Rhodolith Bed Discovered off the South African Coast
Abstract
:1. Introduction
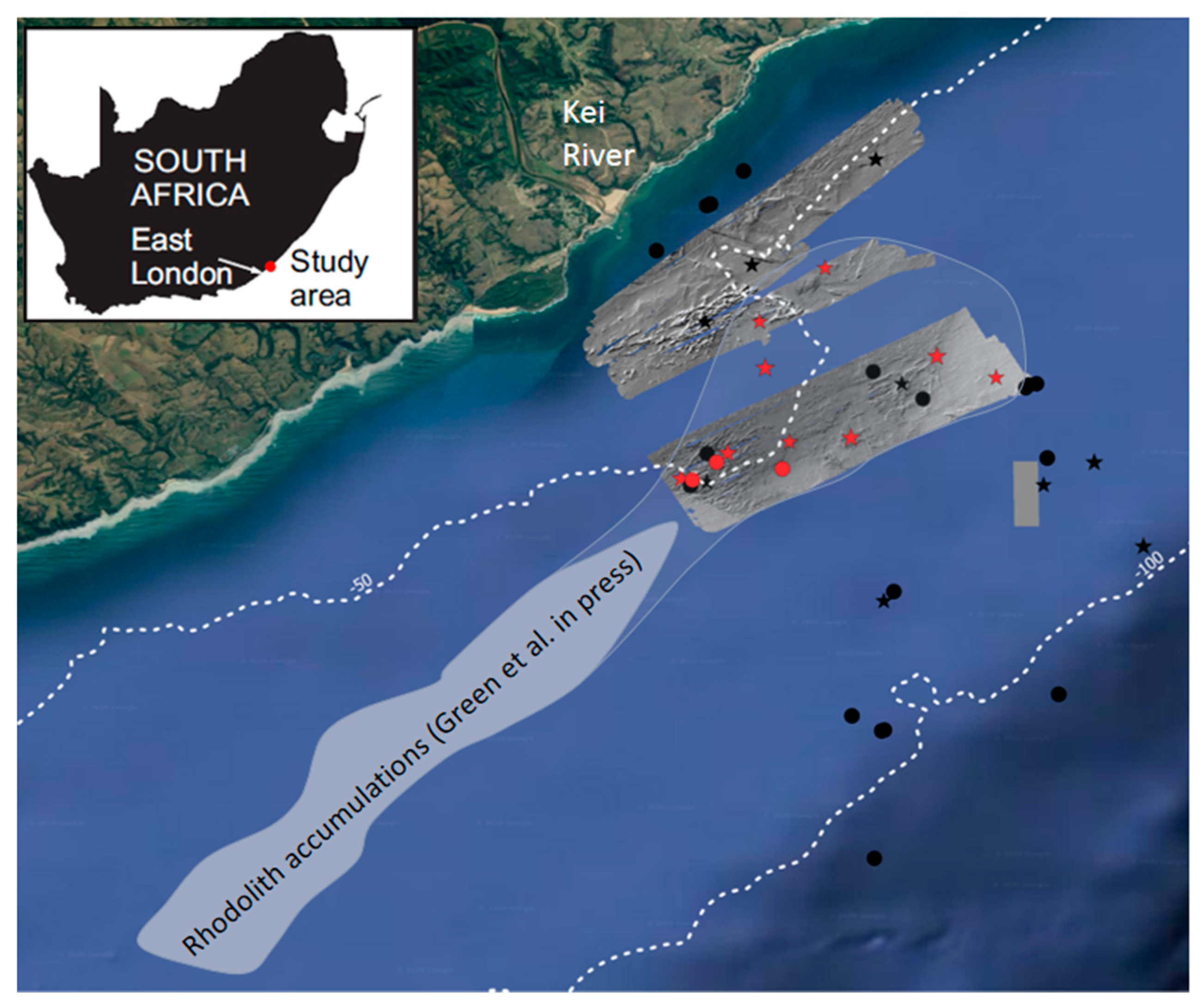
2. Materials and Methods
3. Results
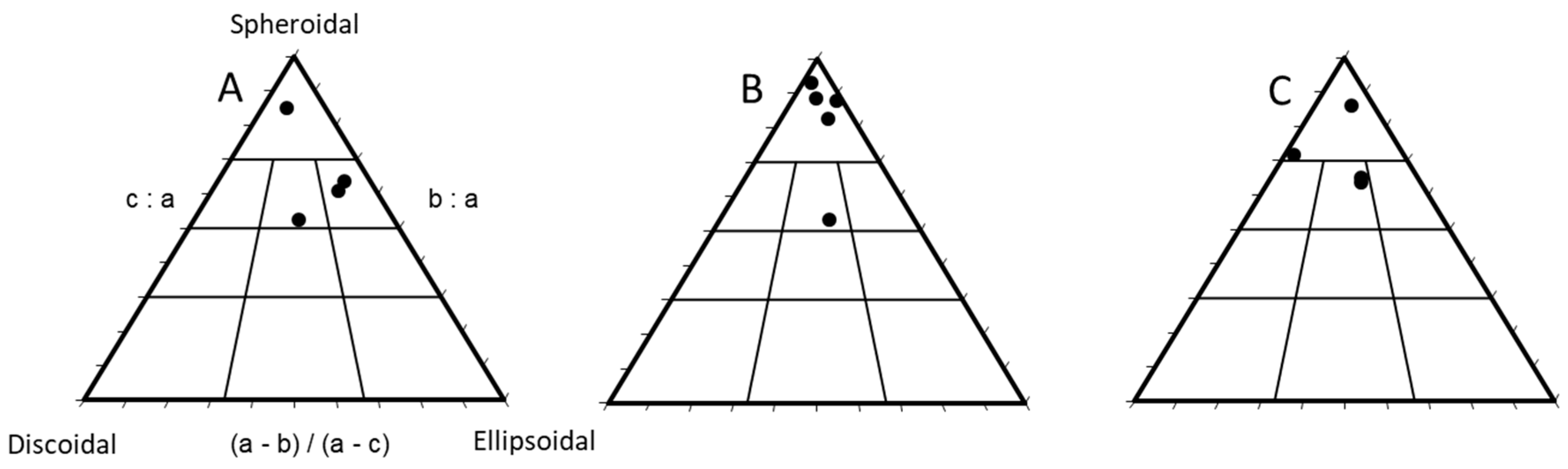
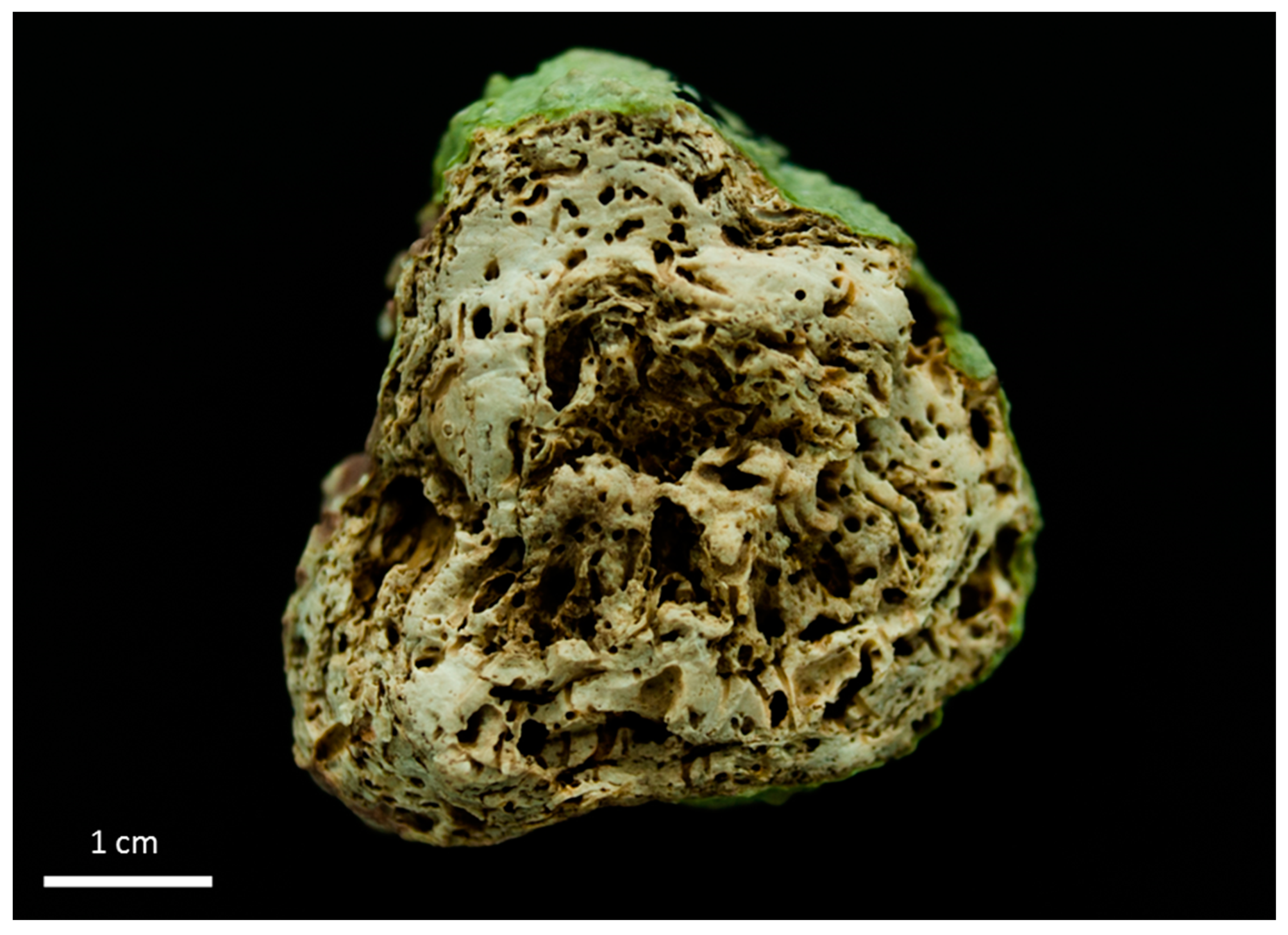
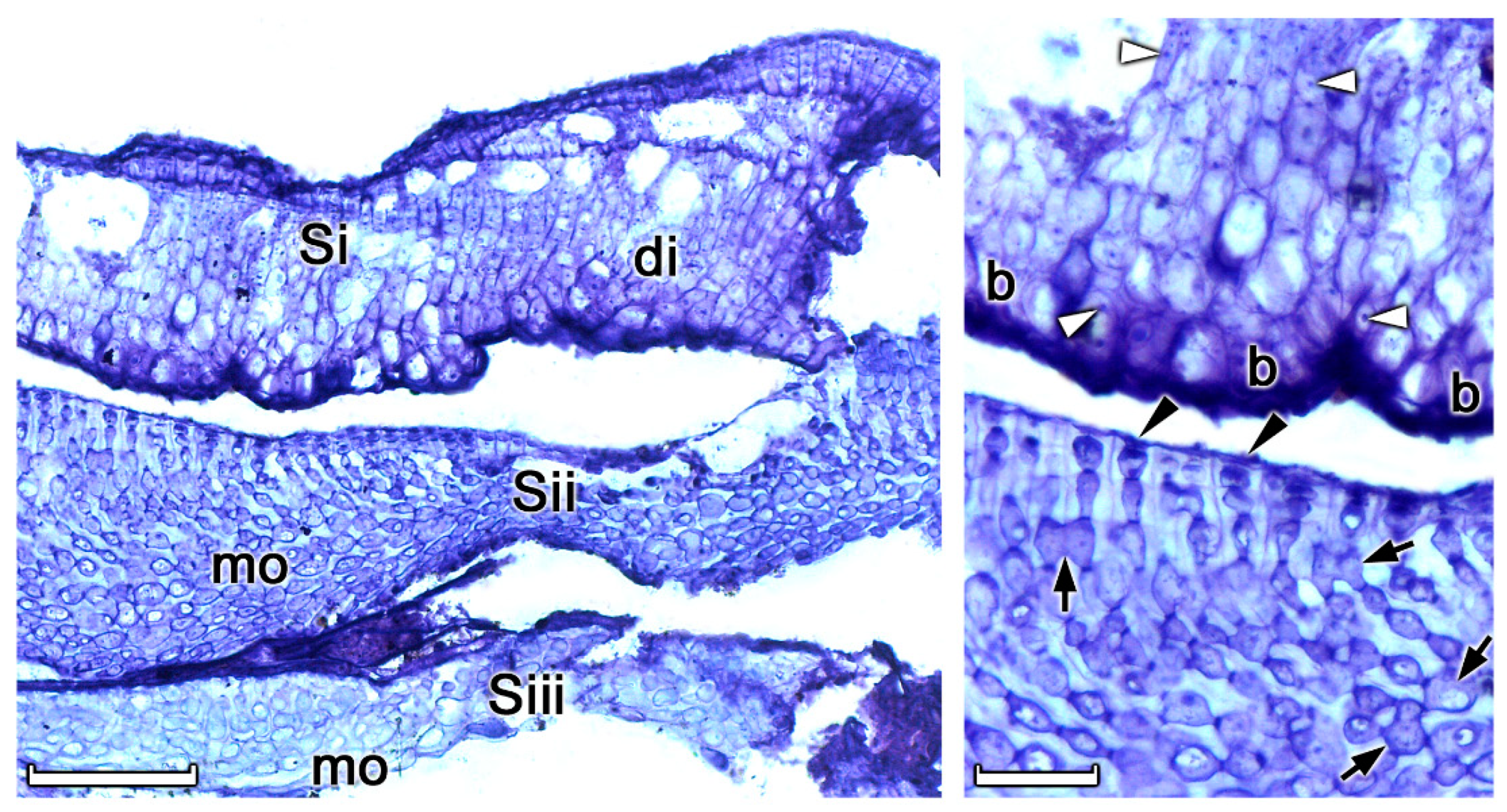
3.1. Rhodolith Morphology and Age
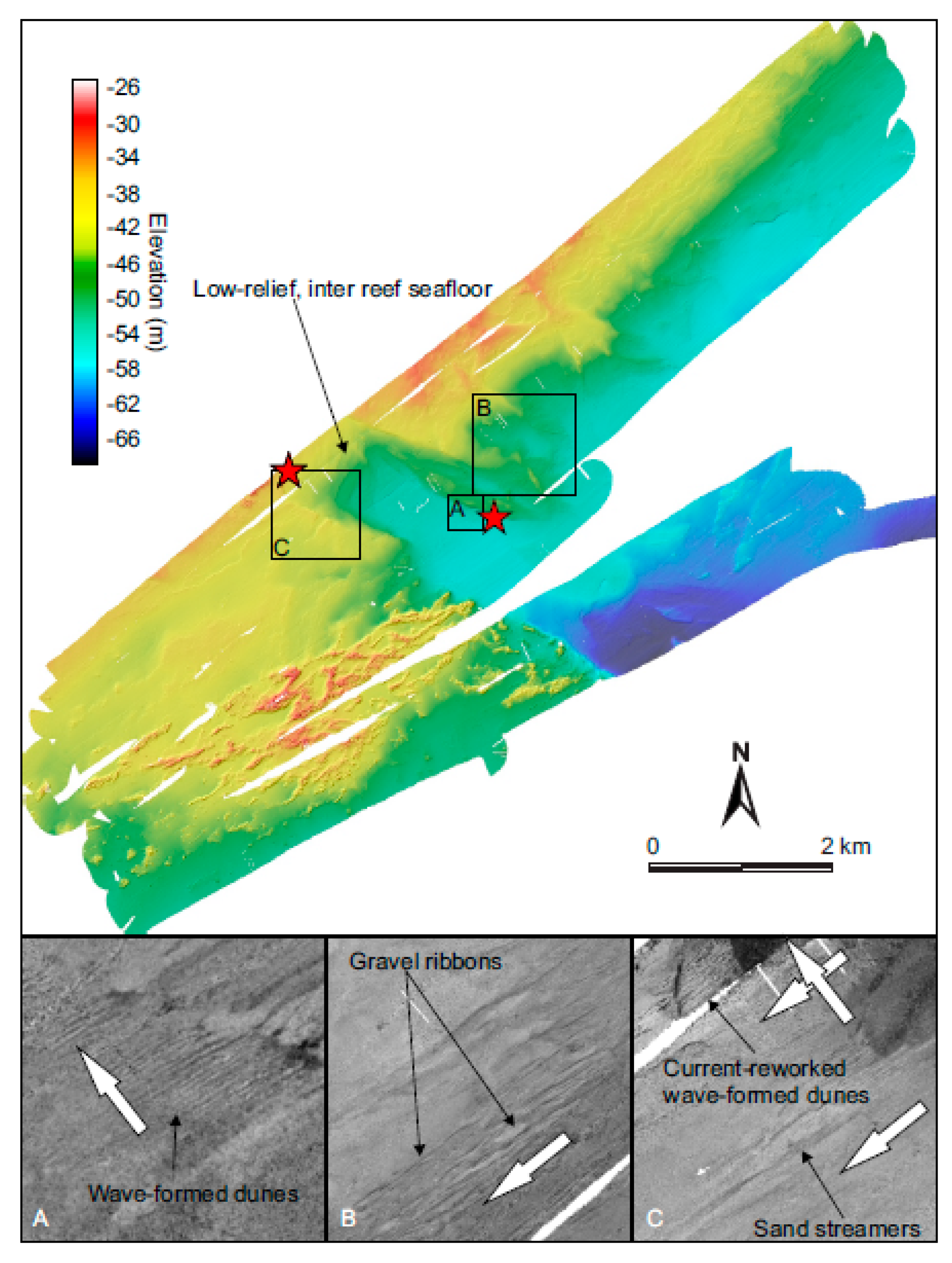
3.2. Depositional Setting and Epibiotic Components
3.3. Non-geniculate Coralline Algal Identifications
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Morphospecies Code | Morphospecies Name |
|---|---|
| ASS | Solitary stalked ascidian |
| AUC | Colonial unstalked ascidians |
| AUS | Solitary unstalked ascidians |
| CB | Black and Octocorals |
| CBBFA | Fleshy arborescent octocorals |
| CBBFM | Fleshy mushroom octocorals |
| CBFR | 2D rigid fan octocorals |
| CBW | Whip corals |
| CNHYD | Hydroids |
| EF | Feather stars |
| EOBSS | Brittle stars |
| MAA | Articulate calcareous macroalgae |
| MAAR | Articulate calcareous red macroalgae |
| MAECR | Erect course branching red macroalgae |
| MAEFR | Erect fine branching red macroalgae |
| MAENR | Encrusting red macroalgae |
| MAENRC | Encrusting red calcareous macroalgae |
| MAENRNC | Encrusting red non-calcareous macroalgae |
| MAF | Filamentous macroalgae |
| MAFR | Filamentous red macroalgae |
| MALAR | Laminate red macroalgae |
| MASR | Sheet-like red macroalgae |
| MAEN | Encrusting macroalgae |
| MOB | Bivalves |
| SPCE | Creeping/ramose sponges |
| SPES | Simple erect sponges |
| SPM | Massive sponges |
