Effects of Upwelling Intensity on Nitrogen and Carbon Fluxes through the Planktonic Food Web off A Coruña (Galicia, NW Spain) Assessed with Stable Isotopes
Abstract
1. Introduction
2. Materials and Methods
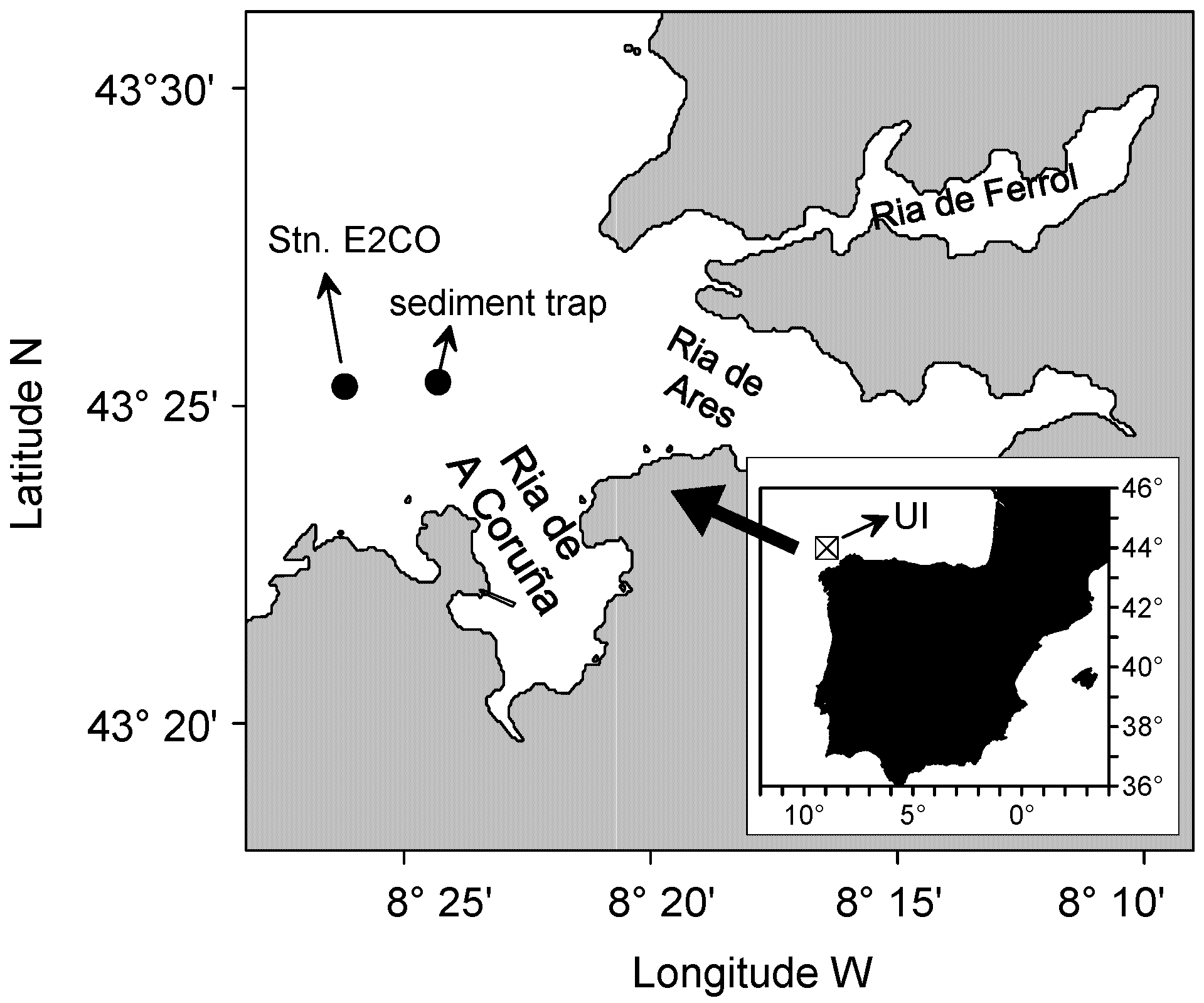
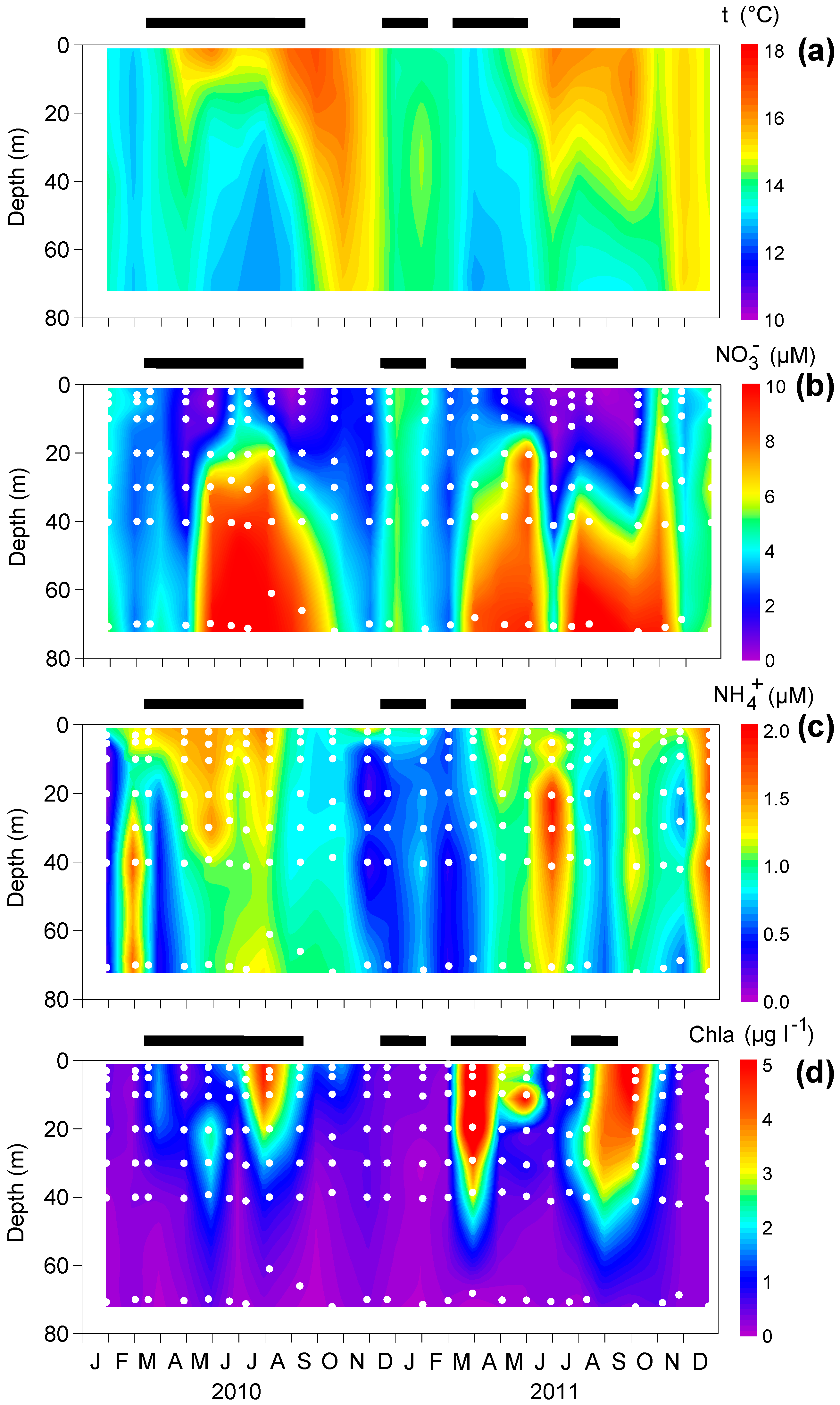
2.1. Sampling and Determination of Environmental Variables
2.2. Stable Isotope Anaysis
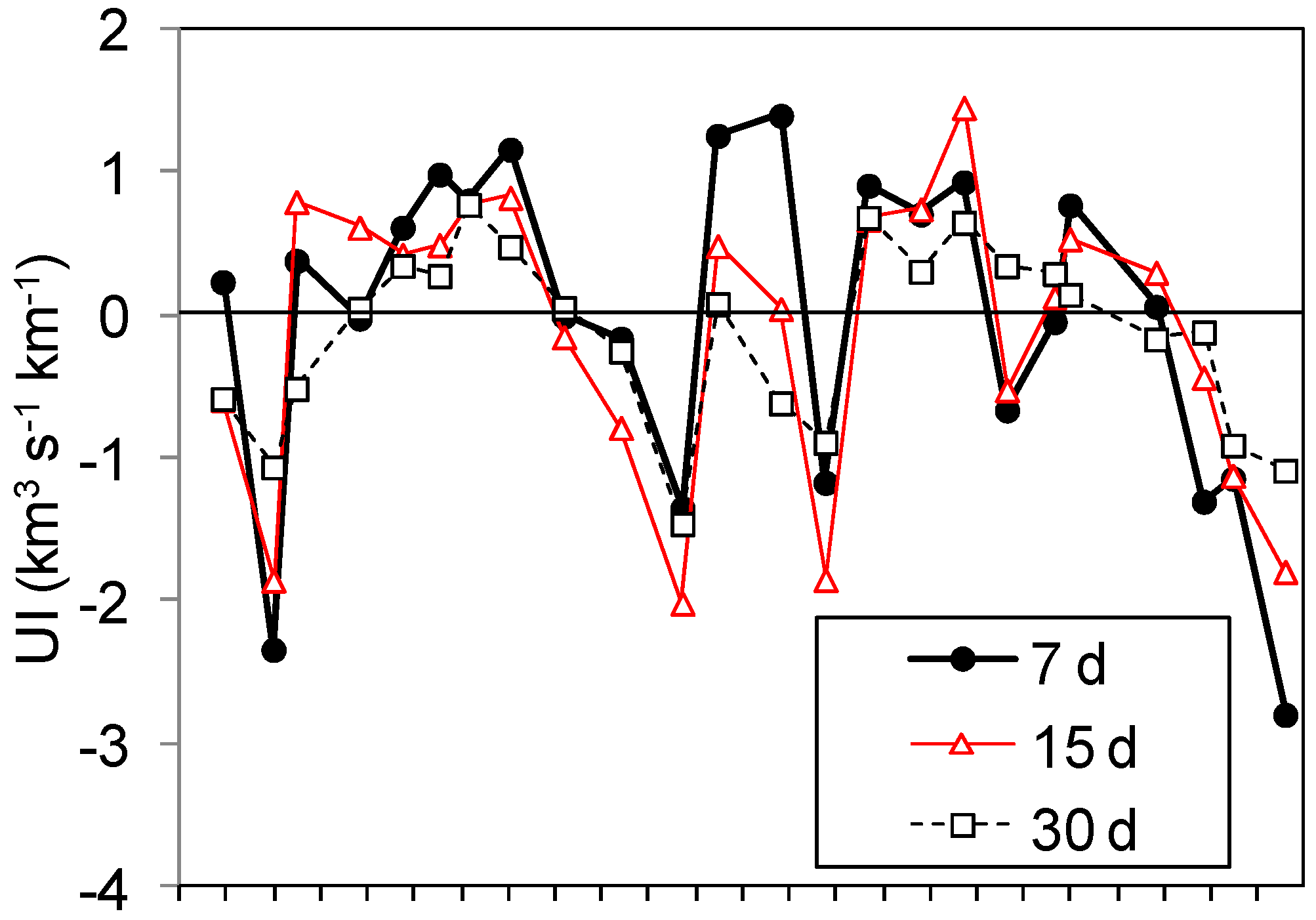
2.3. Upwelling Intensity
2.4. Statistical Analysis
3. Results
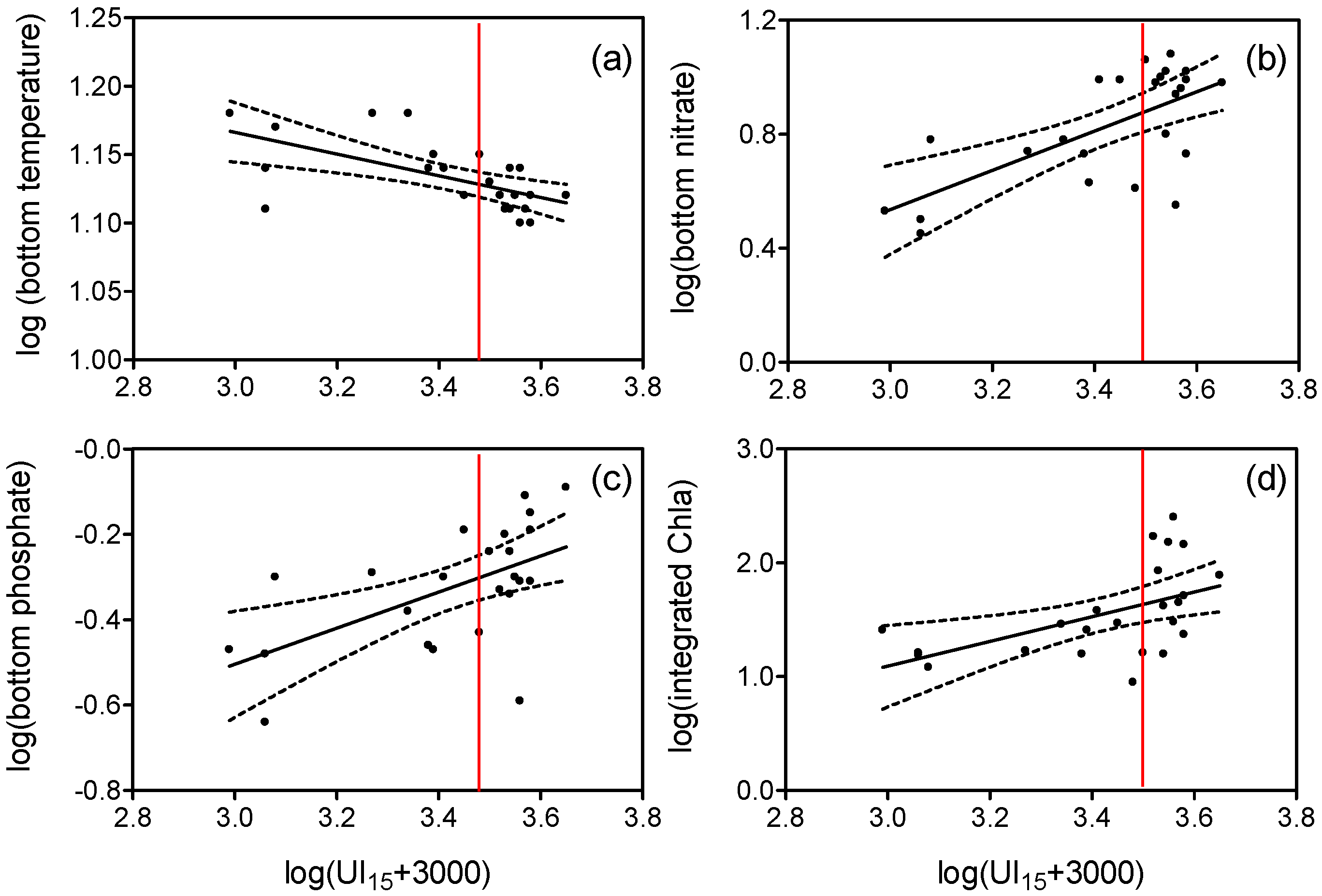
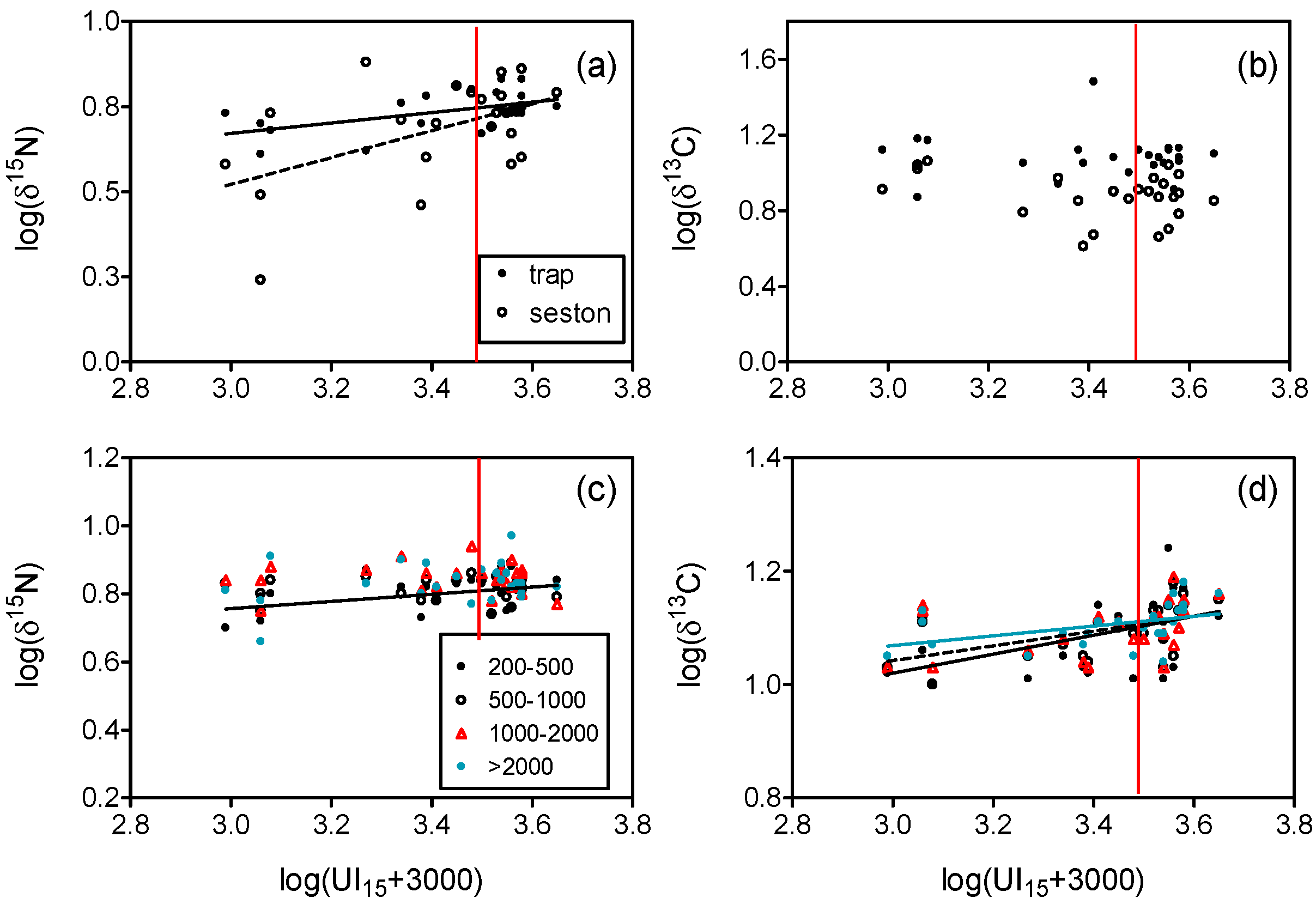
3.1. Upwelling and Nutrient Enrichment
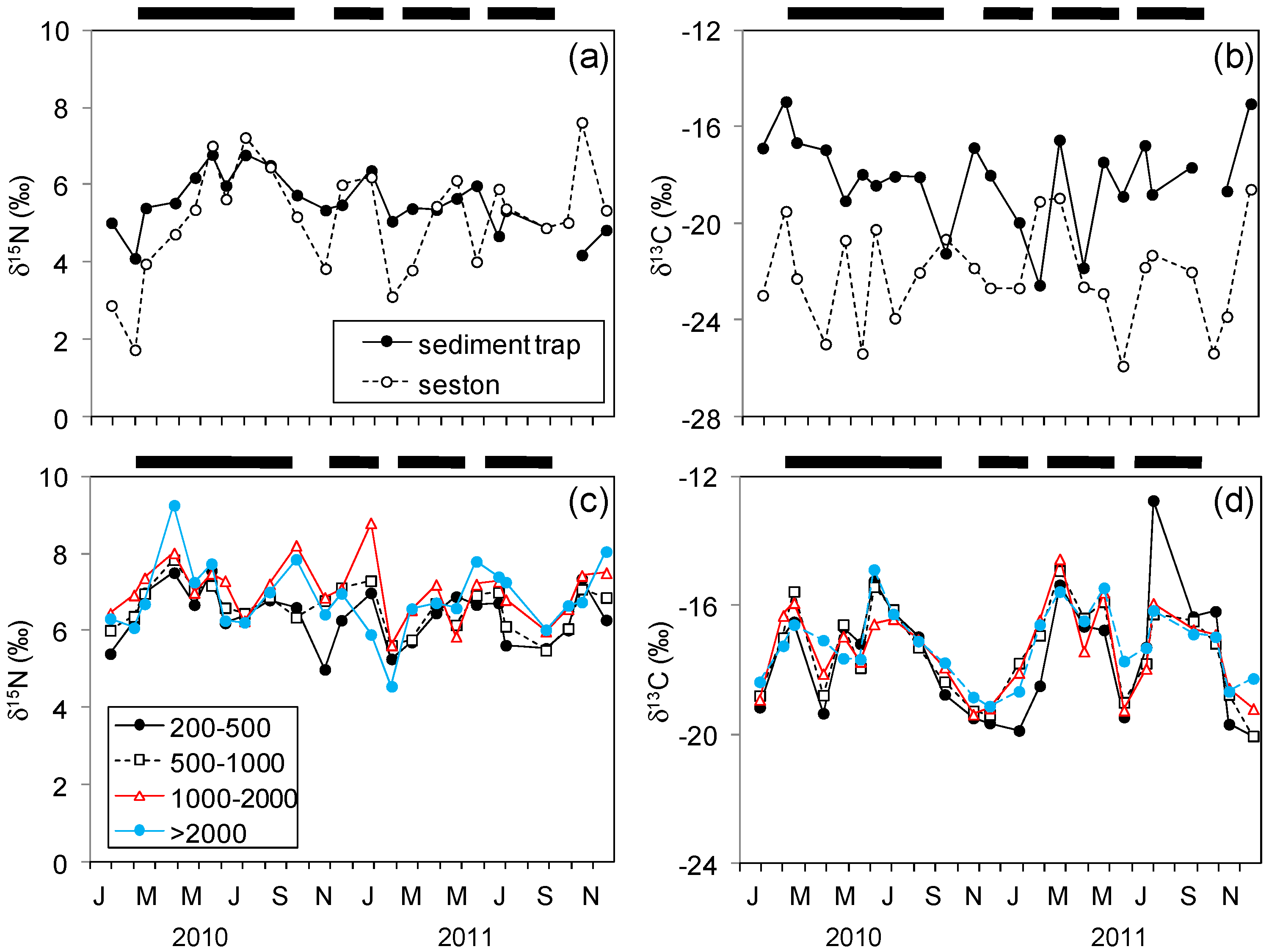
3.2. Stable Isotopes in Seston and Plankton
4. Discussion
4.1. Nitrogen Isotopes and Fluxes
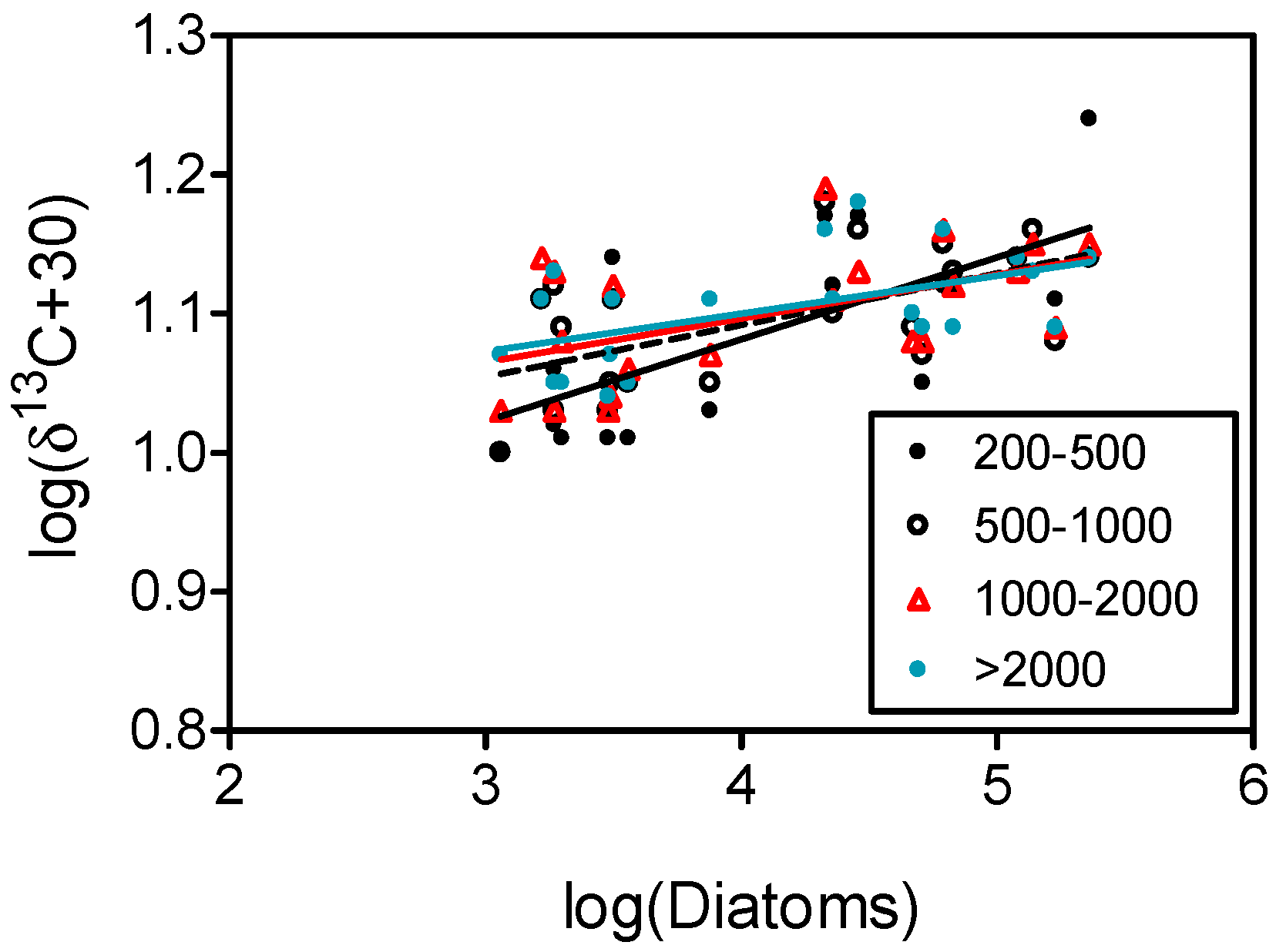
4.2. Carbon Isotopes and Fluxes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demarcq, H. Trends in primary production, sea surface temperature and wind in upwelling systems (1998–2007). Prog. Oceanogr. 2009, 83, 376–385. [Google Scholar] [CrossRef]
