Hummingbird–Plant Interactions Are More Specialized in Forest Compared to Coffee Plantations
Abstract
1. Introduction
2. Methods
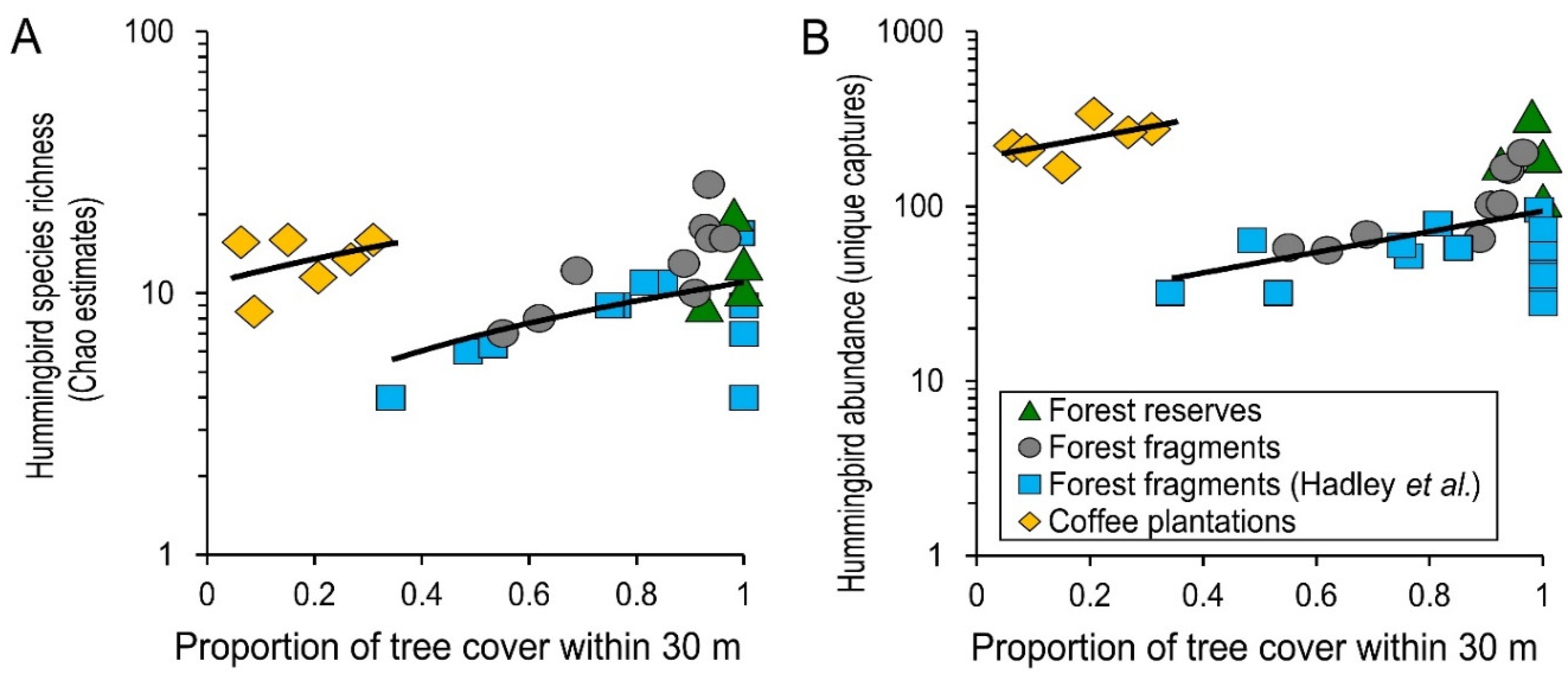
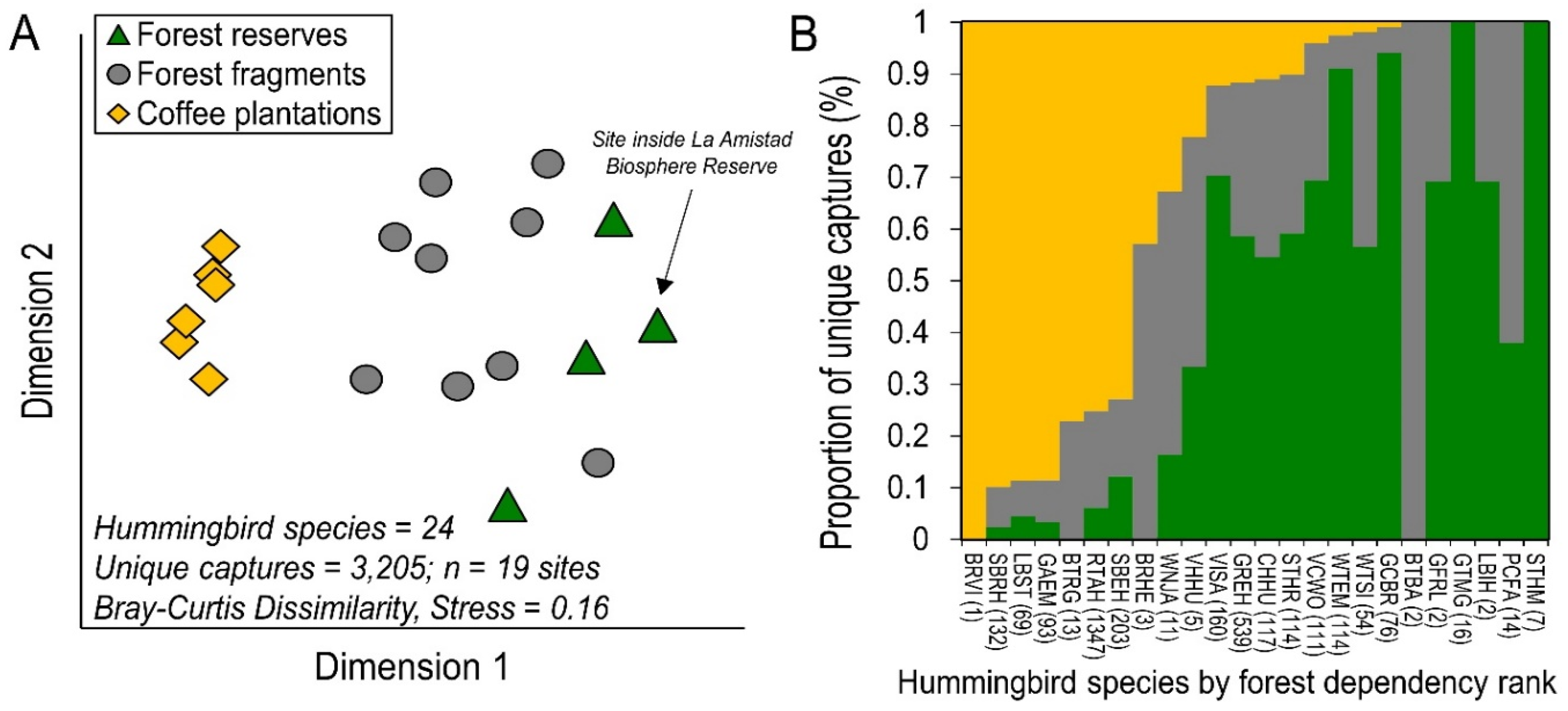
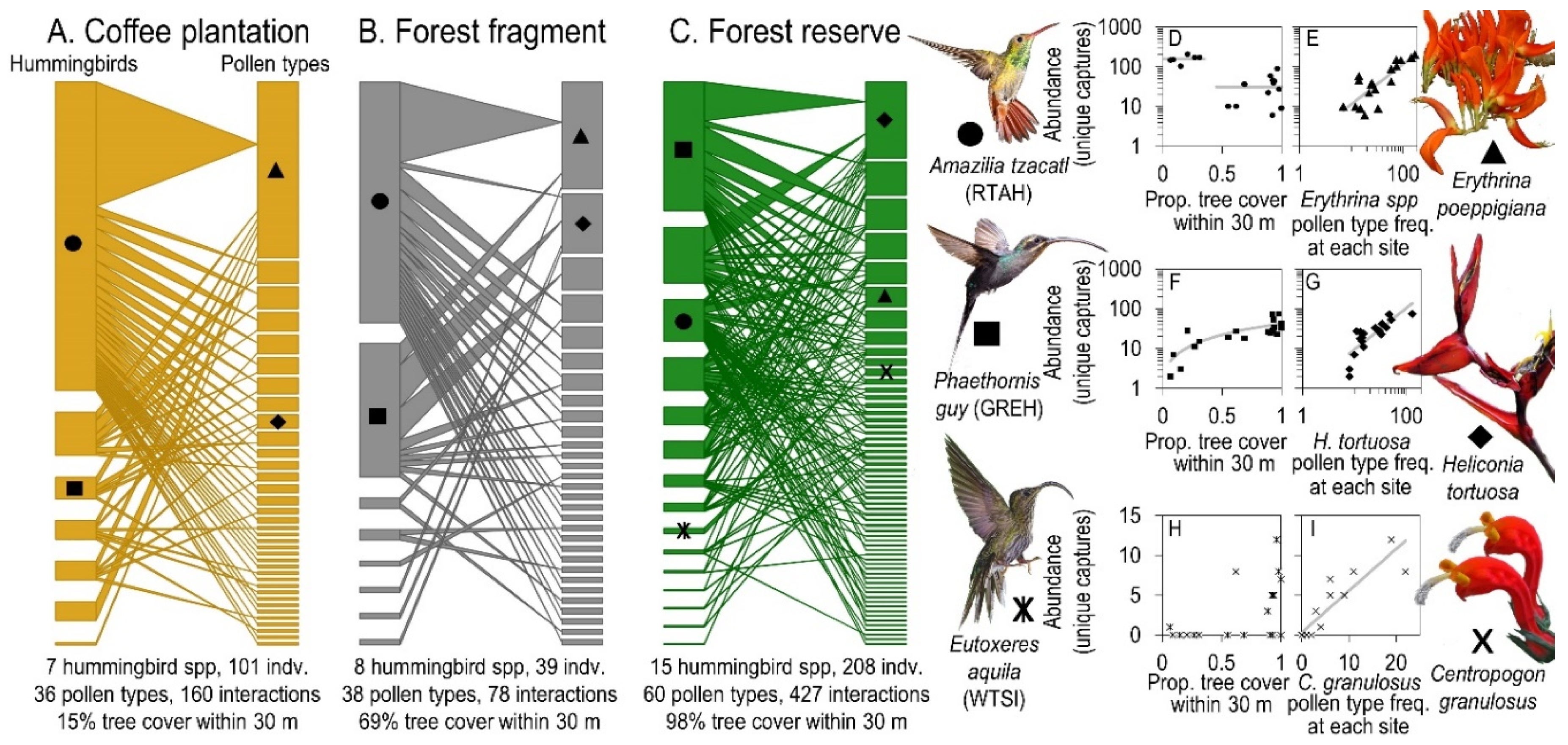
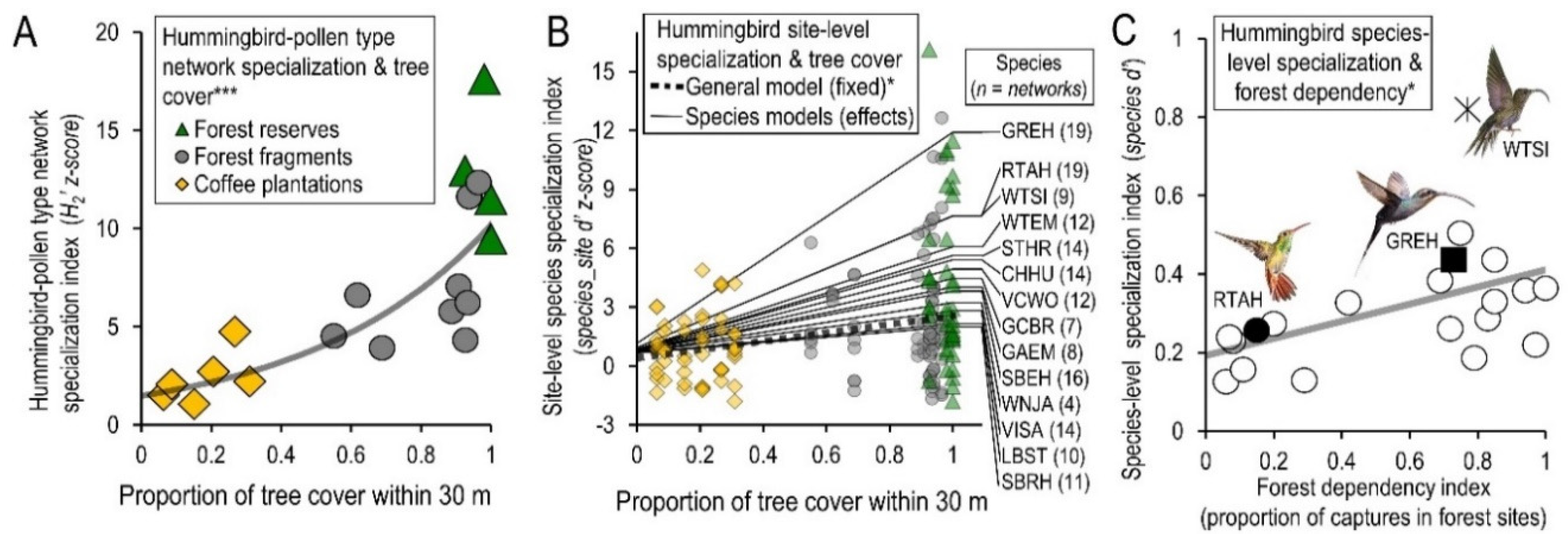
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Carolina Useche, D.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.A.; Sloan, S.P.; Laurance, S.G.; Campbell, M.; Abernethy, K.; Alvarez, P.; et al. Averting biodiversity collapse in tropical forest protected areas. Nature 2012, 489, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Ramankutty, N.; Mehrabi, Z.; Waha, K.; Jarvis, L.; Kremen, C.; Herrero, M.; Rieseberg, L.H. Trends in global agricultural land use: Implications for environmental health and food security. Annu. Rev. Plant Biol. 2018, 69, 789–815. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.C.; Klein Goldewijk, K.; Siebert, S.; Lightman, D.; Ramankutty, N. Anthropogenic transformation of the biomes, 1700 to 2000. Glob. Ecol. Biogeogr. 2010, 19, 586–606. [Google Scholar] [CrossRef]
- Lambin, E.F.; Turner, B.L.; Geist, H.J.; Agbola, S.B.; Angelsen, A.; Folke, C.; Bruce, J.W.; Coomes, O.T.; Dirzo, R.; George, P.S.; et al. The causes of land-use and land-cover change: Moving beyond the myths. Glob. Environ. Chang. 2001, 261–269. [Google Scholar] [CrossRef]
- Mendenhall, C.D.; Shields-estrada, A.; Krishnaswami, A.J.; Daily, G.C. Quantifying and sustaining biodiversity in tropical agricultural landscapes. Proc. Natl. Acad. Sci. USA 2016, 113, 14544–14551. [Google Scholar] [CrossRef]
