006, 30, 409–431. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Traits associated with invasiveness in alien plants: Where do we stand? In Biological Invasions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 97–125. [Google Scholar]
- Moravcová, L.; Pyšek, P.; Jarošík, V.; Pergl, J. Getting the right traits: Reproductive and dispersal characteristics predict the invasiveness of herbaceous plant species. PLoS ONE 2015, 10, e0123634. [Google Scholar] [CrossRef]
- Case, E.J.; Harrison, S.; Cornell, H.V. Do high-impact invaders have the strongest negative effects on abundant and functionally similar resident species? Funct. Ecol. 2016, 30, 1447–1453. [Google Scholar] [CrossRef]
- Carboni, M.; Münkemüller, T.; Lavergne, S.; Choler, P.; Borgy, B.; Violle, C.; Essl, F.; Roquet, C.; Munoz, F.; DivGrass Consortium. What it takes to invade grassland ecosystems: Traits, introduction history and filtering processes. Ecol. Lett. 2016, 19, 219–229. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Gorné, L.D. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Ordonez, A.; Wright, I.J.; Olff, H. Functional differences between native and alien species: A global-scale comparison. Funct. Ecol. 2010, 24, 1353–1361. [Google Scholar] [CrossRef]
- Funk, J.L.; Vitousek, P.M. Resource-use efficiency and plant invasion in low-resource systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef]
- Funk, J.L.; Standish, R.J.; Stock, W.D.; Valladares, F. Plant functional traits of dominant native and invasive species in mediterranean-climate ecosystems. Ecology 2016, 97, 75–83. [Google Scholar] [CrossRef]
- Tecco, P.A.; Díaz, S.; Cabido, M.; Urcelay, C. Functional traits of alien plants across contrasting climatic and land-use regimes: Do aliens join the locals or try harder than them? J. Ecol. 2010, 98, 17–27. [Google Scholar] [CrossRef]
- Diez, J.M.; Sullivan, J.J.; Hulme, P.E.; Edwards, G.; Duncan, R.P. Darwin’s naturalization conundrum: Dissecting taxonomic patterns of species invasions. Ecol. Lett. 2008, 11, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Kraft, N.J.B.; Ackerly, D.D. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol. Monogr. 2010, 80, 401–422. [Google Scholar] [CrossRef]
- Lemoine, N.P.; Shue, J.; Verrico, B.; Erickson, D.; Kress, W.J.; Parker, J.D. Phylogenetic relatedness and leaf functional traits, not introduced status, influence community assembly. Ecology 2015, 96, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.L.; Eviner, V.T.; Jeschke, J.M.; Pace, M.L. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 2006, 21, 645–651. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, A.S.; Gilbert, B.; Levine, J.M. Plant invasions and the niche. J. Ecol. 2009, 97, 609–615. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Thuiller, W.; Gallien, L.; Boulangeat, I.; De Bello, F.; Münkemüller, T.; Roquet, C.; Lavergne, S. Resolving Darwin’s naturalization conundrum: A quest for evidence. Divers. Distrib. 2010, 16, 461–475. [Google Scholar] [CrossRef]
- Gallien, L.; Mazel, F.; Lavergne, S.; Renaud, J.; Douzet, R.; Thuiller, W. Contrasting the effects of environment, dispersal and biotic interactions to explain the distribution of invasive plants in alpine communities. Biol. Invasions 2014, 17, 1407–1423. [Google Scholar] [CrossRef]
- Liu, S.; Streich, J.; Borevitz, J.O.; Rice, K.J.; Li, T.; Li, B.; Bradford, K.J. Environmental resource deficit may drive the evolution of intraspecific trait variation in invasive plant populations. Oikos 2019, 128, 171–184. [Google Scholar] [CrossRef]
- Funk, J.L. The physiology of invasive plants in low-resource environments. Conserv. Physiol. 2013, 1, cot026. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Kuebbing, S.E.; Maynard, D.S.; Bradford, M.A. Linking functional diversity and ecosystem processes: A framework for using functional diversity metrics to predict the ecosystem impact of functionally unique species. J. Ecol. 2018, 106, 687–698. [Google Scholar] [CrossRef]
