A New Species of Paraturbanella Remane, 1927 (Gastrotricha, Macrodasyida) from the Brazilian Coast, and the Molecular Phylogeny of Turbanellidae Remane, 1926
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling and Sample Processing
2.2. Microscopical Study—Differential Interference Contrast (DIC)
2.3. Scanning Electron Microscopy (SEM)
2.4. Granulometric Analysis
2.5. DNA Extraction, Amplification and Sequences
2.6. Phylogenetic Analysis
3. Results
3.1. Taxonomic Account
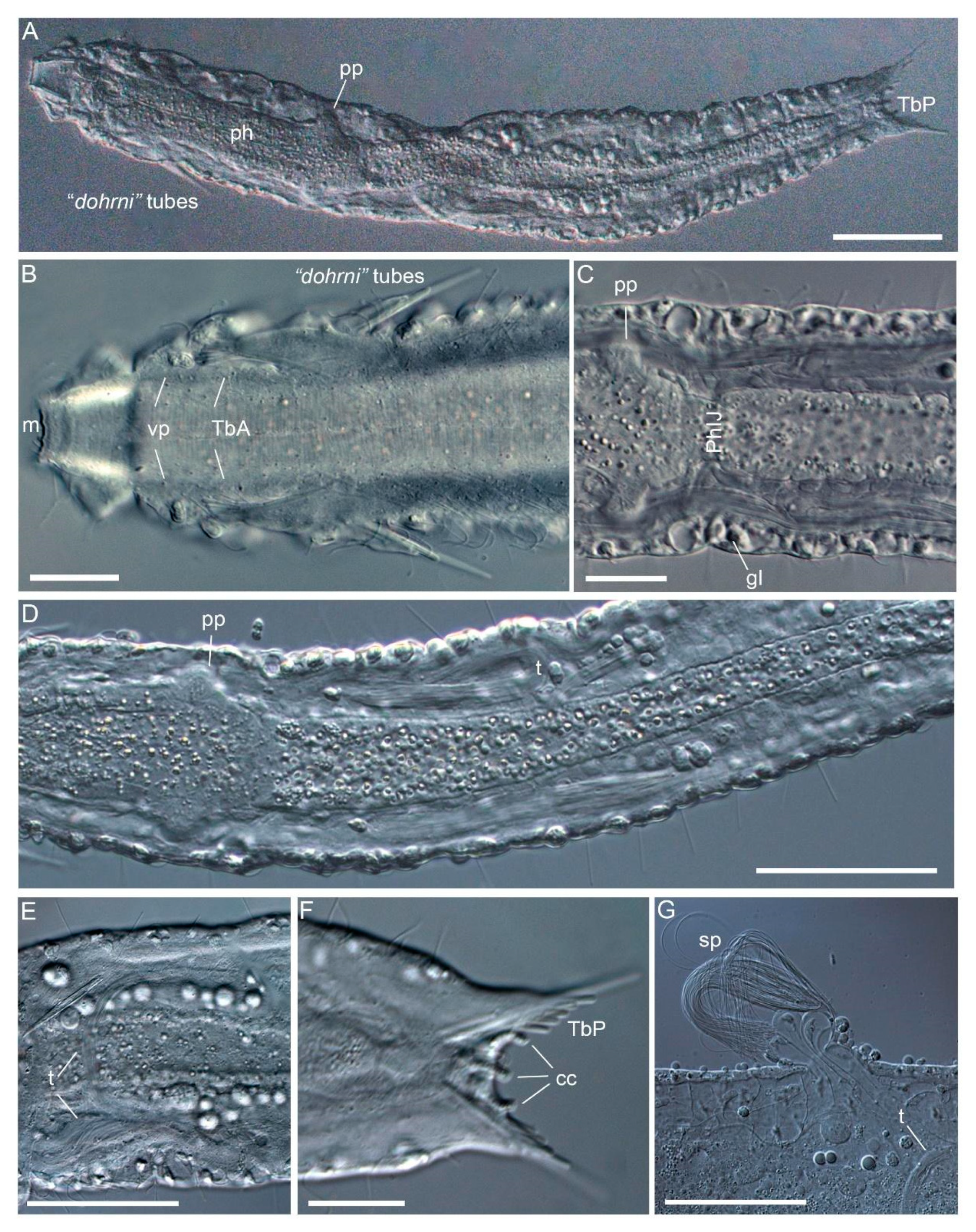
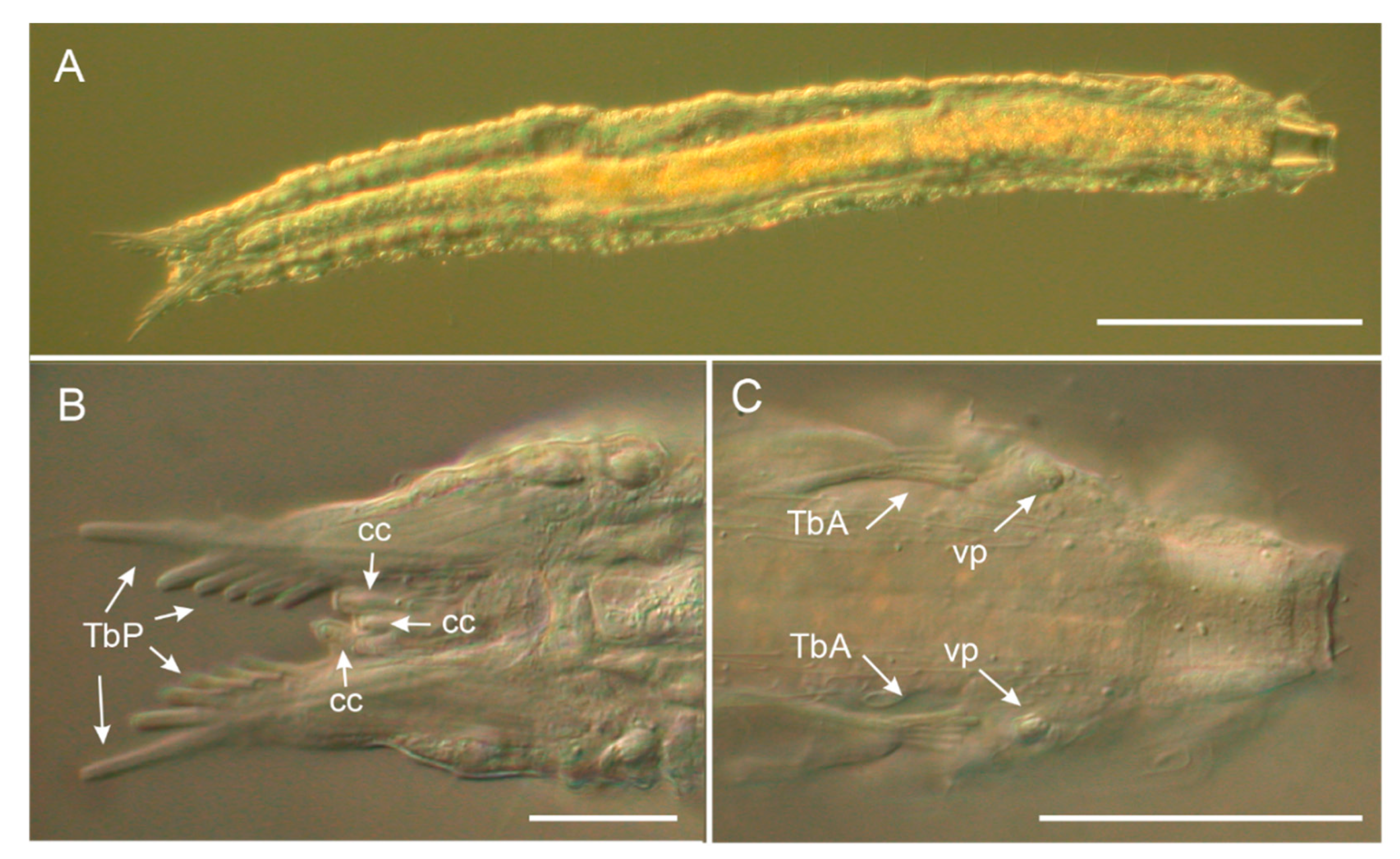
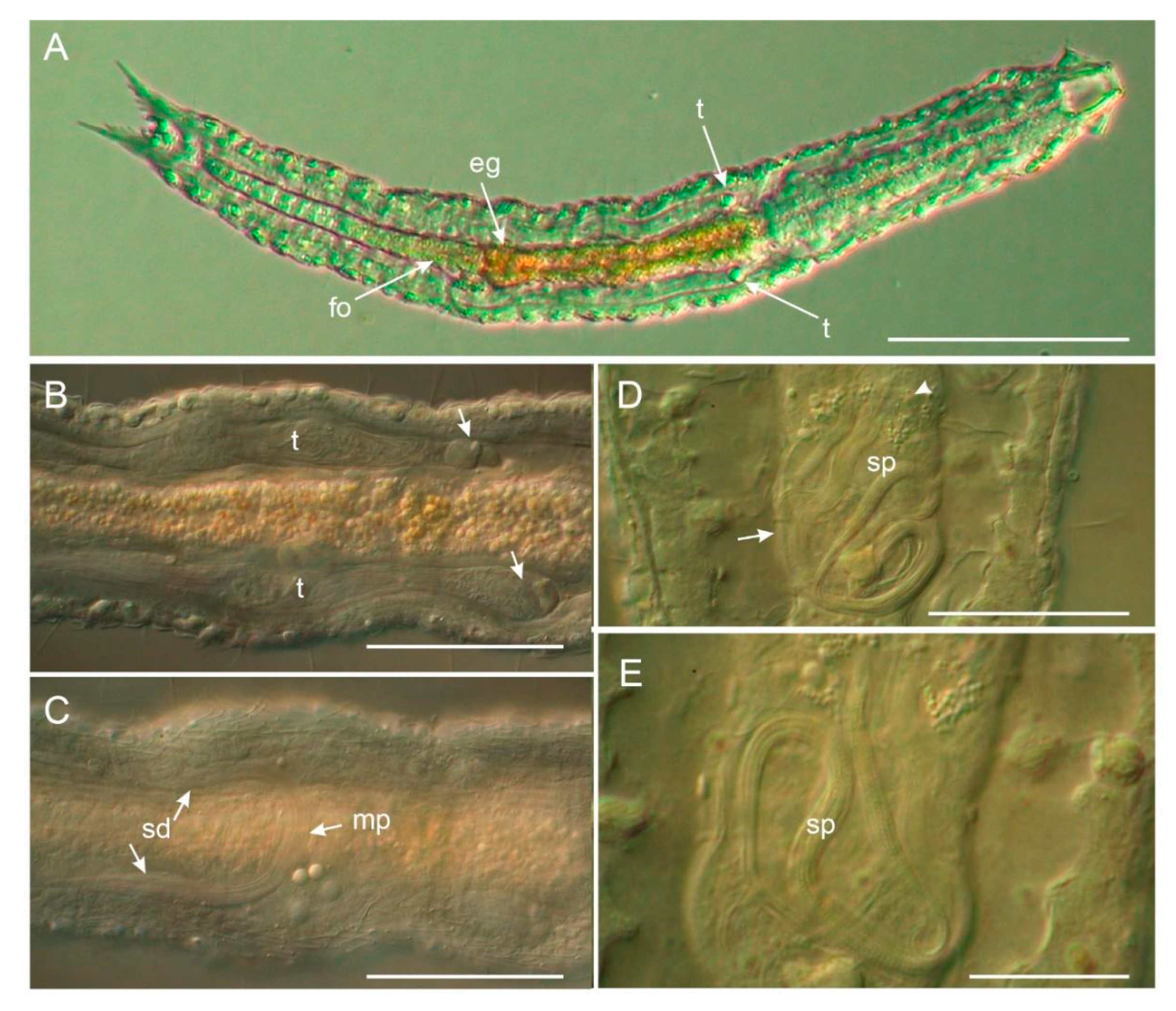
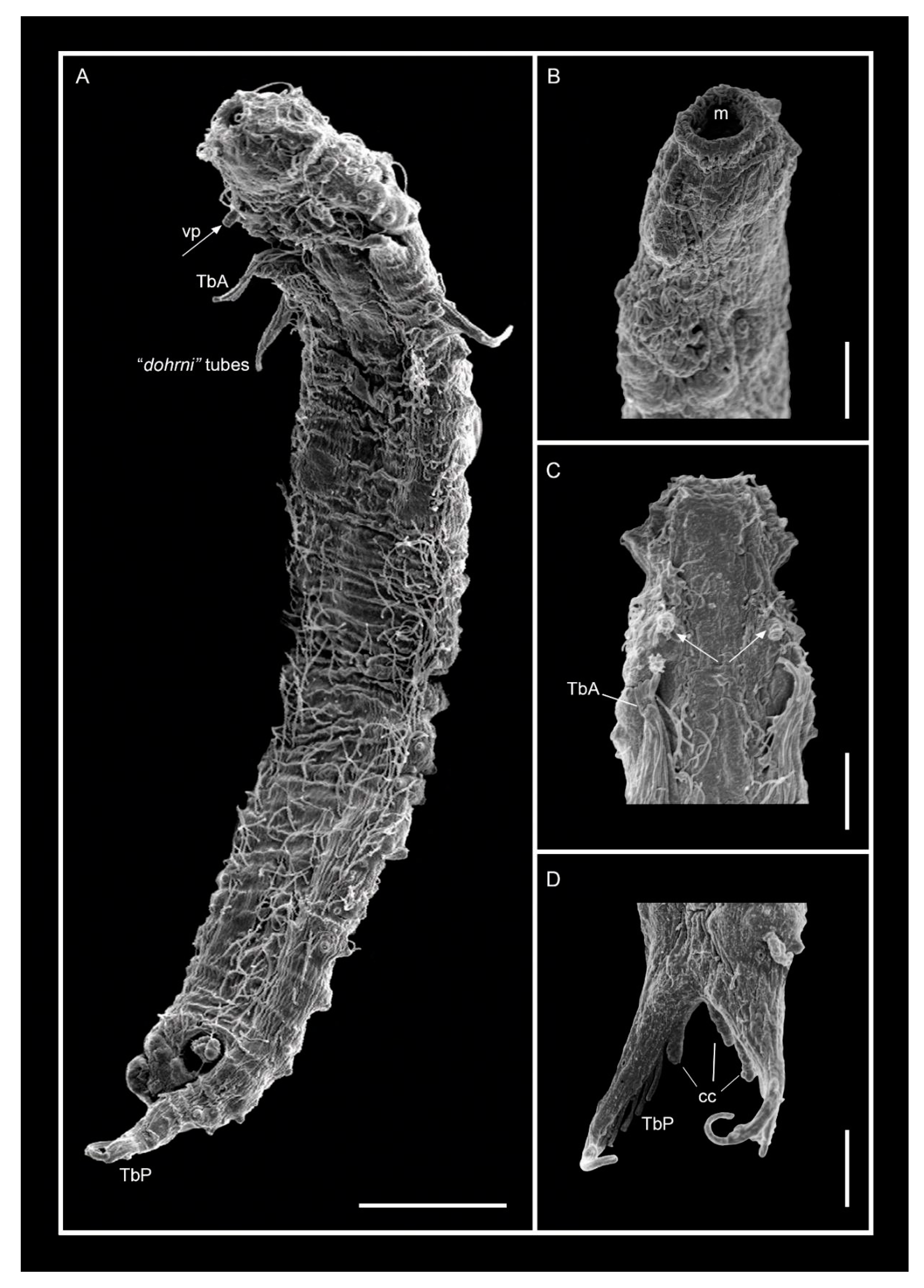
3.1.1. Variability and Remarks on General Morphology
3.1.2. Taxonomic Remarks
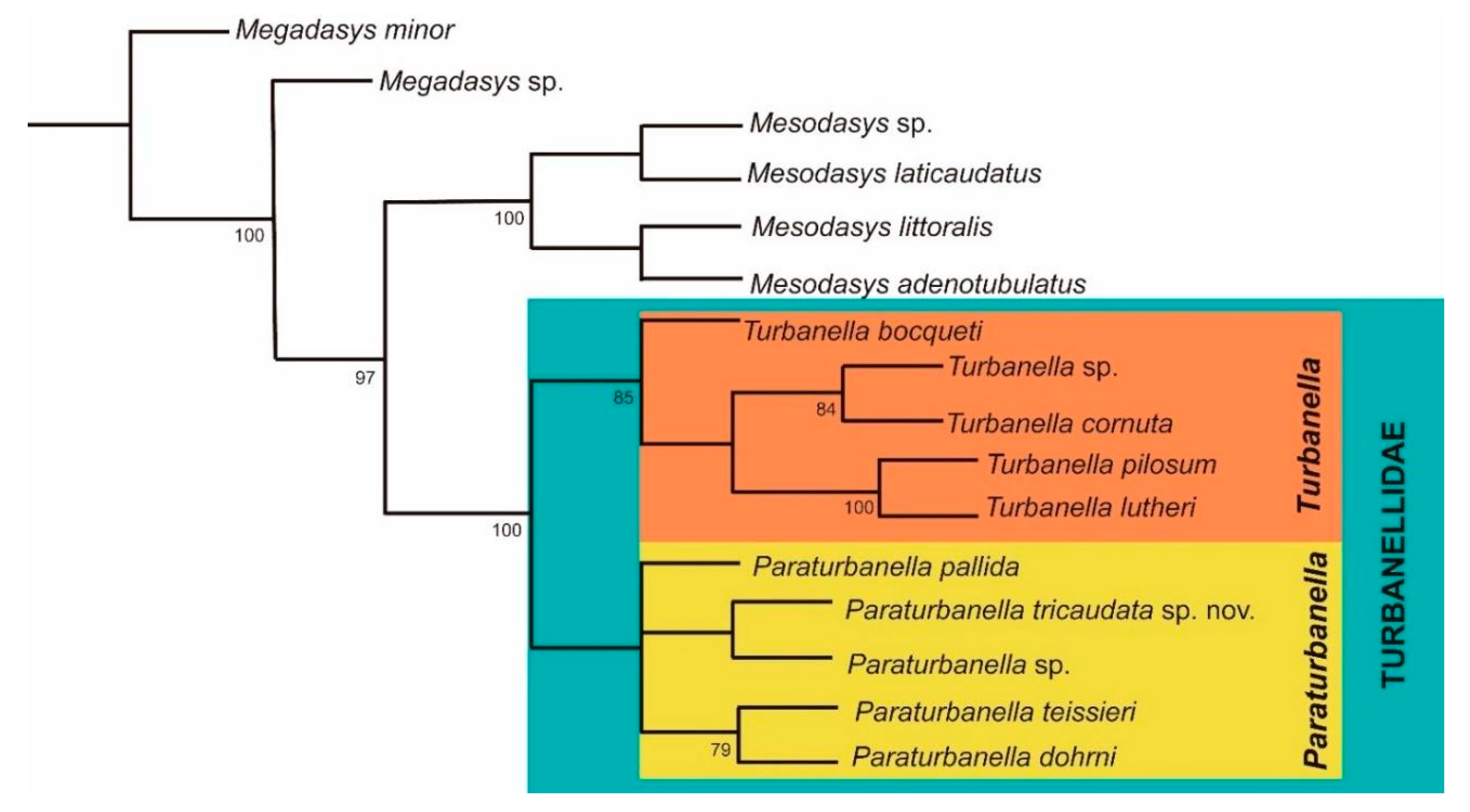
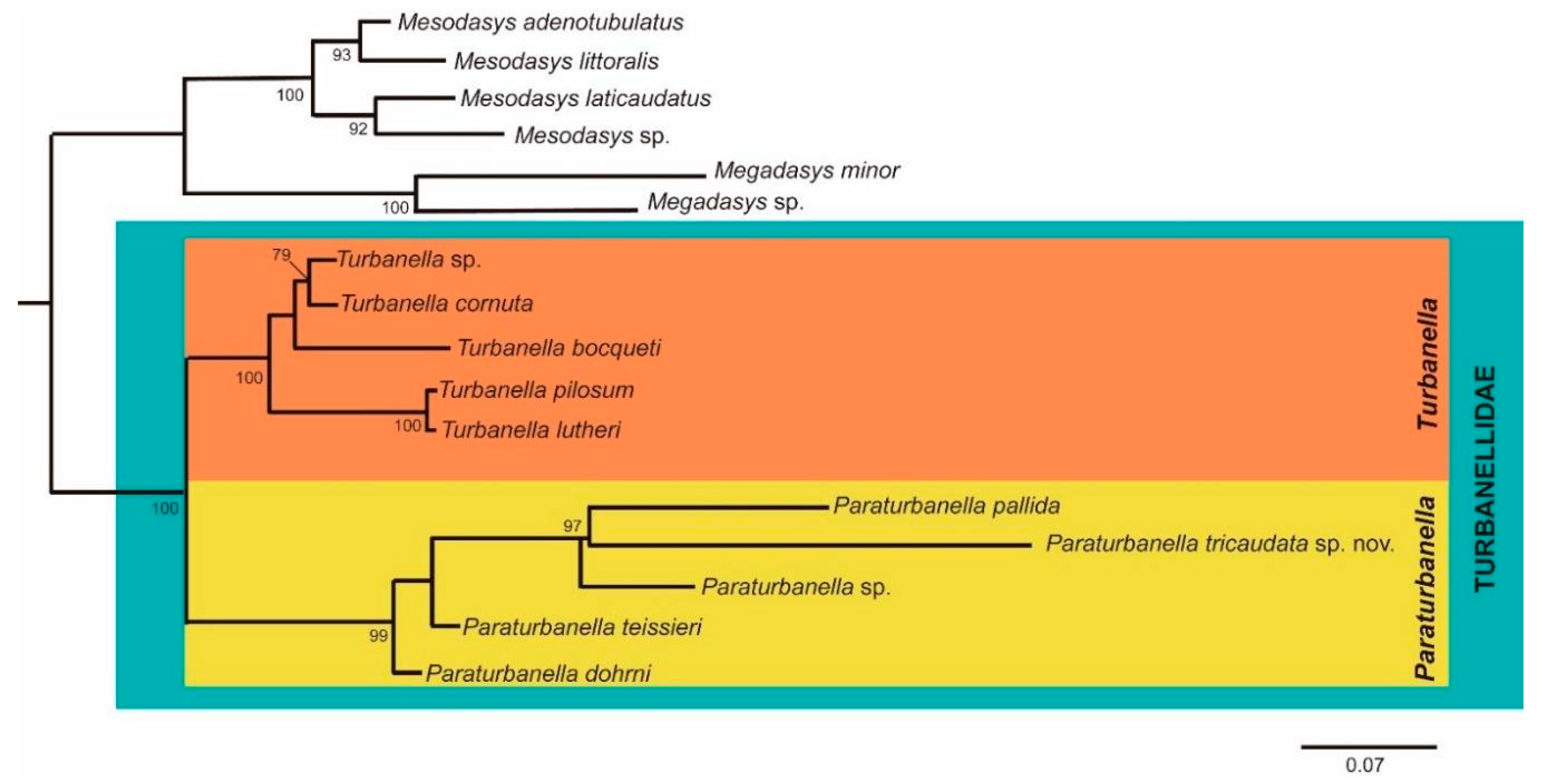
3.1.3. Phylogenetic Analyses