References
- Riosmena-Rodríguez, R.; Nelson, W.; Aguirre, J. Rhodolith/maërl Beds: A Global Perspective; Springer International Publishing: Basel, Switzerland, 2017. [Google Scholar]
- Foster, M.S. Rhodoliths: Between rocks and soft places. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Foster, M.S.; Amado Filho, G.M.; Kamenos, N.A.; Riosmena-Rodriguez, R.; Steller, D.L. Rhodoliths and rhodolith beds. Smithson. Contrib. Mar. Sci. 2013, 39, 143–155. [Google Scholar]
- Steller, D.L.; Foster, M.S. Environmental factors influencing distribution and morphology of rhodoliths in Bahía Concepción, B.C.S., México. J. Exp. Mar. Bio. Ecol. 1995, 194, 201–212. [Google Scholar] [CrossRef]
- Amado-Filho, G.; Maneveldt, G.; Pereira-Filho, G.; Manso, R.; Bahia, R.; Barros-Barreto, M.; Guimarães, S.M.P.B. Seaweed diversity associated with a Brazilian tropical rhodolith bed. Ciencias Mar. 2010, 36, 371–391. [Google Scholar] [CrossRef] [Green Version]
- Vale, N.F.; Amado-Filho, G.M.; Braga, J.C.; Brasileiro, P.S.; Karez, C.S.; Moraes, F.C.; Bahia, R.G.; Bastos, A.C.; Moura, R.L. Structure and composition of rhodoliths from the Amazon River mouth, Brazil. J. South Am. Earth Sci. 2018, 84, 149–159. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Maneveldt, G.; Manso, R.C.C.; Marins-Rosa, B.V.; Pacheco, M.R.; Guimarães, S.M.P.B. Estructura de los mantos de rodolitos de 4 a 55 metros de profundidad en la costa sur del estado de Espírito Santo, Brazil. Ciencias Mar. 2007, 33, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Bahia, R.G.; Abrantes, D.P.; Brasileiro, P.S.; Pereira Filho, G.H.; Amado Filho, G.M. Rhodolith bed structure along a depth gradient on the northern coast of Bahia state, Brazil. Brazilian J. Oceanogr. 