- Brodribb, T.J. Progressing from ‘functional’ to mechanistic traits. New Phytol. 2017, 215, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Larter, M.; Pfautsch, S.; Domec, J.C.; Trueba, S.; Nagalingum, N.; Delzon, S. Aridity drove the evolution of extreme embolism resistance and the radiation of conifer genus Callistris. New Phytol. 2017, 215, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Grotkopp, E.; Erskine-Ogden, J.; Rejmánek, M. Assessing potential invasiveness of woody horticultural plant species using seedling growth rate traits. J. Appl. Ecol. 2010, 47, 1320–1328. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Grewell, B.J.; D’Antonio, C.M.; Funk, J.L.; James, J.J.; Molinari, N.; Richards, C.L. A functional trait perspective on plant invasion. Ann. Bot. 2012, 110, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Belluau, M.; Shipley, B. Linking hard and soft traits: Physiology, morphology and anatomy interact to determine habitat affinities to soil water availability in herbaceous dicots. PLoS ONE 2018, 13, e0193130. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.Y.; Lian, J.; John, R.; Ronghua, L.; Liu, H.; Ye, Q. Using functional trait diversity patterns to disentangle the scale-dependent ecological processes in a subtropical forest. Funct. Ecol. 2018, 32, 1379–1389. [Google Scholar] [CrossRef]
- Tordoni, E.; Petruzzellis, F.; Nardini, A.; Savi, T.; Bacaro, G. Make it simpler: Alien species decrease functional diversity of coastal plant communities. J. Veg. Sci. 2019, 30, 498–509. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Wetland resources: Status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Acosta, A.; Carranza, M.L.; Izzi, C.F. Are there habitats that contribute best to plant species diversity in coastal dunes? Biodiver. Conserv. 2009, 18, 1087. [Google Scholar] [CrossRef]
- Tordoni, E.; Napolitano, R.; Maccherini, S.; Da Re, D.; Bacaro, G. Ecological drivers of plant diversity patterns in remnants coastal sand dune ecosystems along the northern Adriatic coastline. Ecol. Res. 2018, 33, 1157–1168. [Google Scholar] [CrossRef]
- Dawson, W.; Moser, D.; Van Kleunen, M.; Kreft, H.; Pergl, J.; Pyšek, P.; Essl, F. Global hotspots and correlates of alien species richness across taxonomic groups. Nature Ecol. Evol. 2017, 1, 0186. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Fantinato, E.; Silan, G.; Buffa, G. Trade-offs between sampling effort and data quality in habitat monitoring. Biodiver. Conserv. 2019, 28, 55–73. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Boyle, B.; Hopkins, N.; Lu, Z.; Garay, J.A.R.; Mozzherin, D.; Rees, T.; Lowry, S. The taxonomic name resolution service: An online tool for automated standardization of plant names. BMC Bioinform. 2013, 14, 16. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Bartolucci, F. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Villar, R. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.; Jalili, A.; Zak, M.R. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Poorter, H. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aus. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Meth. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Bühler, J.; Rishmawi, L.; Pflugfelder, D.; Huber, G.; Scharr, H.; Hülskamp, M.; Jahnke, S. phenoVein—A tool for leaf vein segmentation and analysis. Plant Physiol. 2015, 169, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Petruzzellis, F.; Savi, T.; Bacaro, G.; Nardini, A. A simplified framework for fast and reliable measurement of leaf turgor loss point. Plant. Physiol. Bioch. 2019, 139, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.I.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 2008. [Google Scholar]
- Gulías, J.; Flexas, J.; Mus, M.; Cifre, J.; Lefi, E.; Medrano, H. Relationship between maximum leaf photosynthesis, nitrogen content and specific leaf area in Balearic endemic and non-endemic Mediterranean species. Ann. Bot. 2003, 92, 215–222. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.G.; Garden, D.; Dorrough, J.; Bonis, A. Assessing functional diversity in the field — methodology matters! Funct. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12. 2014. Available online: https://CRAN.R-project.org/package=FD (accessed on 1 December 2019).