3.1.4. Conclusive Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balsamo, M.; d’Hondt, J.L.; Kisielewski, J.; Todaro, M.A.; Tongiorgi, P.; Guidi, L.; Grilli, P.; de Jong, Y. Fauna Europaea: Gastrotricha. Biodivers. Data J. 2015, 3, e5800. [Google Scholar] [CrossRef]
- Todaro, M.A.; Sibaja-Cordero, J.A.; Segura-Bermúdez, O.A.; Coto-Delgado, G.; Goebel-Otárola, N.; Barquero, J.D.; Cullell-Delgado, M.; Dal Zotto, M. An introduction to the study of Gastrotricha, with a taxonomic key to families and genera of the group. Diversity 2019, 11, 117. [Google Scholar] [CrossRef]
- Remane, A. Neue aberrante Gastrotrichen II: Turbanella cornuta nov.spec. und T. hyalina M. Schultze, 1853. Zool. Anz. 1925, 64, 309–314. [Google Scholar]
- Garraffoni, A.R.S.; Araújo, T.Q.; Lourenço, A.P.; Guidi, L.; Balsamo, M. Integrative taxonomy of a new Redudasys species (Gastrotricha: Macrodasyida) sheds light on the invasion of freshwater habitats by macrodasyids. Sci. Rep. 2019, 9, 2067. [Google Scholar] [CrossRef] [PubMed]