- Capone, D.G.; Hutchins, D.A. Microbial biogeochemistry of coastal upwelling regimes in a changing ocean. Nat. Geosci. 2013, 6, 711–717. [Google Scholar] [CrossRef]
- Dugdale, R.C.; Wilkerson, F.P. New production in the upwelling center at Point Conception, California: Temporal and spatial patterns. Deep Sea Res. 1989, 36, 985–1008. [Google Scholar] [CrossRef]
- Alvarez-Salgado, X.A.; Gago, J.; Míguez, B.M.; Gilcoto, M.; Pérez, F.F. Surface waters of the NW Iberian margin: Upwelling on the shelf versus outwelling of upwelled waters from the Rias Baixas. Estuar. Coast. Shelf Sci. 2000, 51, 821–837. [Google Scholar] [CrossRef]
- Chen, Y.L.L.; Lu, H.B.; Shiah, F.K.; Gong, G.C.; Liu, K.K.; Kanda, J. New production and f-ratio on the continental shelf of the East China Sea: Comparisons between nitrate inputs from the subsurface Kuroshio Current and the Changjiang River. Estuar. Coast. Shelf Sci. 1999, 48, 59–75. [Google Scholar] [CrossRef]
- Vincent, W.F.; Chang, F.H.; Cole, A.; Downes, M.T.; James, M.R.; May, L.; Moore, M.; Woods, P.H. Short-term changes in planktonic community structure and nitrogen transfers in a coastal upwelling system. Estuar. Coast. Shelf Sci. 1989, 29, 131–150. [Google Scholar] [CrossRef]