- Carmona, C.P.; De Bello, F.; Mason, N.W.; Lepš, J. Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef]
- Carmona, C.P. TPD: Methods for Measuring Functional Diversity Based on Trait Probability Density. R package version 1.1.0. 2019. Available online: https://CRAN.R-project.org/package=TPD (accessed on 1 December 2019).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-131.1. 2018. Available online: https://CRAN.R-project.org/package=nlme (accessed on 1 December 2019).
- Barton, K. MuMIn: Multi-Model Inference. R package version1.40.4. 2018. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 December 2019).
- Jaeger, B. r2glmm: Computes R Squared for Mixed (Multilevel) Models. R package version 0.1.2. 2017. Available online: https://CRAN.Rproject.org/package=r2glmm (accessed on 1 December 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Pizzo, L.; Buffa, G. The response of plant community diversity to alien invasion: Evidence from a sand dune time series. Biodiver. Conserv. 2015, 24, 371–392. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Acquavita, A.; Aleffi, I.F.; Benci, C.; Bettoso, N.; Crevatin, E.; Milani, L.; Mattassi, G. Annual characterization of the nutrients and trophic state in a Mediterranean coastal lagoon: The Marano and Grado Lagoon (northern Adriatic Sea). Reg. Stud. Mar. Sci. 2015, 2, 132–144. [Google Scholar] [CrossRef]
- Hart, S.P.; Schreiber, S.J.; Levine, J.M. How variation between individuals affects species coexistence. Ecol. Lett. 2016, 19, 825–838. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Nardini, A.; Savi, T.; Tonet, V.; Castello, M.; Bacaro, G. Less safety for more efficiency: Water relations and hydraulics of the invasive tree Ailanthus altissima (Mill.) Swingle compared with native Fraxinus ornus L. Tree Physiol. 2018, 39, 76–87. [Google Scholar] [CrossRef]
- Divíšek, J.; Chytrý, M.; Beckage, B.; Gotelli, N.J.; Lososová, Z.; Pyšek, P.; Molofsky, J. Similarity of introduced plant species to native ones facilitates naturalization, but differences enhance invasion success. Nat. Commun. 2018, 9, 4631. [Google Scholar] [CrossRef]
- Catford, J.A.; Smith, A.L.; Wragg, P.D.; Clark, A.T.; Kosmala, M.; Cavender-Bares, J.; Tilman, D. Traits linked with species invasiveness and community invasibility vary with time, stage and indicator of invasion in a long-term grassland experiment. Ecol. Lett. 2019, 22, 593–604. [Google Scholar] [CrossRef]