2010, 58, 323–337. [Google Scholar] [CrossRef]
- Villas-Boas, A.B.; Riosmena-Rodriguez, R.; de Oliveira Figueiredo, M.A. Community structure of rhodolith-forming beds on the central Brazilian continental shelf. Helgol. Mar. Res. 2014, 68, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Teichert, S.; Woelkerling, W.; Rüggeberg, A.; Wisshak, M.; Piepenburg, D.; Meyerhöfer, M.; Form, A.; Freiwald, A. Arctic rhodolith beds and their environmental controls (Spitsbergen, Norway). Facies 2014, 60, 15–37. [Google Scholar] [CrossRef]
- Gagnon, P.; Matheson, K.; Stapleton, M. Variation in rhodolith morphology and biogenic potential of newly discovered rhodolith beds in Newfoundland and Labrador (Canada). Bot. Mar. 2012, 55, 85–99. [Google Scholar] [CrossRef]
- Hetzinger, S.; Halfar, J.; Riegl, B.; Godinez, L. Sedimentology and Acoustic Mapping of Modern Rhodolith Facies on a Non- Tropical Carbonate Shelf (Gulf of California, Mexico). J. Sediment. Res. 2006, 76, 670–682. [Google Scholar] [CrossRef]
- Fredericq, S.; Arakaki, N.; Camacho, O.; Gabriel, D.; Krayesky, D.; Self-Krayesky, S.; Rees, G.; Richards, J.; Sauvage, T.; Venera-Ponton, D.; et al. A Dynamic Approach to the Study of Rhodoliths: A Case Study for the Northwestern Gulf of Mexico. Cryptogam. Algol. 2014, 35, 77–98. [Google Scholar] [CrossRef]
