The Role of Climate and Topography in Shaping the Diversity of Plant Communities in Cabo Verde Islands
Abstract
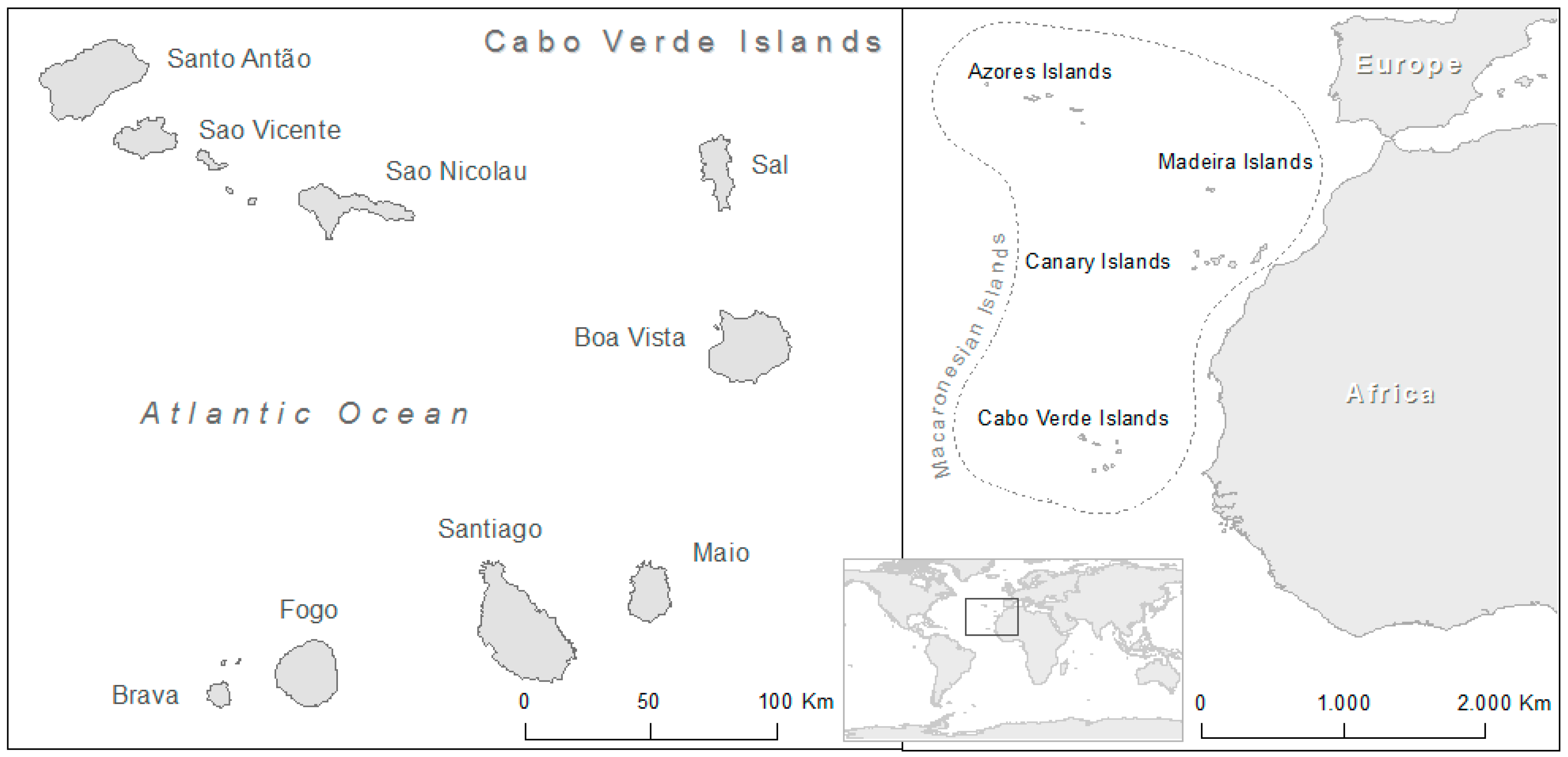
1. Introduction
2. Cabo Verde Islands: Origin and Climate
3. Plant Communities of Cabo Verde
3.1. Ficus and Sideroxylon Woodlands
3.2. Acacia Savannas
3.3. Other Arborescent Communities
3.4. Shrub Vegetation
3.5. Grasslands
3.6. Halophytic and Hydrophytic Communities
3.7. Chasmophytic Communities
4. Aridification of Northern Africa and Its Role in Shaping the Vegetation of Cabo Verde
5. The Lack of Cloud Forests Dominated by Lauroid Taxa
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armitage, S.J.; Bristow, C.S.; Drake, N.A. West African monsoon dynamics inferred from abrupt fluctuations of Lake Mega-Chad. Proc. Natl. Acad. Sci. USA 2015, 112, 8543–8548. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.M.; Rohling, E.J.; Westerhold, T.; Zabel, M.; Heslop, D.; Konijnendijk, T.; Lourens, L. A 3 million year index for North African humidity/aridity and the implication of potential pan-African Humid periods. Quat. Sci. Rev. 2017, 171, 100–118. [Google Scholar] [CrossRef]
- Skonieczny, C.; Paillou, P.; Bory, A.; Bayon, G.; Biscara, L.; Crosta, X.; Eynaud, F.; Malaizé, B.; Revel, M.; Aleman, N.; et al. African humid periods triggered the reactivation of a large river system in Western Sahara. Nat. Commun. 2015, 6, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Z.; Stuut, J.B.W.; Zhao, Y.; Schirone, A.; de Lange, G.J. North-African paleodrainage discharges to the central Mediterranean during the last 18,000 years: A multiproxy characterization. Quat. Sci. Rev. 2017, 163, 95–113. [Google Scholar] [CrossRef]
- Rognon, P.; Coudé-Gaussen, G. Paleoclimates Off Northwest Africa (28°–35°N) about 18,000 yr B.P. Based on Continental Eolian Deposits. Quat. Res. 1996, 46, 118–126. [Google Scholar] [CrossRef]