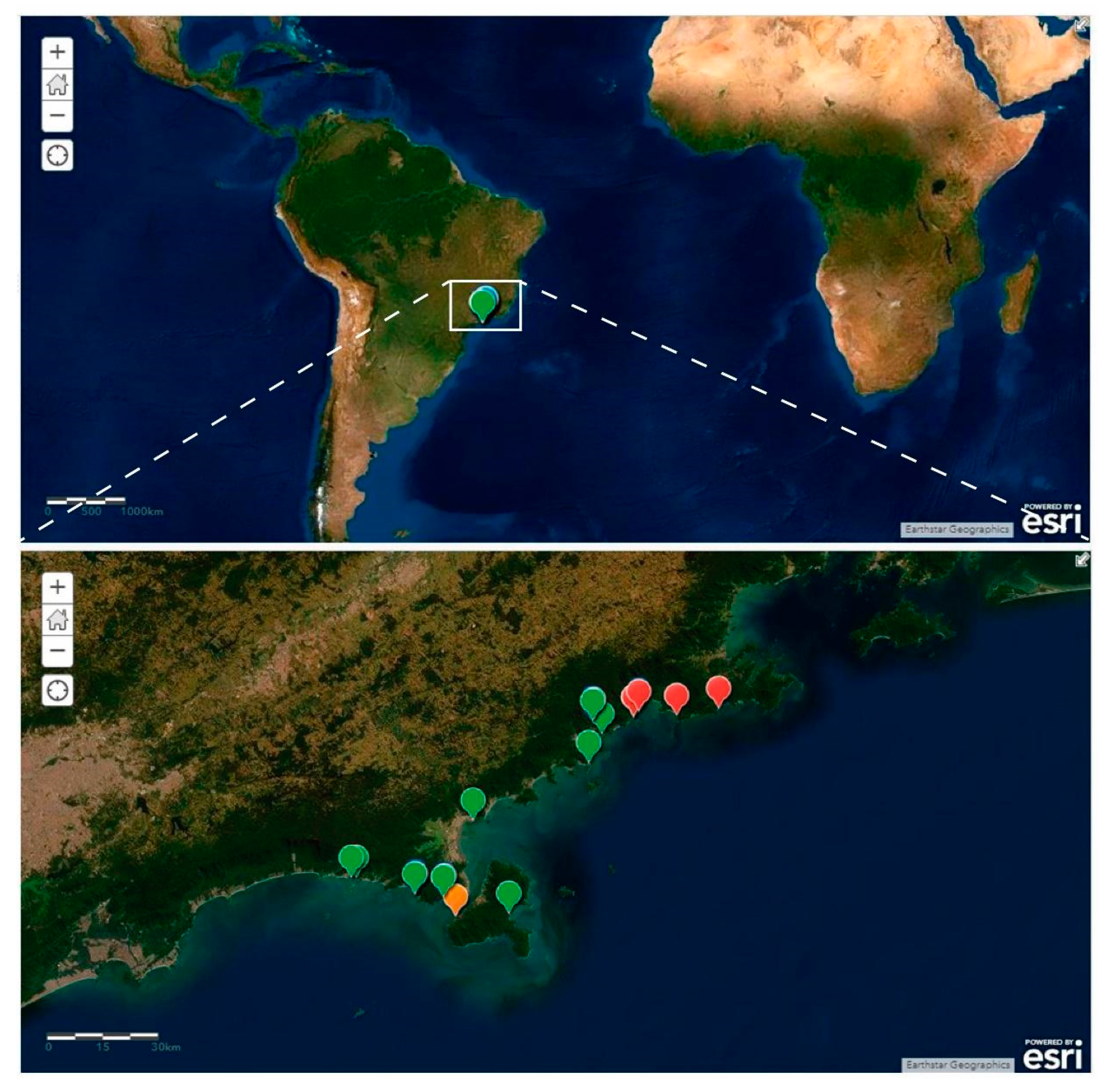
- Campos, A.; Garraffoni, A.R.S. A synopsis of knowledge, zoogeography and an online interactive map of Brazilian marine gastrotrichs. PeerJ 2019, 7, e7898. [Google Scholar] [CrossRef]
- Todaro, M.A. (Ed.) Marine. In Gastrotricha World Portal; 2019; Available online: http://www.gastrotricha.unimore.it/marine.htm (accessed on 30 November 2019).