- Gilroy, J.J.; Edwards, F.A.; Medina Uribe, C.A.; Haugaasen, T.; Edwards, D.P. Surrounding habitats mediate the trade-off between land-sharing and land-sparing agriculture in the tropics. J. Appl. Ecol. 2014, 51, 1337–1346. [Google Scholar] [CrossRef]
- Aizen, M.A.; Sabatino, M.; Tylianakis, J.M. Specialization and rarity predict nonrandom loss of interactions from mutualistic networks. Science 2012, 335, 1486–1489. [Google Scholar] [CrossRef]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Mutualistic Networks; Princeton University Press: Princeton, NJ, USA, 2013; ISBN 9781400848720. [Google Scholar]
- Stiles, F.G. Geographical aspects of bird-flower coevolution, with particular reference to Central America. Ann. Mo. Bot. Gard. 1981, 68, 323–351. [Google Scholar] [CrossRef]
- Feinsinger, P. Coevolution and pollination. In Coevolution; Futuyma, D.J., Slatkin, M., Eds.; Sinauer: Sunderland, MA, USA, 1983; pp. 282–310. [Google Scholar]
- Borgella, R.; Snow, A.A.; Gavin, T.A. Species richness and pollen loads of hummingbirds using forest fragments in southern costa RICA. Biotropica 2001, 33, 90. [Google Scholar] [CrossRef]
- Betts, M.G.; Hadley, A.S.; Kress, W.J. Pollinator recognition by a keystone tropical plant. Proc. Natl. Acad. Sci. USA 2015, 112, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Biesmeijer, J.C.; Roberts, S.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.; Potts, S.; Kleukers, R.; Thomas, C.; et al. Parallel declines in pollinators and insect-pollinated plants in britain and the netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Stouffer, P.C.; Bierregaard, R.O.; Strong, C.; Lovejoy, T.E. Long-term landscape change and bird abundance in Amazonian rainforest fragments. Conserv. Biol. 2006, 20, 1212–1223. [Google Scholar] [CrossRef]