- Kröpelin, S.; Verschuren, D.; Lézine, A.-M.; Eggermont, H.; Cocquyt, C.; Francus, P.; Cazet, J.-P.; Fagot, M.; Rumes, B.; Russell, J.M.; et al. Climate-Driven Ecosystem Succession in the Sahara: The Past 6000 Years. Science 2008, 320, 765–768. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; De Nascimento, L.; Otto, R.; Delgado, J.D.; García-Del-Rey, E.; Arévalo, J.R.; Whittaker, R.J. A reconstruction of Palaeo-Macaronesia, with particular reference to the long-term biogeography of the Atlantic island laurel forests. J. Biogeogr. 2011, 38, 226–246. [Google Scholar] [CrossRef]
- Carrión, J.S.; Fernández, S.; Jiménez-Moreno, G.; Fauquette, S.; Gil-Romera, G.; González-Sampériz, P.; Finlayson, C. The historical origins of aridity and vegetation degradation in southeastern Spain. J. Arid. Environ. 2010, 74, 731–736. [Google Scholar] [CrossRef]
- Patino, J.; Whittaker, R.J.; Borges, P.A.; Fernández-Palacios, J.M.; Ah-Peng, C.; Araújo, M.B.; Ávila, S.P.; Cardoso, P.; Cornuault, J.; de Boer, E.J.; et al. A roadmap for island biology: 50 fundamental questions after 50 years of The Theory of Island Biogeography. J. Biogeogr. 2017, 44, 963–983. [Google Scholar] [CrossRef]
- Carine, M.A. Spatio-Temporal Relationships of the Macaronesian Endemic Flora: A Relictual Series or Window of Opportunity? Taxon 2005, 54, 895–903. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Triantis, K.A.; Ladle, R.J. A general dynamic theory of oceanic island biogeography. J. Biogeogr. 2008, 35, 977–994. [Google Scholar] [CrossRef]
- Kim, S.-C.; McGowen, M.R.; Lubinsky, P.; Barber, J.C.; Mort, M.E.; Santos-Guerra, A. Timing and Tempo of Early and Successive Adaptive Radiations in Macaronesia. PLoS ONE 2008, 3, e2139. [Google Scholar] [CrossRef]
- Bramwell, D. Endemism in the flora of the Canary Islands. In Taxonomy, Phytogeography and Evolution; Valentine, D.H., Ed.; Academic Press: Cambridge, MA, USA, 1972. [Google Scholar]