- Remane, A. Neue Gastrotricha Macrodasyoidea. Zool. Jahrb. Abt. Anat. Ontog. Tiere 1927, 54, 203–242. [Google Scholar]
- Evans, W.A.; Hummon, W.D. A new genus and species of Gastrotricha from the Atlantic coast of Florida, U.S.A. Trans. Am. Microsc. Soc. 1991, 110, 321–327. [Google Scholar] [CrossRef]
- d’Hondt, J.L. Contribution a la connaissance des Gastrotriches intercotidaux du Golfe de Gascogne. Cah. Biol. Mar. 1968, 9, 387–404. [Google Scholar]
- Clausen, C. Desmodasys phocoides gen. et. sp. n., family Turbanellidae (Gastrotricha Macrodasyoidea). Sarsia 1965, 21, 17–21. [Google Scholar] [CrossRef]
- Schultze, M. Über Chaetonotus und Ichthydium (Ehrb.) und eine neue verwandte Gattung Turbanella. Müller’s Arch. Anat. Physiol. 1853, 6, 241–254. [Google Scholar]
- Kieneke, A.; Riemann, O.; Ahlrichs, W.H. Novel implications for the basal internal relationships of Gastrotricha revealed by an analysis of morphological characters. Zool. Scr. 2008, 37, 429–460. [Google Scholar] [CrossRef]
- Kolicka, M.; Kotwicki, L.; Dabert, M. Diversity of Gastrotricha on Spitsbergen (Svalbard Archipelago, Arctic) with a description of seven new species. Ann. Zool. 2018, 68, 609–740. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Perissinotto, R.; Bownes, S.J. First records of Gastrotricha from South Africa, with description of a new species of Halichaetonotus (Chaetonotida, Chaetonotidae). Zookeys 2011, 142, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.A.; Leasi, F.; Hochberg, R. A new species, genus and family of marine Gastrotricha from Jamaica, with a phylogenetic analysis of Macrodasyida based on molecular data. Syst. Biodivers. 2014, 12, 473–488. [Google Scholar] [CrossRef]
- Todaro, M.A.; Perissinotto, R.; Bownes, S.J. Two new marine Gastrotricha from the Indian Ocean coast of South Africa. Zootaxa 2015, 3905, 193–208. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Bownes, S.J.; Perissinotto, R. Two new interesting species of Macrodasyida (Gastrotricha) from KwaZulu-Natal (South Africa). Proc. Biol. Soc. Wash. 2017, 130, 140–155. [Google Scholar] [CrossRef]
- Evans, W.A. Five new species of marine Gastrotricha from the Atlantic coast of Florida. Bull. Mar. Sci. 1992, 51, 315–328. [Google Scholar]
- Swedmark, B. Turbanella armoricana n. sp., nouveau gastrotriche macrodasyoide de la côte nord de Bretagne. Bull. Soc. Zool. Fr. 1954, 79, 469–473. [Google Scholar]
- Rao, G.C.; Ganapati, P.N. Some new interstitial gastrotrichs from the beach sands of Waltair coast. Proc. Sci. Acad. India 1968, 67, 35–53. [Google Scholar]
- Rao, G.C. Meiofauna, In: Fauna of Lakshadweep, State Fauna Series. Zool. Surv. India 1991, 2, 73–75. [Google Scholar]
- Maguire, C. Two new species of Paraturbanella cuanensis and P. eireanna. Cah. Biol. Mar. 1976, 17, 405–410. [Google Scholar]
- Hummon, W.D. Marine Gastrotricha of San Juan Island, Washington, USA, with notes on some species from Oregon and California. Meiofauna Mar. 2010, 18, 11–40. [Google Scholar]
- Wieser, W. Gastrotricha Macrodasyoidea from the intertidal of Puget Sound. Trans. Am. Microsc. Soc. 1957, 76, 372–381. [Google Scholar] [CrossRef]
- Hummon, W.D. Marine Gastrotricha of the Near East: 1. Fourteen new species of Macrodasyida and a redescription of Dactylopodola agadasys Hochberg, 2003. ZooKeys 2011, 94, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Hummon, W.D. Gastrotricha of the North Atlantic Ocean: 1. Twenty-four new and two redescribed species of Macrodasyida. Meiofauna Mar. 2008, 16, 117–174. [Google Scholar]
- Rao, G.C. 3 New interstitial Gastrotrichs from Andhra Coast, India. Cah. Biol. Mar. 1970, 11, 109–120. [Google Scholar]
- Schmidt, P. Interstitielle Fauna von Galapagos. IV. Gastrotricha. Mikrofauna Meeresbodens. 1974, 26, 1–76. [Google Scholar]
- Luporini, P.; Magagnini, G.; Tongiorgi, P. Contribution a la connaissance des gastrotriches des côtes de Toscane. Cah. Biol. Mar. 1971, 12, 433–455. [Google Scholar]