- Hadley, A.S.; Frey, S.J.K.; Robinson, W.D.; Betts, M.G. Forest fragmentation and loss reduce richness, availability, and specialization in tropical hummingbird communities. Biotropica 2018, 50, 74–83. [Google Scholar] [CrossRef]
- Tinoco, B.A.; Graham, C.H.; Aguilar, J.M.; Schleuning, M. Effects of hummingbird morphology on specialization in pollination networks vary with resource availability. Oikos 2017, 126, 52–60. [Google Scholar] [CrossRef]
- Morrison, B.M.L.; Brosi, B.J.; Dirzo, R. Agricultural intensification drives changes in hybrid network robustness by modifying network structure. Ecol. Lett. 2019, 23, 359–369. [Google Scholar] [CrossRef]
- Pianka, E.R. Niche overlap and diffuse competition. Proc. Natl. Acad. Sci. USA 1974, 71, 2141–2145. [Google Scholar] [CrossRef]
- Maglianesi, M.A.; Blüthgen, N.; Böhning-Gaese, K.; Schleuning, M. Functional structure and specialization in three tropical plant-hummingbird interaction networks across an elevational gradient in Costa Rica. Ecography (Cop.) 2015, 38, 1119–1128. [Google Scholar] [CrossRef]
- Spiesman, B.J.; Gratton, C. Flexible foraging shapes the topology of plant-pollinator interaction networks. Ecology 2016, 97, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, P.K.; Vizentin-Bugoni, J.; Oliveira, G.M.; Oliveira, P.E.; Dalsgaard, B. Morphological and spatio-temporal mismatches shape a neotropical savanna plant-hummingbird network. Biotropica 2014, 46, 740–747. [Google Scholar] [CrossRef]
- Maglianesi, M.A.; Blüthgen, N.; Böhning-Gaese, K.; Schleuning, M. Morphological traits determine specialization and resource use in plant-hummingbird networks in the neotropics. Ecology 2014, 95, 3325–3334. [Google Scholar] [CrossRef]