- Bakun, A. Global climate change and intensification of coastal upwelling. Science 1990, 247, 198–201. [Google Scholar] [CrossRef]
- Chavez, F.P.; Ryan, J.P.; Lluch-Cota, S.E.; Ñiquen, M. From anchovies to sardines and back: Multidecadal change in the Pacific Ocean. Science 2003, 299, 217–221. [Google Scholar] [CrossRef]
- Xiu, P.; Chai, F.; Curchitser, E.N.; Castruccio, F.S. Future changes in coastal upwelling ecosystems with global warming: The case of the California Current system. Sci. Rep. 2018, 8, 2866. [Google Scholar] [CrossRef]
- Sigman, D.M.; Karsh, K.L.; Casciotti, K.L. Ocean process tracers: Nitrogen isotopes in the ocean. In Encyclopedia of Ocean Sciences, 2nd ed.; Steele, J.H., Ed.; Academic Press: Oxford, UK, 2009; pp. 40–54. [Google Scholar]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Rasmussen, B. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 1999, 80, 1395–1404. [Google Scholar] [CrossRef]
- Fry, B.; Wainright, S.C. Diatom sources of 13C-rich carbon in marine food webs. Mar. Ecol. Prog. Ser. 1991, 76, 149–157. [Google Scholar] [CrossRef]
- Abrantes, F.; Cermeño, P.; Lopes, C.; Romero, O.; Matos, L.; Van Iperen, J.; Rufino, M.; Magalhães, V. Diatoms si uptake capacity drives carbon export in coastal upwelling systems. Biogeosciences 2016, 13, 4099–4109. [Google Scholar] [CrossRef]
- Arístegui, J.; Alvarez-Salgado, X.A.; Barton, E.D.; Figueiras, F.G.; Hernández-León, S.; Roy, C.; Santos, A.M.P. Chapter 23. Oceanography and fisheries of the canary current/iberian region of the eastern north atlantic (18a, e). In The Global Coastal Ocean: Interdisciplinary Regional Studies and Syntheses; Robinson, A.R., Brink, K., Eds.; Harvard University Press: Boston, MA, USA, 2006; Volume 14, pp. 877–931. [Google Scholar]
- Pérez, F.F.; Padin, X.A.; Pazos, Y.; Gilcoto, M.; Cabanas, M.; Pardo, P.C.; Doval, M.D.; Farina-Bustos, L. Plankton response to weakening of the iberian coastal upwelling. Glob. Chang. Biol. 2010, 16, 1258–1267. [Google Scholar] [CrossRef]