- Conti, L.; Block, S.; Parepa, M.; Münkemüller, T.; Thuiller, W.; Acosta, A.T.; Carboni, M. Functional trait differences and trait plasticity mediate biotic resistance to potential plant invaders. J. Ecol. 2018, 106, 1607–1620. [Google Scholar] [CrossRef]
- Fried, G.; Carboni, M.; Mahaut, L.; Violle, C. Functional traits modulate plant community responses to alien plant invasion. Perspect. Plant Ecol. Evol. Syst. 2019, 37, 53–63. [Google Scholar] [CrossRef]
- Stanisci, A.; Acosta, A.T.R.; Di Iorio, A.; Vergalito, M. Leaf and root trait variability of alien and native species along Adriatic coastal dunes (Italy). Plant Biosyst. 2010, 144, 47–52. [Google Scholar] [CrossRef]
- Mahdavi, P.; Bergmeier, E. Plant functional traits and diversity in sand dune ecosystems across different biogeographic regions. Acta Oecol. 2016, 74, 37–45. [Google Scholar] [CrossRef]
- Kooyman, R.; Cornwell, W.; Westoby, M. Plant functional traits in Australian subtropical rain forest: Partitioning within-community from cross-landscape variation. J. Ecol. 2010, 98, 517–525. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B.J.; Pither, J.; Kerkhoff, A.J.; Boyle, B.; Weiser, M.D.; Nolting, K.M. The biogeography and filtering of woody plant functional diversity in North and South America. Global Ecol. Biogeogr. 2012, 21, 798–808. [Google Scholar] [CrossRef]
- Coyle, J.R.; Halliday, F.W.; Lopez, B.E.; Palmquist, K.A.; Wilfahrt, P.A.; Hurlbert, A.H. Using trait and phylogenetic diversity to evaluate the generality of the stress-dominance hypothesis in eastern North American tree communities. Ecography 2014, 37, 814–826. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Bradley, B.A.; Laginhas, B.B.; Whitlock, R.; Allen, J.M.; Bates, A.E.; Bernatchez, G.; Sorte, C.J. Disentangling the abundance–impact relationship for invasive species. Proc. Nat. Acad. Sci. USA 2019, 116, 9919–9924. [Google Scholar] [CrossRef]
- Knappová, J.; Knapp, M.; Münzbergová, Z. Spatio-temporal variation in contrasting effects of resident vegetation on establishment, growth and reproduction of dry grassland plants: Implications for seed addition experiments. PLoS ONE 2013, 8, e65879. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Ubierna, N.; Winter, K.; Holtum, J.A.; Marshall, J.D.; Farquhar, G.D. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 2013, 200, 950–965. [Google Scholar] [CrossRef]
- Dawson, W.; Rohr, R.P.; Van Kleunen, M.; Fischer, M. Alien plant species with a wider global distribution are better able to capitalize on increased resource availability. New Phytol. 2012, 194, 859–867. [Google Scholar] [CrossRef]