- Böhle, U.-R.; Hilger, H.H.; Martin, W.F. Island colonization and evolution of the insular woody habit in Echium, L. (Boraginaceae). Proc. Natl. Acad. Sci. USA 1996, 93, 11740–11745. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-C.; Crawford, D.J.; Francisco-Ortega, J.; Santos-Guerra, A. A common origin for woody Sonchus and five related genera in the Macaronesian Islands: Molecular evidence for extensive radiation. Proc. Natl. Acad. Sci. USA 1996, 93, 7743–7748. [Google Scholar] [CrossRef]
- Lens, F.; Davin, N.; Smets, E.; del Arco, M. Insular woodiness on the Canary Islands: A remarkable case of convergent evolution. Int. J. Plant. Sci. 2013, 174, 992–1013. [Google Scholar] [CrossRef]
- Pokorny, L.; Riina, R.; Mairal, M.; Meseguer, A.S.; Culshaw, V.; Cendoya, J.; Sanmartín, I. Living on the edge: Timing of Rand Flora disjunctions congruent with ongoing aridification in Africa. Front. Genet. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Holmes, J.A. How the Sahara became dry. Science 2008, 320, 752–753. [Google Scholar] [CrossRef]
- Plesner, S.; Holm, P.M.; Wilson, J.R. 40-39 Ar geochronology of Santo Antão, Cape Verde Islands. J. Volcanol. Geoth. Res. 2003, 120, 103–121. [Google Scholar] [CrossRef]
- Victória, S. Caracterização geológica e geotécnica das unidades litológicas da Cidade da Praia, ilha de Santiago, Cabo Verde. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, 2013. Available online: http://www.portaldoconhecimento.gov.cv/handle/10961/2471 (accessed on 17 February 2020).
- Correia, E. Contribuições para o conhecimento do clima de Cabo Verde. Garcia de Orta Sér. Geogr. 1996, 15, 81–107. [Google Scholar]
- Soares, E. Variabilidade climática na região de Cabo Verde. Master’s Thesis, University of Évora, Évora, Portugal, 2004. Available online: http://www.icterra.pt/ (accessed on 17 February 2020).
- Furtado, F.J.R. A Captação de Água no Nevoeiro no Parque Natural de Serra Malagueta. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 2009. Available online: http://hdl.handle.net/10773/629 (accessed on 17 February 2020).