- Rindi, F.; Braga, J.C.; Martin, S.; Peña, V.; Le Gall, L.; Caragnano, A.; Aguirre, J. Coralline Algae in a Changing Mediterranean Sea: How Can We Predict Their Future, if We Do Not Know Their Present? Front. Mar. Sci. 2019, 6, 723. [Google Scholar] [CrossRef]
- Basso, D. Deep rhodolith distribution in the Pontian Islands, Italy: A model for the paleoecology of a temperate sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 137, 173–187. [Google Scholar] [CrossRef]
- Sañé, E.; Chiocci, F.L.; Basso, D.; Martorelli, E. Environmental factors controlling the distribution of rhodoliths: An integrated study based on seafloor sampling, ROV and side scan sonar data, offshore the W-Pontine Archipelago. Cont. Shelf Res. 2016, 129, 10–22. [Google Scholar] [CrossRef]
- Goldberg, N. Age estimates and description of rhodoliths from Esperance Bay, Western Australia. J. Mar. Biol. Assoc. U.K. 2006, 86, 1291–1296. [Google Scholar] [CrossRef]
- Bassi, D.; Nebelsick, J.H.; Checconi, A.; Hohenegger, J.; Iryu, Y. Present-day and fossil rhodolith pavements compared: Their potential for analysing shallow-water carbonate deposits. Sediment. Geol. 2009, 214, 74–84. [Google Scholar] [CrossRef]
- Steller, D.L.; Riosmena-Rodríguez, R.; Foster, M.S.; Roberts, C.A. Rhodolith bed diversity in the Gulf of California: The importance of rhodolith structure and consequences of disturbance. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 5–20. [Google Scholar] [CrossRef]