- Clausen, C. Three new species of Gastrotricha Macrodasyida from the Bergen area, western Norway. Sarsia 1996, 81, 119–129. [Google Scholar] [CrossRef]
- Todaro, M.A. Paraturbanella solitaria, a new psammic species (Gastrotricha: Macrodasyida: Turbanellidae), from the coast of California. Proc. Biol. Soc. Wash. 1995, 108, 553–559. [Google Scholar]
- Hochberg, R. Two new species of Turbanellidae (Gastrotricha: Macrodasyida) from a high-energy beach on North Stradbroke Island, Australia. N. Zeal. J. Mar. Fresh. 2002, 36, 311–319. [Google Scholar] [CrossRef]
- Dal Zotto, M.; Leasi, F.; Todaro, M.A. A new species of Turbanellidae (Gastrotricha, Macrodasyida) from Jamaica, with a key to species of Paraturbanella. ZooKeys 2018, 734, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.A.; Rocha, C.E. Diversity and distribution of marine Gastrotricha along the northern beaches of the State of São Paulo (Brazil), with description of a new species of Macrodasys (Macrodasyida, Macrodasyidae). J. Nat. Hist. 2004, 38, 1605–1634. [Google Scholar] [CrossRef]
- Todaro, M.A.; Rocha, C.E. Further data on marine gastrotrichs from the State of São Paulo and the first records from the State of Rio de Janeiro (Brazil). Meiofauna Mar. 2005, 14, 27–31. [Google Scholar]
- ArcGis. Paraturbanella in Brazil [basemap], Scale Not Given. 2019. Available online: https://www.arcgis.com/home/webmap/viewer.html?webmap=fafb1d0942b4483a99ddf828fc24039a (accessed on 17 November 2019).
- Hummon, W.D.; Todaro, M.A.; Tongiorgi, P. Italian Marine Gastrotricha: II. One new genus and ten new species of Macrodasyida. Boll. Zool. 1993, 60, 109–127. [Google Scholar] [CrossRef]
- Suguio, K. Introdução à Sedimentologia; Edgard Blucher: São Paulo, Brazil, 1973; p. 307. [Google Scholar]
- Balsillie, J.H.; Donoghue, J.F.; Butler, K.M.; Koch, J.L. Plotting equation for Gaussian percentiles and a spreadsheet program for generating probability plots. J. Sediment. Res. 2002, 72, 929–933. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Kånneby, T.; Hochberg, R. Integrated data analysis allows the establishment of a new, cosmopolitan genus of marine Macrodasyida (Gastrotricha). Sci. Rep. 2019, 9, 7989. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Todaro, M.A.; Kånneby, T.; Dal Zotto, M.; Jondelius, U. Phylogeny of Thaumastodermatidae (Gastrotricha: Macrodasyida) inferred from nuclear and mitochondrial sequence data. PLoS ONE 2011, 6, e17892. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.A.; Littlewood, D.T.J.; Balsamo, M.; Herniou, E.A.; Cassanelli, S.; Manicardi, G.; Tongiorgi, P. The interrelationships of the Gastrotricha using nuclear small rRNA subunit sequence data, with an interpretation based on morphology. Zool. Anz. 2003, 242, 145–156. [Google Scholar] [CrossRef]
- Petrov, N.B.; Pegova, A.N.; Manylov, O.G.; Vladychenskaya, N.S.; Mugue, N.S.; Aleshin, V.V. Molecular phylogeny of Gastrotricha on the basis of a comparison of the 18S rRNA genes: Rejection of the hypothesis of a relationship between Gastrotricha and Nematoda. Mol. Biol. 2007, 41, 445–452. [Google Scholar] [CrossRef]
- Paps, J.; Riutort, M. Molecular phylogeny of the phylum Gastrotricha: New data brings together molecules and morphology. Mol. Phylogenet. Evol. 2012, 63, 208–212. [Google Scholar] [CrossRef]
- Rao, G.C.; Clausen, C. Planodasys marginalis gen. et sp. nov. and Planodasyidae fam. nov. (Gastrotricha Macrodasyoidea). Sarsia 1970, 42, 73–82. [Google Scholar] [CrossRef]
- Remane, A. Morphologie und Verwandtschaftbeziehungen der aberranten Gastrotrichen I. Z. Morph. Oekol. Tiere 1926, 5, 625–754. [Google Scholar] [CrossRef]
- Garraffoni, A.R.; Kieneke, A.; Kolicka, M.; Corgosinho, P.H.; Prado, J.; Nihei, S.S.; Freitas, A.V. ICZN Declaration 45: A remedy for the nomenclatural and typification dilemma regarding soft-bodied meiofaunal organisms? Mar. Biodivers. 2019, 49, 2199–2207. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; Balsamo, M. Is the ubiquitous distribution real for marine gastrotrichs? Detection of areas of endemism using Parsimony Analysis of Endemicity (PAE). Proc. Biol. Soc. Wash. 2017, 130, 197–210. [Google Scholar] [CrossRef]