- Ritter, A.; Regalado, C.M.; Aschan, G. Fog reduces transpiration in tree species of the Canarian relict heath-laurel cloud forest (Garajonay National Park, Spain). Tree Physiol. 2009, 29, 517–528. [Google Scholar] [CrossRef]
- Prada, S.; de Sequeira, M.M.; Figueira, C.; Vasconcelos, R. Cloud water interception in the high altitude tree heath forest (Erica arborea L.) of Paul da Serra Massif (Madeira, Portugal). Hydrol. Process. 2012, 26, 202–212. [Google Scholar] [CrossRef]
- Acosta Baladón, A.; Gioda, A. L’importance des précipitations occultes sous les tropiques secs. Sécheresse 1991, 2, 132–134. [Google Scholar]
- Cunha, F.R. Problema da Captação da Água de Nevoeiro em Cabo Verde. Garcia de Orta 1964, 12, 719–754. [Google Scholar]
- Rivas-Martínez, S.; Lousã, M.; Costa, J.C.; Duarte, M.C. Geobotanical survey of Cabo Verde Islands (West Africa). Int. J. Geobot. Res. 2017, 7, 1–103. [Google Scholar]
- Duarte, M.C.; Rego, F.; Moreira, I. Distribution patterns of plant communities on Santiago Island, Cape Verde. J. Veg. Sci. 2005, 16, 283–292. [Google Scholar] [CrossRef]
- Díaz-Pérez, A.; Sequeira, M.; Santos-Guerra, A.; Catalán, P. Multiple colonizations, in situ speciation, and volcanism-associated stepping-stone dispersals shaped the phylogeography of the Macaronesian red fescues (Festuca, L., Gramineae). Syst. Biol. 2008, 57, 732–749. [Google Scholar] [CrossRef]
- Duarte, M.C.; Moreira, I. A vegetação de Santiago (Cabo Verde). Apontamento histórico. Garcia de Orta Sér. Bot. 2002, 16, 51–80. [Google Scholar]
- Caujapé-Castells, J.; Tye, A.; Crawford, D.J.; Santos-Guerra, A.; Sakai, A.; Beaver, K.; Lobin, W.; Vincent Florens, F.B.; Moura, M.; Jardim, R.; et al. Conservation of oceanic island floras: Present and future global challenges. Perspect. Plant Ecol. 2010, 12, 107–129. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Duarte, M.C.; Santos-Guerra, A.; Carine, M.; Francisco-Ortega, J. Botanical exploration of the Cape Verde Islands: From the pre-Linnaean records and collections to late 18th century floristic accounts and expeditions. Taxon 2014, 63, 625–640. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Catarino, S.; Gomes, I.; Fernandes, C.; Costa, J.C.; Caujapé-Castells, J.; Duarte, M.C. IUCN Red List assessment of the Cape Verde endemic flora: Towards a Global Strategy for Plant Conservation in Macaronesia. Bot. J. Linn. Soc. 2016, 180, 413–425. [Google Scholar] [CrossRef]
- Rico, L.; Duarte, M.C.; Romeiras, M.M.; Santos-Guerra, A.; Nepi, C.; Francisco-Ortega, J.; Joseph, D. Hooker’s 1839 Cabo Verde Collections. Curtis’s Bot. Mag. 2017, 34, 146–168. [Google Scholar] [CrossRef]
- Brochmann, C.; Rustan, Ø.H.; Lobin, W.; Kilian, N. The endemic vascular plants of the Cape Verde Islands, W Africa. Sommerfeltia 1997, 24, 1–356. [Google Scholar]
- POWO. Plants of the World Online. Royal Botanic Gardens, Kew. Available online: www.plantsoftheworldonline.org (accessed on 8 January 2020).