- Siniscalco, C.; Barni, E.; Bacaro, G. Non-native species distribution along the elevation gradient in the western Italian Alps. Plant Biosyst. 2011, 145, 150–158. [Google Scholar] [CrossRef]
- Barni, E.; Bacaro, G.; Falzoi, S.; Spanna, F.; Siniscalco, C. Establishing climatic constrains shaping the distribution of alien plant species along the elevation gradient in the Alps. Plant Ecol. 2012, 213, 757–767. [Google Scholar] [CrossRef]
- Bacaro, G.; Maccherini, S.; Chiarucci, A.; Jentsch, A.; Rocchini, D.; Torri, D.; Gioria, M.; Tordoni, E.; Martellos, S.; Altobelli, A.; et al. Distributional patterns of endemic, native and alien species along a roadside elevation gradient in Tenerife, Canary Island. Comm. Ecol. 2015, 16, 223–234. [Google Scholar] [CrossRef]
- Tordoni, E.; Napolitano, R.; Nimis, P.; Castello, M.; Altobelli, A.; Da Re, D.; Zago, S.; Chines, A.; Martellos, S.; Maccherini, S.; et al. Diversity patterns of alien and native plant species in Trieste port area: Exploring the role of urban habitats in biodiversity consevation. Urban Ecosyst. 2017, 20, 1151–1160. [Google Scholar] [CrossRef]
- Landi, S.; Tordoni, E.; Amici, V.; Bacaro, G.; Carboni, M.; Filibeck, G.; Scoppola, A.; Bagella, S. Contrasting patterns of native and non-native plants in a network of protected areas across spatial scales. Biodiv. Conserv. 2020, 29, 2035–2053. [Google Scholar] [CrossRef]
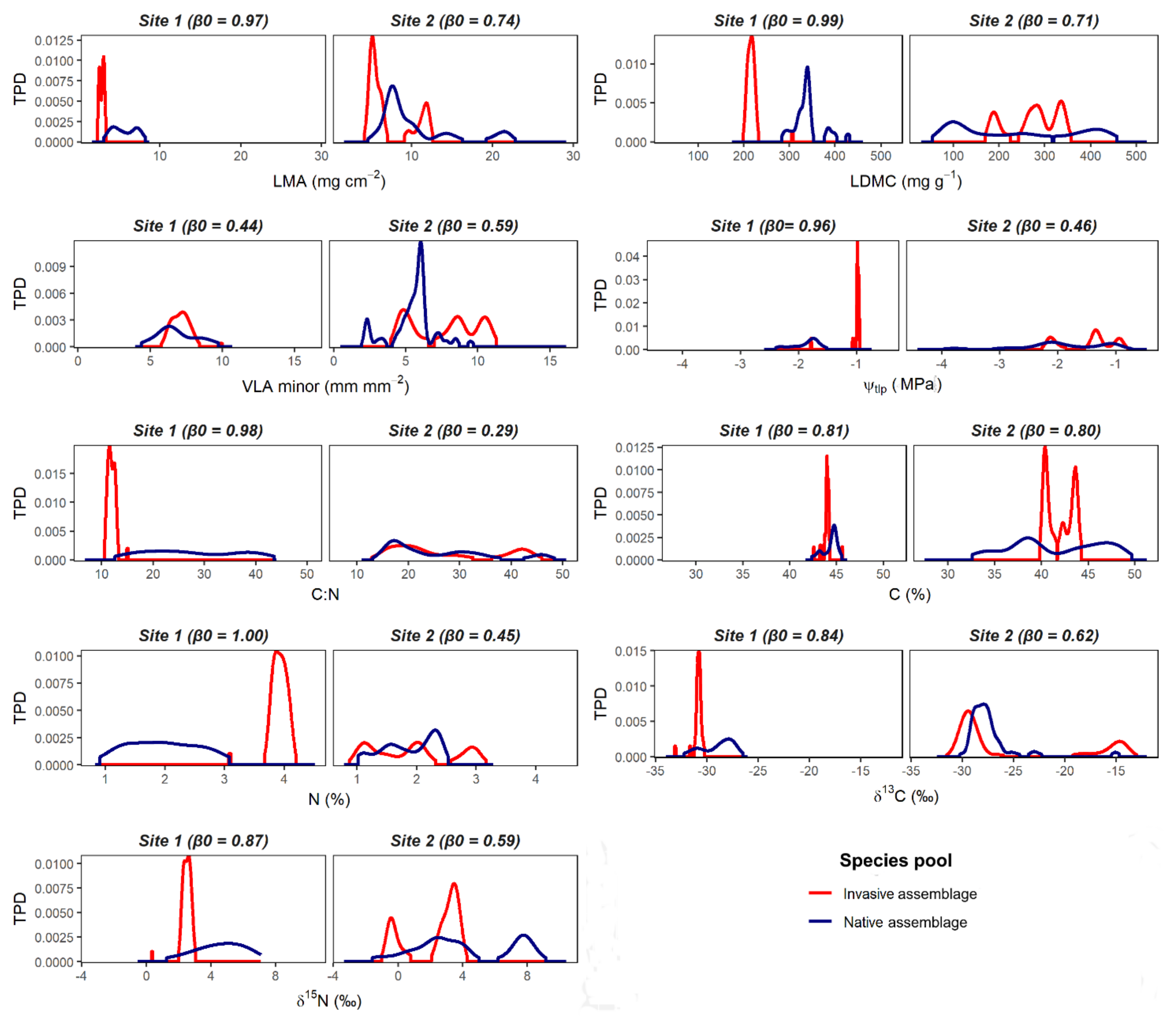
| Functional Trait | Abbreviation | Units | Functional Significance |
|---|---|---|---|
| Leaf dry mass per unit area | LMA | mg cm−2 | Indicative of the leaf-level cost of light interception, correlated with relative growth rate, photosynthetic rate, and nutrient concentration [53,54] |
| Leaf dry matter content | LDMC | mg g−1 | Indicator of plant resource use [52] |
| Vein length per unit area (minor) * | VLAmin | mm mm−2 | Structural feature influencing plant performance, correlated with leaf hydraulic conductance and gas exchange rates [56] |
| Leaf water potential at turgor loss point * | Ψtlp | −MPa | Indicative of species’ drought tolerance [58] |
| Leaf C:N ratio * | C:N | - | Indicative of carbon and nitrogen investment costs [62] |
| Leaf C content * | C | % | |
| Leaf N content * | N | % | |
| Leaf 13C isotopic composition * | δ13C | ‰ | Indicative of photosynthetic water-use efficiency (lower values indicate greater stomatal aperture [61] |
