The Coupled Influence of Thermal Physiology and Biotic Interactions on the Distribution and Density of Ant Species along an Elevational Gradient
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Assessing Thermal Tolerance
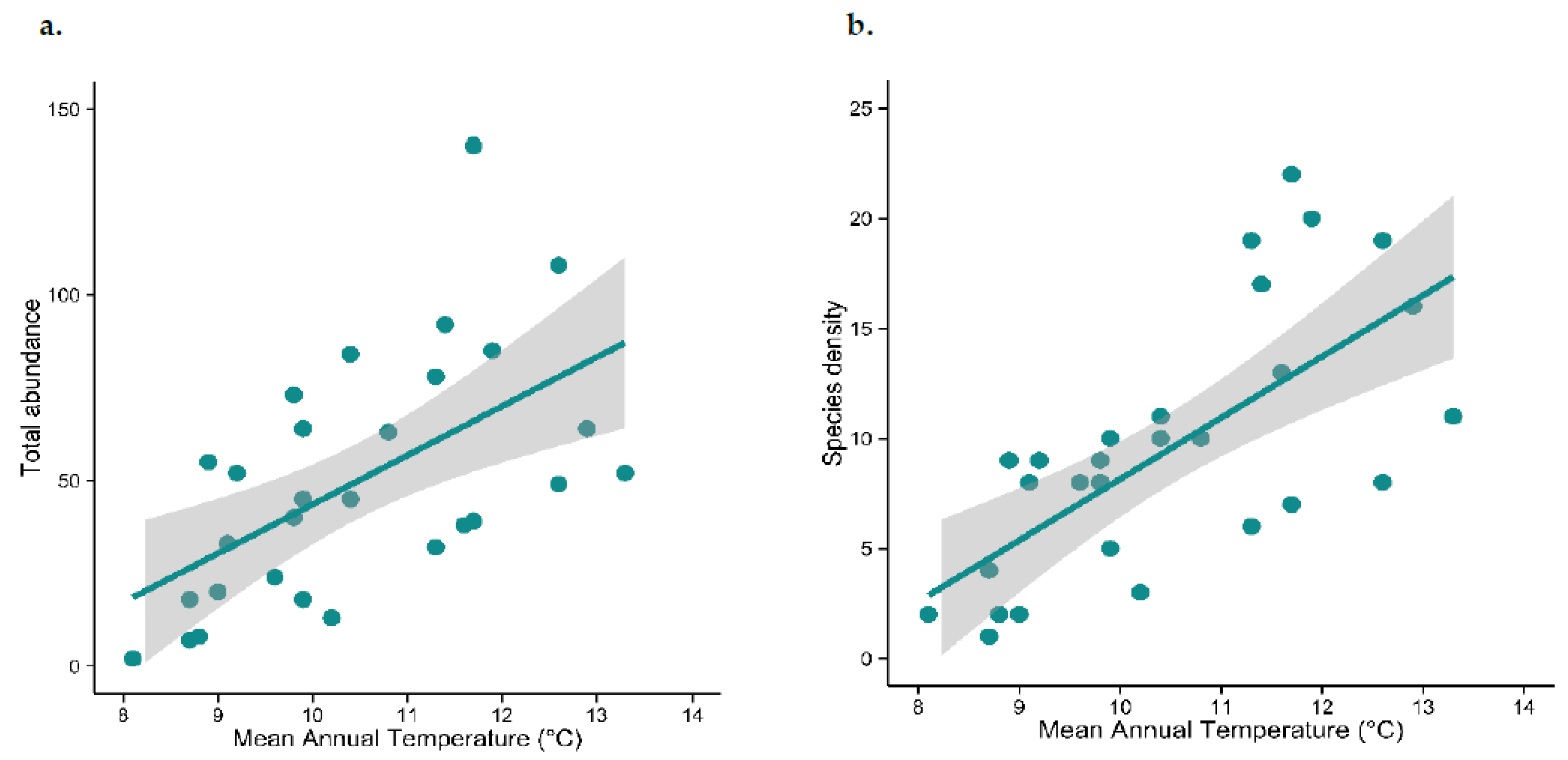