- Cubas, J.; Martín-Esquivel, J.L.; Nogales, M.; Irl, S.D.; Hernández-Hernández, R.; López-Darias, M.; González-Mancebo, J.M. Contrasting effects of invasive rabbits on endemic plants driving vegetation change in a subtropical alpine insular environment. Biol. Invasions 2018, 20, 793–807. [Google Scholar] [CrossRef]
- Irl, S.D.; Steinbauer, M.J.; Messinger, J.; Blume-Werry, G.; Palomares-Martínez, Á.; Beierkuhnlein, C.; Jentsch, A. Burned and devoured-introduced herbivores, fire, and the endemic flora of the high-elevation ecosystem on La Palma, Canary Islands. Arct. Antarct. Alp. Res. 2018, 46, 859–869. [Google Scholar] [CrossRef]
- Burrows, J.; Burrows, S. Figs of Southern and South.-Central Africa; Umdaus Press: Hatfield, South Africa, 2003. [Google Scholar]
- Esler, K.J.; Milton, S.; Dean, W.R.J. Karoo Veld: Ecology and Management; Briza Publications: Pretoria, South Africa, 2006; p. 214. [Google Scholar]
- Andrus, N.; Trusty, J.; Santos-Guerra, A.; Jansen, R.K.; Francisco-Ortega, J. Using molecular phylogenies to test phytogeographical links between East/South Africa–Southern Arabia and the Macaronesian islands—A review, and the case of Vierea and Pulicaria section Vieraeopsis (Asteraceae). Taxon 2004, 53, 333–346. [Google Scholar] [CrossRef]
- Sanmartin, I.; Anderson, C.L.; Alarcon, M.; Ronquist, F.; Aldasoro, J.J. Bayesian island biogeography in a continental setting: The Rand Flora case. Biol. Lett. 2010, 6, 703–707. [Google Scholar] [CrossRef]
- Mairal, M.; Sanmartín, I.; Herrero, A.; Pokorny, L.; Vargas, P.; Aldasoro, J.; Alarcón, M. Geographic barriers and Pleistocene climate change shaped patterns of genetic variation in the Eastern Afromontane biodiversity hotspot. Sci. Rep. 2017, 7, 45749. [Google Scholar] [CrossRef]
- Wood, P.J. The Botany and Distribution of Faidherbia albida. In Faidherbia albida in the West African Semi-Arid Tropics; Vandenbeldt, R.J., Ed.; International Centre for Research in Agroforestry: Niamey, Niger, 1992. [Google Scholar]
- Joly, H. The Genetics of Acacia albida (syn. Faidherbia albida). In Faidherbia albida in the West African Semi-Arid Tropics; Vandenbeldt, R.J., Ed.; International Centre for Research in Agroforestry: Niamey, Niger, 1992.albida). In Faidherbia albida in the West African Semi-Arid Tropics; Vandenbeldt, R.J., Ed.; International Centre for Research in Agroforestry: Niamey, Niger, 1992; Vandenbeldt, R.J., Ed. [Google Scholar]
- Roupsard, O.; Ferhi, A.; Granier, A.; Pallo, F.; Depommier, D.; Mallet, B.; Joly, H.I.; Dreyer, E. Reverse phenology and dry season water uptake by Fadherbia albida (Del.) A. Chev. in an agroforestry parkland of Sudanense Africa. Funct. Ecol. 1999, 13, 460–472. [Google Scholar] [CrossRef]
- Kirmse, R.; Norton, B. The potential of Acacia albida for desertification control and increased productivity in Chad. Biol. Conserv. 1984, 29, 121–141. [Google Scholar] [CrossRef]
- Chevalier, A. Les Iles du Cap Vert. Géographie, biogéographie, agriculture. Flore de l’archipel. Rev. Bot. Appl. Agric. Trop. 1935, 15, 733–1090. [Google Scholar] [CrossRef]
- Schulz, E.; Abichou, A.; Adamou, A.; Ousseïni, I.; Ballouche, A. The desert in the Sahara. Transitions and boundaries. In Palaeoecology of Africa and the Surrounding Islands; Baumhauer, R., Runge, J., Eds.; Taylor & Francis Group: London, UK, 2009; Volume 29, pp. 63–89. [Google Scholar]