| Leaf 15N isotopic composition * | δ15N | ‰ | Indicative of the ability of plant species in resource acquisition and use [61] |
| Site 1 | Site 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Trait | β0 | Overlap (1- β0) | PN | PU | β0 | Overlap (1- β0) | PN | PU |
| LMA | 0.97 | 0.03 | 0.13 | 0.87 | 0.74 | 0.26 | 0.89 | 0.11 |
| LDMC | 0.99 | 0.01 | 0.00 | 1.00 | 0.71 | 0.29 | 0.94 | 0.06 |
| VLAmin | 0.44 | 0.56 | 0.95 | 0.05 | 0.59 | 0.41 | 0.69 | 0.31 |
| Ψtlp | 0.96 | 0.04 | 0.00 | 1.00 | 0.46 | 0.54 | 1.00 | 0.00 |
| C | 0.81 | 0.19 | 0.95 | 0.05 | 0.80 | 0.20 | 1.00 | 0.00 |
| N | 1.00 | 0.00 | 1.00 | 0.00 | 0.45 | 0.55 | 0.53 | 0.47 |
| C:N | 0.98 | 0.02 | 0.08 | 0.92 | 0.29 | 0.71 | 0.54 | 0.46 |
| δ15N | 0.87 | 0.13 | 0.97 | 0.03 | 0.59 | 0.41 | 1.00 | 0.00 |
| δ13C | 0.84 | 0.16 | 0.93 | 0.07 | 0.62 | 0.38 | 0.83 | 0.17 |
| Model | logLik | AICc | ΔAICc | Weight | R2 |
|---|---|---|---|---|---|
| y~1 (Intercept-only) | −51.10 | 110.49 | 42.95 | - | - |
| C:N + δ13C + LDMC + Ψtlp | −25.23 | 67.54 | 0.00 | 0.50 | 0.42 |
| C:N + δ13C + LDMC + N + Ψtlp | −24.58 | 68.52 | 0.98 | 0.31 | 0.41 |
| C:N + δ13C + LDMC + C + Ψtlp | −25.07 | 69.50 | 1.96 | 0.19 | 0.42 |
| Coefficient | Std. Value | S.E. | t-Value | p-Value | Partial R2 |
|---|---|---|---|---|---|
| (Intercept) | 0.19 | 0.32 | 0.61 | ||
| δ13C | 0.28 | 0.05 | 5.21 | *** | 0.13 |
| LDMC | 0.20 | 0.04 | 5.50 | *** | 0.19 |
| Ψtlp | −0.14 | 0.03 | −4.46 | *** | 0.17 |
| C:N | −0.12 | 0.05 | −2.58 | ** | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tordoni, E.; Petruzzellis, F.; Nardini, A.; Bacaro, G. Functional Divergence Drives Invasibility of Plant Communities at the Edges of a Resource Availability Gradient. Diversity 2020, 12, 148. https://doi.org/10.3390/d12040148
Tordoni E, Petruzzellis F, Nardini A, Bacaro G. Functional Divergence Drives Invasibility of Plant Communities at the Edges of a Resource Availability Gradient. Diversity. 2020; 12(4):148. https://doi.org/10.3390/d12040148
Chicago/Turabian StyleTordoni, Enrico, Francesco Petruzzellis, Andrea Nardini, and Giovanni Bacaro. 2020. "Functional Divergence Drives Invasibility of Plant Communities at the Edges of a Resource Availability Gradient" Diversity 12, no. 4: 148. https://doi.org/10.3390/d12040148
APA StyleTordoni, E., Petruzzellis, F., Nardini, A., & Bacaro, G. (2020). Functional Divergence Drives Invasibility of Plant Communities at the Edges of a Resource Availability Gradient. Diversity, 12(4), 148. https://doi.org/10.3390/d12040148