- Figueiras, F.G.; Labarta, U.; Fernández Reiriz, M.J. Coastal upwelling, primary production and mussel growth in the Rías Baixas of Galicia. Hydrobiologia 2002, 484, 121–131. [Google Scholar] [CrossRef]
- Viana, I.G.; Bode, A. Variability in δ15N of intertidal brown algae along a salinity gradient: Differential impact of nitrogen sources. Sci. Tot. Environ. 2015, 512–513, 167–176. [Google Scholar] [CrossRef]
- Bode, A.; Álvarez, M.; Ruíz-Villarreal, M.; Varela, M.M. Changes in phytoplankton production and upwelling intensity off A Coruña (NW Spain) for the last 28yr. Ocean Dyn. 2019, 69, 861–873. [Google Scholar] [CrossRef]
- Buttay, L.; Cazelles, B.; Miranda, A.; Casas, G.; Nogueira, E.; González-Quirós, R. Environmental multi-scale effects on zooplankton inter-specific synchrony. Limnol. Oceanogr. 2017, 62, 1355–1365. [Google Scholar] [CrossRef]
- Bode, A.; Varela, M.; Barquero, S.; Alvarez-Ossorio, M.T.; González, N. Preliminary studies on the export of organic matter during phytoplankton blooms off La Coruña (North Western Spain). J. Mar. Biol. Ass. UK 1998, 78, 1–15. [Google Scholar] [CrossRef]
- Ng, J.S.S.; Wai, T.C.; Williams, G.A. The effects of acidification on the stable isotope signatures of marine algae and molluscs. Mar. Chem. 2007, 103, 97–102. [Google Scholar] [CrossRef]
- Coplen, T.B. Guidelines and recommended terms for expression of stable isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Marañón, E.; Bode, A. Large-scale meridional and zonal variability in the nitrogen isotopic composition of plankton in the Atlantic Ocean. J. Plankton Res. 2014, 36, 1060–1073. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef] [PubMed]
- González-Nuevo, G.; Gago, J.; Cabanas, J.M. Upwelling index: A powerful tool for marine research in the nw iberian upwelling system. J. Oper. Oceanogr. 2014, 7, 45–55. [Google Scholar] [CrossRef]
- Nogueira, E.; Perez, F.F.; Rios, A.F. Seasonal patterns and long-term trends in an estuarine upwelling ecosystem (Ria de Vigo, NW Spain). Estuar. Coast. Shelf Sci. 1997, 44, 285–300. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 6 March 2020).