- Steinbauer, M.J.; Field, R.; Grytnes, J.A.; Trigas, P.; Ah-Peng, C.; Attorre, F.; Beierkuhnlein, C. Topography-driven isolation, speciation and a global increase of endemism with elevation. Glob. Ecol. Biogeogr. 2016, 25, 1097–1107. [Google Scholar] [CrossRef]
- Irl, S.D.H.; Schweiger, A.H.; Medina, F.M.; Fernández-Palacios, J.M.; Harter, D.E.; Jentsch, A.; Beierkuhnlein, C. An island view of endemic rarity—Environmental drivers and consequences for nature conservation. Divers. Distrib. 2017, 23, 1132–1142. [Google Scholar] [CrossRef]
- Otto, R.; Whittaker, R.J.; von Gaisberg, M.; Stierstorfer, C.; Naranjo-Cigala, A.; Steinbauer, M.J.; Fernández-Palacios, J.M. Transferring and implementing the general dynamic model of oceanic island biogeography at the scale of island fragments: The roles of geological age and topography in plant diversification in the Canaries. J. Biogeogr. 2016, 43, 911–922. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Monteiro, F.; Duarte, M.C.; Schaefer, H.; Carine, M. Patterns of genetic diversity in three plant lineages endemic to the Cape Verde Islands. AoB PLANTS 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Senut, B.; Pickford, M.; Ségalen, L. Neogene desertification of Africa. C. R. Geosci. 2009, 341, 591–602. [Google Scholar] [CrossRef]
- Adkins, J.; DeMenocal, P.; Eshel, G. The “African humid period” and the record of marine upwelling from excess 230Th in Ocean Drilling Program Hole 658C. Paleoceanography 2006, 21, 1–14. [Google Scholar] [CrossRef]
- Pausata, F.S.R.; Messori, G.; Zhang, Q. Impacts of dust reduction on the northward expansion of the African monsoon during the Green Sahara period. Earth Planet. Sci. Lett. 2016, 434, 298–307. [Google Scholar] [CrossRef]
- Larrasoaña, J.C.; Roberts, A.P.; Rohling, E.J. Dynamics of Green Sahara Periods and Their Role in Hominin Evolution. PLoS ONE 2013, 8, e76514. [Google Scholar] [CrossRef]
- Jung, S.J.A.; Davies, G.R.; Ganssen, G.M.; Kroon, D. Stepwise Holocene aridification in NE Africa deduced from dust-borne radiogenic isotope records. Earth Planet. Sci. Lett. 2004, 221, 27–37. [Google Scholar] [CrossRef]
- Kuper, R.; Kröpelin, S. Climate-Controlled Holocene Occupation in the Sahara: Motor of Africa’s Evolution. Science 2006, 313, 803–807. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Gallimore, R.; Gasse, F.; Johnson, T.; DeMenocal, P.; Adkins, J.; Notaro, M.; Prentice, I.C.; Kutzbach, J.; et al. Simulating the transient evolution and abrupt change of Northern Africa atmosphere-ocean-terrestrial ecosystem in the Holocene. Quat. Sci. Rev. 2007, 26, 1818–1837. [Google Scholar] [CrossRef]
- Wright, D.K. Humans as Agents in the Termination of the African Humid Period. Front. Earth Sci. 2017, 5, 4. [Google Scholar] [CrossRef]
- Mairal, M.; Pokorny, L.; Aldasoro, J.J.; Alarcõn, M.; Sanmartín, I. Ancient vicariance and climate-driven extinction continental-wide disjunctions in Africa: The case of the Rand Flora genus Canarina (Campanulaceae). Mol. Ecol. 2015, 24, 1335–1354. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pérez, M.L.; Vargas, P.; Fernández-Mazuecos, M.; López, J.; Valtueña, F.J.; Ortega-Olivencia, A. Multiple windows of colonization to Macaronesia by the dispersal-unspecialized Scrophularia since the Late Miocene. Perspect. Plant. Ecol. 2015, 17, 263–273. [Google Scholar] [CrossRef]