- Fontaine, C.; Collin, C.L.; Dajoz, I. Generalist foraging of pollinators: Diet expansion at high density. J. Ecol. 2008, 96, 1002–1010. [Google Scholar] [CrossRef]
- Alarcón, R.; Waser, N.M.; Ollerton, J. Year-to-year variation in the topology of a plant-pollinator interaction network. Oikos 2008, 117, 1796–1807. [Google Scholar] [CrossRef]
- Brosi, B.J.; Briggs, H.M. Single pollinator species losses reduce floral fidelity and plant reproductive function. Proc. Natl. Acad. Sci. USA 2013, 110, 13044–13048. [Google Scholar] [CrossRef]
- Zahawi, R.A.; Duran, G.; Kormann, U. Sixty-seven years of land-use change in southern Costa Rica. PLoS ONE 2015, 10, e0143554. [Google Scholar] [CrossRef]
- Mendenhall, C.D.; Wrona, A.M. Improving tree cover estimates for fine-scale landscape ecology. Landsc. Ecol. 2018, 33, 1691–1696. [Google Scholar] [CrossRef]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783. [Google Scholar] [CrossRef]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.-J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2004, 8, 148–159. [Google Scholar] [CrossRef]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. R Version 2.4.4. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 27 March 2020).
- Fahrig, L. Rethinking patch size and isolation effects: The habitat amount hypothesis. J. Biogeogr. 2013, 40, 1649–1663. [Google Scholar] [CrossRef]
- Diamond, J.M.; Jones, H.L. Breeding land birds of the Channel Islands. In The California Islands: Proceedings of a Multidisciplinary Symposium; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1980; pp. 597–612. [Google Scholar]