- Berlandi, R.M.; de O. Figueiredo, M.A.; Paiva, P.C. Rhodolith Morphology and the Diversity of Polychaetes Off the Southeastern Brazilian Coast. J. Coast. Res. 2012, 279, 280–287. [Google Scholar] [CrossRef]
- Botha, T.P.A.; Griffiths, C.L.; Maneveldt, G.W. Coralline red algae — a new host taxon for burrowing barnacles (Cirripedia, Acrothoracica). Mar. Biodivers. 2020, 50, 1–5. [Google Scholar] [CrossRef]
- Pascelli, C.; Riul, P.; Riosmena-rodríguez, R.; Scherner, F.; Nunes, M.; Hall-spencer, J.M.; Cabral, E.; Oliveira, D.; Horta, P. Seasonal and depth-driven changes in rhodolith bed structure and associated macroalgae off Arvoredo island ( southeastern Brazil ). Aquat. Bot. 2013, 111, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Steller, D.L.; Cáceres-Martínez, C. Coralline algal rhodoliths enhance larval settlement and early growth of the pacific calico scallop Argopecten ventricosus. Mar. Ecol. Prog. Ser. 2009, 396, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.J.; van der Lingen, C.D.; Whittle, C.; van den Berg, M. Shelf currents, lee-trapped and transient eddies on the inshore boundary of the agulhas current, South Africa: Their relevance to the kwaZulu-Natal sardine run. African J. Mar. Sci. 2010, 32, 423–447. [Google Scholar] [CrossRef]
- Dixon, S.; Green, A.; Cooper, A. Storm Swash Deposition On An Embayed Rock Coastline: Facies, Formative Mechanisms, and Preservation. J. Sediment. Res. 2015, 85, 1155–1165. [Google Scholar] [CrossRef]