- Wu, J.P.; Calvert, S.E.; Wong, C.S. Carbon and nitrogen isotope ratios in sedimenting particulate organic matter at an upwelling site off Vancouver Island. Estuar. Coast. Shelf Sci. 1999, 48, 193–203. [Google Scholar] [CrossRef]
- Montoya, J.P.; McCarthy, J.J. Isotopic fractionation during nitrate uptake by phytoplankton grown in continuous culture. J. Plankton Res. 1995, 17, 439–464. [Google Scholar] [CrossRef]
- Altabet, M.A. Variations in nitrogen isotopic composition between sinking and suspended particles: Implications for nitrogen cycling and particle transformation in the open ocean. Deep Sea Res. 1988, 35, 535–554. [Google Scholar] [CrossRef]
- Mahaffey, C.; Williams, R.G.; Wolff, G.A.; Anderson, W.T. Physical supply of nitrogen to phytoplankton in the Atlantic Ocean. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Fawcett, S.E.; Lomas, M.W.; Casey, J.R.; Ward, B.B.; Sigman, D.M. Assimilation of upwelled nitrate by small eukaryotes in the Sargasso Sea. Nat. Geosci. 2011, 4, 717–722. [Google Scholar] [CrossRef]
- Needoba, J.A.; Waser, N.A.; Harrison, P.J.; Calvert, S.E. Nitrogen isotope fractionation in 12 species of marine phytoplankton during growth on nitrate. Mar. Ecol. Prog. Ser. 2003, 255, 81–91. [Google Scholar] [CrossRef]
- Mompeán, C.; Bode, A.; Benítez-Barrios, V.M.; Domínguez-Yanes, J.F.; Escánez, J.; Fraile-Nuez, E. Spatial patterns of plankton biomass and stable isotopes reflect the influence of the nitrogen-fixer Trichodesmium along the subtropical North Atlantic. J. Plankton Res. 2013, 35, 513–525. [Google Scholar] [CrossRef]
- McKinney, R.A.; Oczkowski, A.J.; Prezioso, J.; Hyde, K.J.W. Spatial variability of nitrogen isotope ratios of particulate material from northwest Atlantic continental shelf waters. Estuar. Coast. Shelf Sci. 2010, 89, 287–293. [Google Scholar] [CrossRef]
- Bode, A.; Alvarez-Ossorio, M.T.; Varela, M. Phytoplankton and macrophyte contributions to littoral food webs in the Galician upwelling (NW Spain) estimated from stable isotopes. Mar. Ecol. Prog. Ser. 2006, 318, 89–102. [Google Scholar] [CrossRef]
- Bode, A.; Álvarez, M.; Ruiz-Villarreal, M.; Varela, M.M. Time Series of Hydrographic, Chemical and Primary Production Variables for a Shelf Station off A Coruña (NW Spain): 1989–2016; PANGAEA: Bremerhaven, Germany, 2018. [Google Scholar] [CrossRef]
- Moreira-Coello, V.; Mouriño-Carballido, B.; Marañón, E.; Fernández-Carrera, A.; Chouciño, P.; Varela, M.M.; Bode, A. Biological N2 fixation in the upwelling region off NW Iberia: Magnitude, relevance and abundance of diazotrophs. Front. Mar. Sci. 2017, 4, 303. [Google Scholar] [CrossRef]
- Casas, B.; Varela, M.; Bode, A. Seasonal succession of phytoplankton species on the coast of A Coruña (Galicia, Northwest Spain). Bol. Inst. Esp. Oceanogr. 1999, 15, 413–429. [Google Scholar]
- Walker, B.D.; McCarthy, M.D. Elemental and isotopic characterization of dissolved and particulate organic matter in a unique California upwelling system: Importance of size and composition in the export of labile material. Limnol. Oceanogr. 2012, 57, 1757–1774. [Google Scholar] [CrossRef]
- Saino, T. 15N and 13C natural abundance in suspended particulate organic matter from a Kuroshio warm-core ring. Deep-Sea Res. 1992, 39 (Suppl. 1), 5347–5362. [Google Scholar] [CrossRef]
- Jennings, S.; Maxwell, T.A.D.; Schratzberger, M.; Milligan, S.P. Body-size dependent temporal variations in nitrogen stable isotope ratios in food webs. Mar. Ecol. Prog. Ser. 2008, 370, 199–206. [Google Scholar] [CrossRef]
- Bode, A.; Alvarez-Ossorio, M.T. Taxonomic versus trophic structure of mesozooplankton: A seasonal study of species succession and stable carbon and nitrogen isotopes in a coastal upwelling ecosystem. ICES J. Mar. Sci. 2004, 61, 563–571. [Google Scholar] [CrossRef]
- Sweeting, C.J.; Barry, J.; Barnes, C.; Polunin, N.V.C.; Jennings, S. Effects of body size and environment on diet-tissue δ15N fractionation in fishes. J. Exp. Mar. Biol. Ecol. 2007, 340, 1–10. [Google Scholar] [CrossRef]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (δ15N and δ13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.; Décima, M.; Popp, B.N.; Landry, M.R. Isotopic invisibility of protozoan trophic steps in marine food webs. Limnol. Oceanogr. 2014, 59, 1590–1598. [Google Scholar] [CrossRef]