- Lack, D. The numbers of species of hummingbirds in the West Indies. Evolution (N.Y.) 1973, 27, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Feeley, K. Analysis of avian communities in Lake Guri, Venezuela, using multiple assembly rule models. Oecologia 2003, 137, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J. How isolation affects rates of turnover of species on islands. Oikos 1985, 44, 331. [Google Scholar] [CrossRef]
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D.; Kelling, S. eBird: A citizen-based bird observation network in the biological sciences. Biol. Conserv. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef][Green Version]
- Traveset, A.; Castro-Urgal, R.; Rotllàn-Puig, X.; Lázaro, A. Effects of habitat loss on the plant-flower visitor network structure of a dune community. Oikos 2018, 127, 45–55. [Google Scholar] [CrossRef]
- Blüthgen, N. Why network analysis is often disconnected from community ecology: A critique and an ecologist’s guide. Basic Appl. Ecol. 2010, 11, 185–195. [Google Scholar] [CrossRef]
- Joppa, L.N.; Bascompte, J.; Montoya, J.M.; Solé, R.V.; Sanderson, J.; Pimm, S.L. Reciprocal specialization in ecological networks. Ecol. Lett. 2009, 12, 961–969. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Patefield, W. Algorithm AS 159: An efficient method of generating random R× C tables with given row and column totals. J. R. Stat. Soc. Ser. C Appl. Stat. 1981, 30, 91–97. [Google Scholar] [CrossRef]
- Grass, I.; Jauker, B.; Steffan-Dewenter, I.; Tscharntke, T.; Jauker, F. Past and potential future effects of habitat fragmentation on structure and stability of plant–pollinator and host–parasitoid networks. Nat. Ecol. Evol. 2018, 2, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Zook, J. Las Cruces Birds. Available online: https://tropicalstudies.org/wp-content/uploads/2018/12/Las-Cruces-Birds-2018.xlsx (accessed on 10 January 2020).