- Russo, C.S.; Lamont, T.; Tutt, G.C.O.; van den Berg, M.A.; Barlow, R.G. Hydrography of a shelf ecosystem inshore of a major Western Boundary Current. Estuar. Coast. Shelf Sci. 2019, 228, 106363. [Google Scholar] [CrossRef]
- Sneed, E.D.; Folk, R.L. Pebbles in the Lower Colorado River, Texas a Study in Particle Morphogenesis. J. Geol. 1958, 66, 114–150. [Google Scholar] [CrossRef]
- Hogg, A.G.; Hua, Q.; Blackwell, P.G.; Niu, M.; Buck, C.E.; Guilderson, T.P.; Heaton, T.J.; Palmer, J.G.; Reimer, P.J.; Reimer, R.W.; et al. SHCal13 Southern Hemisphere Calibration, 0–50,000 Years cal BP. Radiocarbon 2013, 55, 1889–1903. [Google Scholar] [CrossRef] [Green Version]
- Althaus, F.; Hill, N.; Ferrari, R.; Edwards, L.; Przeslawski, R.; Schönberg, C.H.L.; Stuart-Smith, R.; Barrett, N.; Edgar, G.; Colquhoun, J.; et al. A standardised vocabulary for identifying benthic biota and substrata from underwater imagery: The CATAMI classification scheme. PLoS ONE 2015, 10, e0141039. [Google Scholar] [CrossRef]
- Maneveldt, G.W.; van der Merwe, E. Heydrichia cerasina sp. nov. (Sporolithales, Corallinophycidae, Rhodophyta) from the southernmost tip of Africa. Phycologia 2012, 51, 11–21. [Google Scholar] [CrossRef]
- Maneveldt, G.W.; Van der Merwe, E.; Keats, D.W. Updated keys to the non-geniculate coralline red algae (Corallinophycidae, Rhodophyta) of South Africa. South African J. Bot. 2016, 106, 158–164. [Google Scholar] [CrossRef]
- Wilson, S.; Blake, C.; Berges, J.A.; Maggs, C.A. Environmental tolerances of free-living coralline algae (maerl): Implications for European marine conservation. Biol. Conserv. 2004, 120, 279–289. [Google Scholar] [CrossRef]