- Dupont, L.M.; Jahns, S.; Marret, F.; Ning, S. Vegetation change in equatorial West Africa: Time-slices for the last 150 ka. Palaeogeogr. Palaeocl. 2000, 155, 95–122. [Google Scholar] [CrossRef]
- Dalibard, M.; Popescu, S.M.; Maley, J.; Baudin, F.; Melinte-Dobrinescu, M.C.; Pittet, B.; Marsset, T.; Bernard, D.; Laurence, D.; Suc, J.P. High-resolution vegetation history of West Africa during the last 145 ka. Geobios 2014, 47, 183–198. [Google Scholar] [CrossRef][Green Version]
- Zazo, C.; Goy, J.L.; Dabrio, C.J.; Soler, V.; Hillaire-Marcel, C.; Ghaleb, B.; González-Delgado, J.A.; Bardají, T.; Cabrero, A. Quaternary marine terraces on Sal Island (Cape Verde archipelago). Quat. Sci. Rev. 2007, 26, 876–893. [Google Scholar] [CrossRef]
- Zazo, C.; Goy, J.L.; Dabrio, C.J.; Cabero, A.; Bardaji, T.; Ghaleb, B.; Soler, V. Sea level changes during the last and present interglacials in Sal Island (Cape Verde archipelago). Glob. Planet. Chang. 2010, 72, 302–317. [Google Scholar] [CrossRef]
- Hooghiemstra, H.; Lézine, A.-M.; Leroy, S.A.G.; Dupont, L.; Marret, F. Late Quaternary palynology in marine sediments: A synthesis of the understanding of pollen distribution patterns in the NW African setting. Quat. Int. 2006, 148, 29–44. [Google Scholar] [CrossRef]
- Dupont, L. Orbital scale vegetation change in Africa. Quat. Sci. Rev. 2011, 30, 3589–3602. [Google Scholar] [CrossRef]
- Figueiredo, A. Assessing Climate Change Impacts on the Distribution of Flora and Vegetation at Madeira Island. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 2013. [Google Scholar]
- Barres, L.; Galbany-Casals, M.; Hipp, A.; Molero, J.; Vilatersana, R. Phylogeography and character evolution of Euphorbia sect. Aphyllis subsect. Macaronesicae (Euphorbiaceae). Taxon 2017, 2, 324–342. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Paulo, O.S.; Duarte, M.C.; Pina-Martins, F.; Cotrim, M.H.; Carine, M.A.; Pais, M.S. Origin and diversification of genus Echium (Boraginaceae) in Cape Verde islands: A phylogenetic study based on ITS (rDNA) and cpDNA sequences. Taxon 2011, 60, 1375–1385. [Google Scholar] [CrossRef]
- Alarcón, M.; Roquet, C.; García-Fernández, A.; Vargas, P.; Aldasoro, J.J. Phylogenetic and phylogeographic evidence for a Pleistocene disjunction between Campanula jacobaea (Cape Verde Islands) and C. balfourii (Socotra). Mol. Phylogenet. Evol. 2013, 69, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Menezes, T.; Romeiras, M.M.; de Sequeira, M.M.; Moura, M. Phylogenetic relationships and phylogeography of relevant lineages within the complex Campanulaceae family in Macaronesia. Ecol. Evol. 2017, 1–21. [Google Scholar] [CrossRef]
- Migliore, J.; Baumel, A.; Juin, M.; Fady, B.; Roig, A.; Duong, N.; Médail, F. Surviving in Mountain Climate Refugia: New Insights from the Genetic Diversity and Structure of the Relict Shrub Myrtus nivellei (Myrtaceae) in the Sahara Desert. PLoS ONE 2013, 8, e73795. [Google Scholar] [CrossRef][Green Version]
- Weigelt, P.; Steinbauer, M.J.; Cabral, J.S.; Kreft, H. Late Quaternary climate change shapes island biodiversity. Nature 2016, 532, 99. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Pena, A.R.; Menezes, T.; Vasconcelos, R.; Monteiro, F.; Paulo, O.S.; Moura, M. Shortcomings of Phylogenetic Studies on Recent Radiated Insular Groups: A Meta-Analysis Using Cabo Verde Biodiversity. Int. J. Mol. Sci. 2019, 20, 2782. [Google Scholar] [CrossRef]