- Araújo, T.Q.; Balsamo, M.; Garraffoni, A.R.S. A new species of Pseudostomella (Gastrotricha, Thaumastodermatidae) from Brazil. Mar. Biodivers. 2014, 44, 243–248. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; Di Domenico, M.; Amaral, A.C.Z. Patterns of diversity in marine Gastrotricha from Southeastern Brazilian Coast is predicted by sediment textures. Hydrobiologia 2016, 773, 105–116. [Google Scholar] [CrossRef]
- Hochberg, R.; Atherton, S.; Kieneke, A. Marine Gastrotricha of Little Cayman Island with the description of one new species and an initial assessment of meiofaunal diversity. Mar. Biodivers. 2014, 44, 89–113. [Google Scholar] [CrossRef]

| Species | 18S | 28S | References |
|---|---|---|---|
| Megadasys sp. 1 | JF357656 | JF357704 | Todaro et al. [44] |
| Megadasysminor Kisielewski, 1987 | AY228131 | - | Todaro et al. [45] |
| Mesodasys littoralis Remane, 1951 | JF357658 | JF357706 | Todaro et al. [44] |
| Mesodasys laticaudatus Remane, 1951 | JF357657 | JF357705 | Todaro et al. [44] |
| Mesodasys sp. | AY963690 | KF921011 | Petrov et al. [46] |
| Mesodasys adenotubulatus Hummon, Todaro & Tongiorgi, 1993 | AM231780 | - | Todaro et al. [45] |
| Paraturbanella sp. | KF921017 | - | Petrov et al. [46] |
| Paraturbanellateissieri Swedmark, 1954 | JF357661 | JF357709 | Todaro et al. [44,45] |
| Paraturbanellapallida Luporini, Magagnini & Tongiorgi, 1973 | JF357660 | JF357708 | Todaro et al. [44] |
| Paraturbanelladohrni Remane, 1927 | JF357659 | JF357707 | Todaro et al. [44] |
| Paraturbanellatricaudata sp. nov. | SUB6819988 | SUB6819996 | Present study |
| Turbanella sp. | JF970238 | - | Paps & Riutort [47] |
| Turbanellacornuta Remane, 1925 | AF157007 | JF357711 | Todaro et al. [44] |
| Turbanellapilosum Kolicka, 2018 | MF325920 | MF325905 | Kolicka et al. [13] |
| Turbanellalutheri Remane, 1952 | JF357669 | - | Todaro et al. [44] |
| Turbanellabocqueti Kaplan, 1958 | JF357662 | JF357710 | Todaro et al. [44] |
| Character | Min–Max (µm) | Average (µm) | SD (µm) | N |
|---|---|---|---|---|
| Lt: total body length | 417–480 | 449.00 | 31.51 | 5 |
| Lbc: length of buccal cavity | 18.6–22.3 | 19.9 | 1.2 | 10 |
| Diameter of mouth opening | 13.3–17.4 | 15.0 | 1.3 | 10 |
| Head width at cephalic swellings | 35.8–51.5 | 40.4 | 5.1 | 8 |
| Neck constriction width | 30.1–34.1 | 32.3 | 1.7 | 8 |
| Maximum trunk width | 40.9–49.1 | 43.2 | 2.8 | 8 |
| Lph: length of pharynx | 127.1–141.9 | 136.9 | 4.9 | 6 |
| Distance of the pharyngeal pores from PhJIn | 20.2–27.1 | 23.5 | 2.8 | 6 |
| “Dohrni” longer tube length | 22.6–33.8 | 27.8 | 4.9 | 6 |
| “Dohrni” shorter tube length | 15.6–19.4 | 18.5 | 2.5 | 5 |
| Maximum length of TbA | 8.7–9.6 | 9.2 | 0.4 | 4 |
| Length of TbP | 6.2–7.9 | 7.0 | 0.9 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, A.; Todaro, M.A.; Garraffoni, A.R.S. A New Species of Paraturbanella Remane, 1927 (Gastrotricha, Macrodasyida) from the Brazilian Coast, and the Molecular Phylogeny of Turbanellidae Remane, 1926. Diversity 2020, 12, 42. https://doi.org/10.3390/d12020042
Campos A, Todaro MA, Garraffoni ARS. A New Species of Paraturbanella Remane, 1927 (Gastrotricha, Macrodasyida) from the Brazilian Coast, and the Molecular Phylogeny of Turbanellidae Remane, 1926. Diversity. 2020; 12(2):42. https://doi.org/10.3390/d12020042
Chicago/Turabian StyleCampos, Ariane, M. Antonio Todaro, and André Rinaldo Senna Garraffoni. 2020. "A New Species of Paraturbanella Remane, 1927 (Gastrotricha, Macrodasyida) from the Brazilian Coast, and the Molecular Phylogeny of Turbanellidae Remane, 1926" Diversity 12, no. 2: 42. https://doi.org/10.3390/d12020042
APA StyleCampos, A., Todaro, M. A., & Garraffoni, A. R. S. (2020). A New Species of Paraturbanella Remane, 1927 (Gastrotricha, Macrodasyida) from the Brazilian Coast, and the Molecular Phylogeny of Turbanellidae Remane, 1926. Diversity, 12(2), 42. https://doi.org/10.3390/d12020042