- Flemming, B.W. Sand transport and bedform patterns on the continental shelf between Durban and Port Elizabeth (southeast African continental margin). Sediment. Geol. 1980, 26, 179–205. [Google Scholar] [CrossRef]
- Figueiredo, M.A.O.; Coutinho, R.; Villas-Boas, A.B.; Tâmega, F.T.S.; Mariath, R. Deep-water rhodolith productivity and growth in the southwestern Atlantic. J. Appl. Phycol. 2012, 24, 487–493. [Google Scholar] [CrossRef]
- Frantz, B.R.; Kashgarian, M.; Coale, K.H.; Foster, M.S. Growth rate and potential climate record from a rhodolith using 14C accelerator mass spectrometry. Limnol. Oceanogr. 2000, 45, 1773–1777. [Google Scholar] [CrossRef]
- Bosence, D.; Wilson, J. Maerl growth, carbonate production rates and accumulation rates in the northeast Atlantic. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 21–31. [Google Scholar] [CrossRef]
- Cooper, J.A.G.; Green, A.N.; Compton, J.S. Sea-level change in southern Africa since the Last Glacial Maximum. Quat. Sci. Rev. 2018, 201, 303–318. [Google Scholar] [CrossRef]
- Holz, V.L.; Bahia, R.G.; Karez, C.S.; Vieira, F.V.; Moraes, F.C.; Vale, N.F.; Sudatti, D.B.; Salgado, L.T.; Moura, R.L.; Amado-filho, G.M.; et al. Structure of Rhodolith Beds and Surrounding Habitats at the Doce River Shelf (Brazil). Diversity 2020, 12, 75. [Google Scholar] [CrossRef] [Green Version]