- Harter, D.E.; Irl, S.D.; Seo, B.; Steinbauer, M.J.; Gillespie, R.; Triantis, K.A.; Beierkuhnlein, C. Impacts of global climate change on the floras of oceanic islands–Projections, implications and current knowledge. Perspect. Plant. Ecol. Evol. Syst. 2015, 17, 160–183. [Google Scholar] [CrossRef]
- Patiño, J.; Mateo, R.G.; Zanatta, F.; Marquet, A.; Aranda, S.C.; Borges, P.A.; Muñoz, J. Climate threat on the Macaronesian endemic bryophyte flora. Sci. Rep. 2016, 6, 29156. [Google Scholar] [CrossRef]
- Monteiro, F.; Fortes, A.; Ferreira, V.; Pereira Essoh, A.; Gomes, I.; Correia, A.M.; Romeiras, M.M. Current Status and Trends in Cabo Verde Agriculture. Agronomy 2020, 10, 74. [Google Scholar] [CrossRef]
- Caujapé-Castells, J.; García-Verdugo, C.; Marrero-Rodríguez, Á.; Fernández-Palacios, J.M.; Crawford, D.J.; Mort, M.E. Island ontogenies, syngameons, and the origins and evolution of genetic diversity in the Canarian endemic flora. Perspect. Plant. Ecol. 2017, 27, 9–22. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Arévalo, J.R.; Balguerías, E.; Barone, R.; Nascimento, L.; de Elias, R.B.; Delgado, J.D.; Fernández-Lugo, S.; Méndez, J.; Naranjo Cigala, A.; et al. La Laurisilva. Canarias, Madeira y Azores; Macaronesia Editorial: Santa Cruz de Tenerife, Spain, 2017. [Google Scholar]
- Castilla-Beltrán, A.; Duarte, I.; de Nascimento, L.; Fernández-Palacios, J.M.; Romeiras, M.; Whittaker, R.J.; Jambrina-Enríquezf, M.; Mallolf, C.; Cundyg, A.B.; Edwardsa, M.; et al. Using multiple palaeoecological indicators to guide biodiversity conservation in tropical dry islands: The case of São Nicolau, Cabo Verde. Biol. Conserv. 2020, 242, 108397. [Google Scholar] [CrossRef]
- Freitas, R.; Romeiras, M.; Silva, L.; Cordeiro, R.; Madeira, P.; González, J.A.; Wirtz, P.; Falcón, J.M.; Brito, A.; Floeter, S.R.; et al. Restructuring of the ‘Macaronesia’ biogeographic unit: A marine multi-taxon biogeographical approach. Sci. Rep. 2019, 9, 15792. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, C.; Costa, J.C.; Figueiredo, A.; Capelo, J.; Gomes, I.; Vitória, S.; Semedo, J.M.; Lopes, A.; Dinis, H.; Correia, E.; et al. The Role of Climate and Topography in Shaping the Diversity of Plant Communities in Cabo Verde Islands. Diversity 2020, 12, 80. https://doi.org/10.3390/d12020080
Neto C, Costa JC, Figueiredo A, Capelo J, Gomes I, Vitória S, Semedo JM, Lopes A, Dinis H, Correia E, et al. The Role of Climate and Topography in Shaping the Diversity of Plant Communities in Cabo Verde Islands. Diversity. 2020; 12(2):80. https://doi.org/10.3390/d12020080
Chicago/Turabian StyleNeto, Carlos, José Carlos Costa, Albano Figueiredo, Jorge Capelo, Isildo Gomes, Sónia Vitória, José Maria Semedo, António Lopes, Herculano Dinis, Ezequiel Correia, and et al. 2020. "The Role of Climate and Topography in Shaping the Diversity of Plant Communities in Cabo Verde Islands" Diversity 12, no. 2: 80. https://doi.org/10.3390/d12020080
APA StyleNeto, C., Costa, J. C., Figueiredo, A., Capelo, J., Gomes, I., Vitória, S., Semedo, J. M., Lopes, A., Dinis, H., Correia, E., Duarte, M. C., & Romeiras, M. M. (2020). The Role of Climate and Topography in Shaping the Diversity of Plant Communities in Cabo Verde Islands. Diversity, 12(2), 80. https://doi.org/10.3390/d12020080