- Fogden, M.; Fogden, P. Hummingbirds of Costa Rica; Firefly Books: Buffalo, NY, USA, 2006. [Google Scholar]
- Stiles, F.G.; Skutch, A.F. A Guide to the Birds of Costa Rica; Comstock Cornell University Press, Instituto Nacional de Biodiversidad: Heredia, Costa Rica, 1989; ISBN 0801496004. [Google Scholar]
- Mendenhall, C.D.; Frishkoff, L.O.; Santos-Barrera, G.; Pacheco, J.; Mesfun, E.; Quijano, F.M.; Ehrlich, P.R.; Ceballos, G.; Daily, G.C.; Pringle, R.M. Countryside biogeography of Neotropical reptiles and amphibians. Ecology 2014, 95, 856–870. [Google Scholar] [CrossRef]
- Vaast, P.; van Kanten, R.; Siles, P.; Angrand, J.; Aguilar, A. Biophysical interactions between timber trees and arabica coffee in suboptimal conditions of Central America. In Advances in Agroforestry; Springer: Berlin/Heidelberg, Germany, 2008; pp. 133–146. [Google Scholar]
- Hurlbert, A.H. Species-energy relationships and habitat complexity in bird communities. Ecol. Lett. 2004, 7, 714–720. [Google Scholar] [CrossRef]
- Storch, D.; Evans, K.L.; Gaston, K.J. The species-area-energy relationship. Ecol. Lett. 2005, 8, 487–492. [Google Scholar] [CrossRef]
- Smithson, A.; MacNair, M.R. Negative frequency-dependent selection by pollinators on artificial flowers without rewards. Evolution (N.Y.) 1997, 51, 715–723. [Google Scholar] [CrossRef]
- Bernhardt, C.E.; Mitchell, R.J.; Michaels, H.J. Effects of population size and density on pollinator visitation, pollinator behavior, and pollen tube abundance in lupinus perennis. Int. J. Plant Sci. 2008, 169, 944–953. [Google Scholar] [CrossRef]
- Spiesman, B.J.; Inouye, B.D. Habitat loss alters the architecture of plant-pollinator interaction networks. Ecology 2013, 94, 2688–2696. [Google Scholar] [CrossRef]
- Valdovinos, F.S.; Brosi, B.J.; Briggs, H.M.; Moisset de Espanés, P.; Ramos-Jiliberto, R.; Martinez, N.D. Niche partitioning due to adaptive foraging reverses effects of nestedness and connectance on pollination network stability. Ecol. Lett. 2016, 19, 1277–1286. [Google Scholar] [CrossRef]
- Schluter, D. Speciation, ecological opportunity, and latitude. Am. Nat. 2016, 187, 1–15. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrison, B.M.L.; Mendenhall, C.D. Hummingbird–Plant Interactions Are More Specialized in Forest Compared to Coffee Plantations. Diversity 2020, 12, 126. https://doi.org/10.3390/d12040126
Morrison BML, Mendenhall CD. Hummingbird–Plant Interactions Are More Specialized in Forest Compared to Coffee Plantations. Diversity. 2020; 12(4):126. https://doi.org/10.3390/d12040126
Chicago/Turabian StyleMorrison, Beth M. L., and Chase D. Mendenhall. 2020. "Hummingbird–Plant Interactions Are More Specialized in Forest Compared to Coffee Plantations" Diversity 12, no. 4: 126. https://doi.org/10.3390/d12040126
APA StyleMorrison, B. M. L., & Mendenhall, C. D. (2020). Hummingbird–Plant Interactions Are More Specialized in Forest Compared to Coffee Plantations. Diversity, 12(4), 126. https://doi.org/10.3390/d12040126