- Teichert, S. Hollow rhodoliths increase Svalbard’s shelf biodiversity. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Bassi, D.; Iryu, Y.; Braga, J.C.; Takayanagi, H.; Tsuji, Y. Bathymetric distribution of ichnocoenoses from recent subtropical algal nodules off Fraser Island, eastern Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 369, 58–66. [Google Scholar] [CrossRef]
- Basso, D.; Nalin, R.; Nelson, C.S. Shallow-Water Sporolithon Rhodoliths From North Island (New Zealand). Palaios 2009, 24, 92–103. [Google Scholar] [CrossRef]
- Leal, R.N.; Bassi, D.; Posenato, R.; Amado-Filho, G.M. Tomographic Analysis for Bioerosion Signatures in Shallow-Water Rhodoliths from the Abrolhos Bank, Brazil. J. Coast. Res. 2012, 279, 306–309. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, L.A.; Maneveldt, G.W.; Green, A.; Karenyi, N.; Parker, D.; Samaai, T.; Kerwath, S. Rhodolith Bed Discovered off the South African Coast. Diversity 2020, 12, 125. https://doi.org/10.3390/d12040125
Adams LA, Maneveldt GW, Green A, Karenyi N, Parker D, Samaai T, Kerwath S. Rhodolith Bed Discovered off the South African Coast. Diversity. 2020; 12(4):125. https://doi.org/10.3390/d12040125
Chicago/Turabian StyleAdams, Luther A., Gavin W. Maneveldt, Andrew Green, Natasha Karenyi, Denham Parker, Toufiek Samaai, and Sven Kerwath. 2020. "Rhodolith Bed Discovered off the South African Coast" Diversity 12, no. 4: 125. https://doi.org/10.3390/d12040125
APA StyleAdams, L. A., Maneveldt, G. W., Green, A., Karenyi, N., Parker, D., Samaai, T., & Kerwath, S. (2020). Rhodolith Bed Discovered off the South African Coast. Diversity, 12(4), 125. https://doi.org/10.3390/d12040125