- Bode, A.; Barquero, S.; González, N.; Alvarez-Ossorio, M.T.; Varela, M. Contribution of heterotrophic plankton to nitrogen regeneration in the upwelling ecosystem of A Coruña (NW Spain). J. Plankton Res. 2004, 26, 1–18. [Google Scholar] [CrossRef]
- Hill, J.M.; McQuaid, C.D. δ13C and δ15N biogeographic trends in rocky intertidal communities along the coast of South Africa: Evidence of strong environmental signatures. Estuar. Coast. Shelf Sci. 2008, 80, 261–268. [Google Scholar] [CrossRef]
- Reddin, C.J.; Docmac, F.; O’Connor, N.E.; Bothwell, J.H.; Harrod, C. Coastal upwelling drives intertidal assemblage structure and trophic ecology. PLoS ONE 2015, 10, e0130789. [Google Scholar] [CrossRef]
- Vokhshoori, N.L.; McCarthy, M.D. Compound-specific δ15N amino acid measurements in littoral mussels in the california upwelling ecosystem: A new approach to generating baseline delta n-15 isoscapes for coastal ecosystems. PLoS ONE 2014, 9, e98087. [Google Scholar] [CrossRef]
- Rau, G.H.; Ohman, M.D.; Pierrot-Bults, A. Linking nitrogen dynamics to climate variability off central California: A 51 year based on 15N/14N in CALCOFI zooplankton. Deep Sea Res. II 2003, 50, 2431–2447. [Google Scholar] [CrossRef]
- Varela, M. Upwelling and phytoplankton ecology in Galician (NW Spain) rías and shelf waters. Bol. Inst. Esp. Oceanogr. 1992, 8, 57–74. [Google Scholar]
- Otero, J.; Bode, A.; Álvarez-Salgado, X.A.; Varela, M. Role of functional traits variability in the response of individual phytoplankton species to changing environmental conditions in a coastal upwelling zone. Mar. Ecol. Prog. Ser. 2018, 596, 33–47. [Google Scholar] [CrossRef]
- Roland, L.A.; McCarthy, M.D.; Guilderson, T. Sources of molecularly uncharacterized organic carbon in sinking particles from three ocean basins: A coupled δ14C and δ13C approach. Mar. Chem. 2008, 111, 199–213. [Google Scholar] [CrossRef]
- Bowen, G. Isoscapes: Spatial pattern in isotopic biogeochemistry. Annu. Rev. Earth Planet. Sci. 2010, 38, 161–187. [Google Scholar] [CrossRef]
- Bode, A.; Alvarez-Ossorio, M.T.; Carrera, P.; Lorenzo, J. Reconstruction of trophic pathways between plankton and the North Iberian sardine (Sardina pilchardus) using stable isotopes. Sci. Mar. 2004, 68, 165–178. [Google Scholar] [CrossRef]
- Méndez-Fernandez, P.; Bustamante, P.; Bode, A.; Chouvelon, T.; Ferreira, M.; López, A.; Pierce, G.J.; Santos, M.B.; Spitz, J.; Vingada, J.V.; et al. Foraging ecology of five toothed whale species in the northwest Iberian Peninsula, inferred using carbon and nitrogen isotope ratios. J. Exp. Mar. Biol. Ecol. 2012, 413, 150–158. [Google Scholar] [CrossRef]
| δ15N | δ13C | C:N | ||||
|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | |
| seston | 5.12 | 1.43 | −22.18 | 2.08 | 7.46 | 1.31 |
| sediment trap particles | 5.50 | 0.73 | −18.14 | 1.91 | 10.38 | 1.65 |
| plankton 200–500 µm | 6.38 | 0.70 | −17.55 | 0.38 | 4.92 | 0.46 |
| plankton 500–1000 µm | 6.61 | 0.58 | −17.42 | 0.29 | 4.96 | 0.37 |
| plankton 1000–2000 µm | 7.04 | 0.73 | −17.38 | 0.27 | 4.71 | 0.31 |
| plankton >2000 µm | 6.85 | 0.92 | −17.22 | 0.23 | 4.97 | 0.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bode, A.; Lamas, A.F.; Mompeán, C. Effects of Upwelling Intensity on Nitrogen and Carbon Fluxes through the Planktonic Food Web off A Coruña (Galicia, NW Spain) Assessed with Stable Isotopes. Diversity 2020, 12, 121. https://doi.org/10.3390/d12040121
Bode A, Lamas AF, Mompeán C. Effects of Upwelling Intensity on Nitrogen and Carbon Fluxes through the Planktonic Food Web off A Coruña (Galicia, NW Spain) Assessed with Stable Isotopes. Diversity. 2020; 12(4):121. https://doi.org/10.3390/d12040121
Chicago/Turabian StyleBode, Antonio, Angel F. Lamas, and Carmen Mompeán. 2020. "Effects of Upwelling Intensity on Nitrogen and Carbon Fluxes through the Planktonic Food Web off A Coruña (Galicia, NW Spain) Assessed with Stable Isotopes" Diversity 12, no. 4: 121. https://doi.org/10.3390/d12040121
APA StyleBode, A., Lamas, A. F., & Mompeán, C. (2020). Effects of Upwelling Intensity on Nitrogen and Carbon Fluxes through the Planktonic Food Web off A Coruña (Galicia, NW Spain) Assessed with Stable Isotopes. Diversity, 12(4), 121. https://doi.org/10.3390/d12040121