2.3. Are Abundance and Species Density Related to Environmental Temperature?
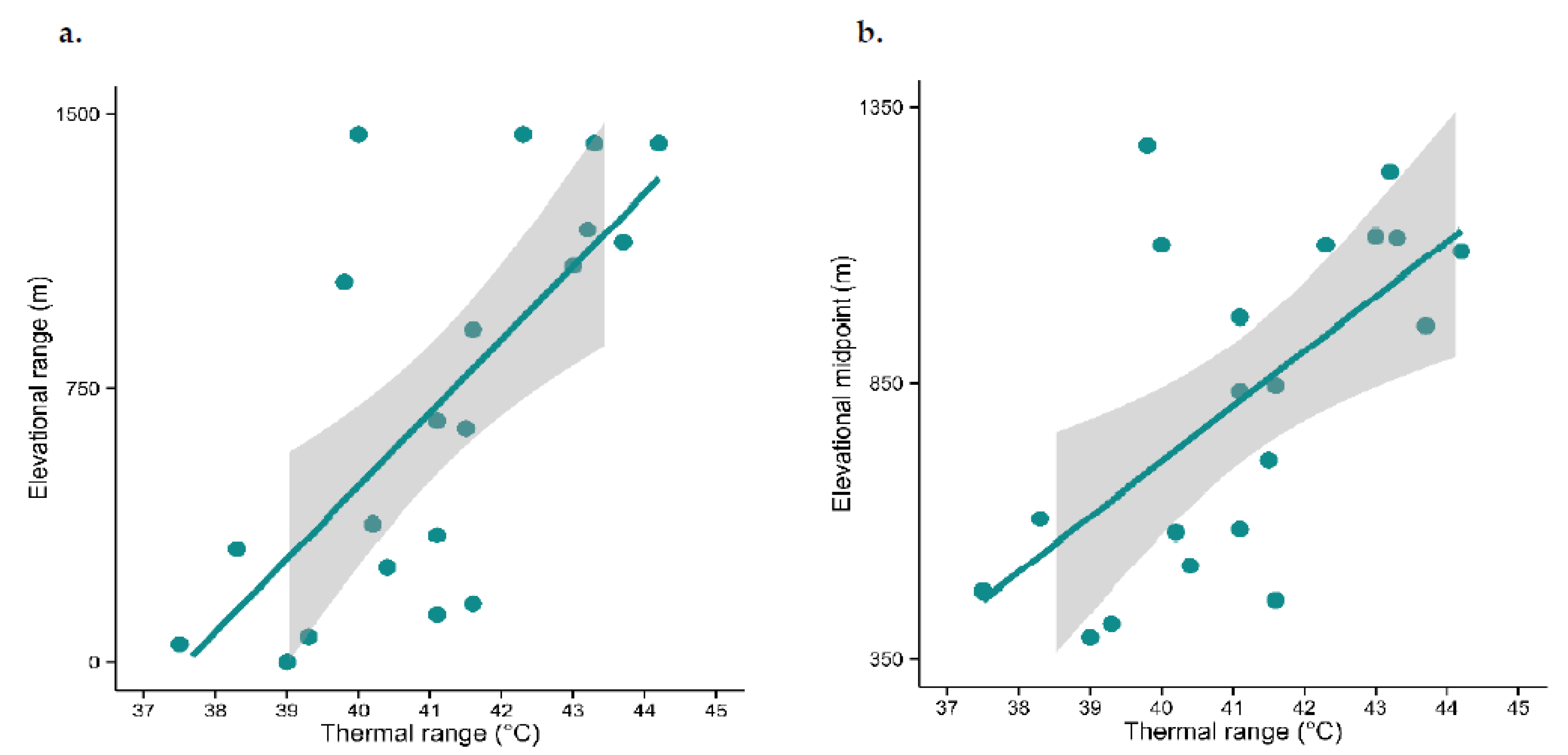
2.4. Does Thermal Tolerance Predict Elevational Range Size and Species Density as Would Be the Case If Temperature Were the Sole Driver of Species Occurrence?
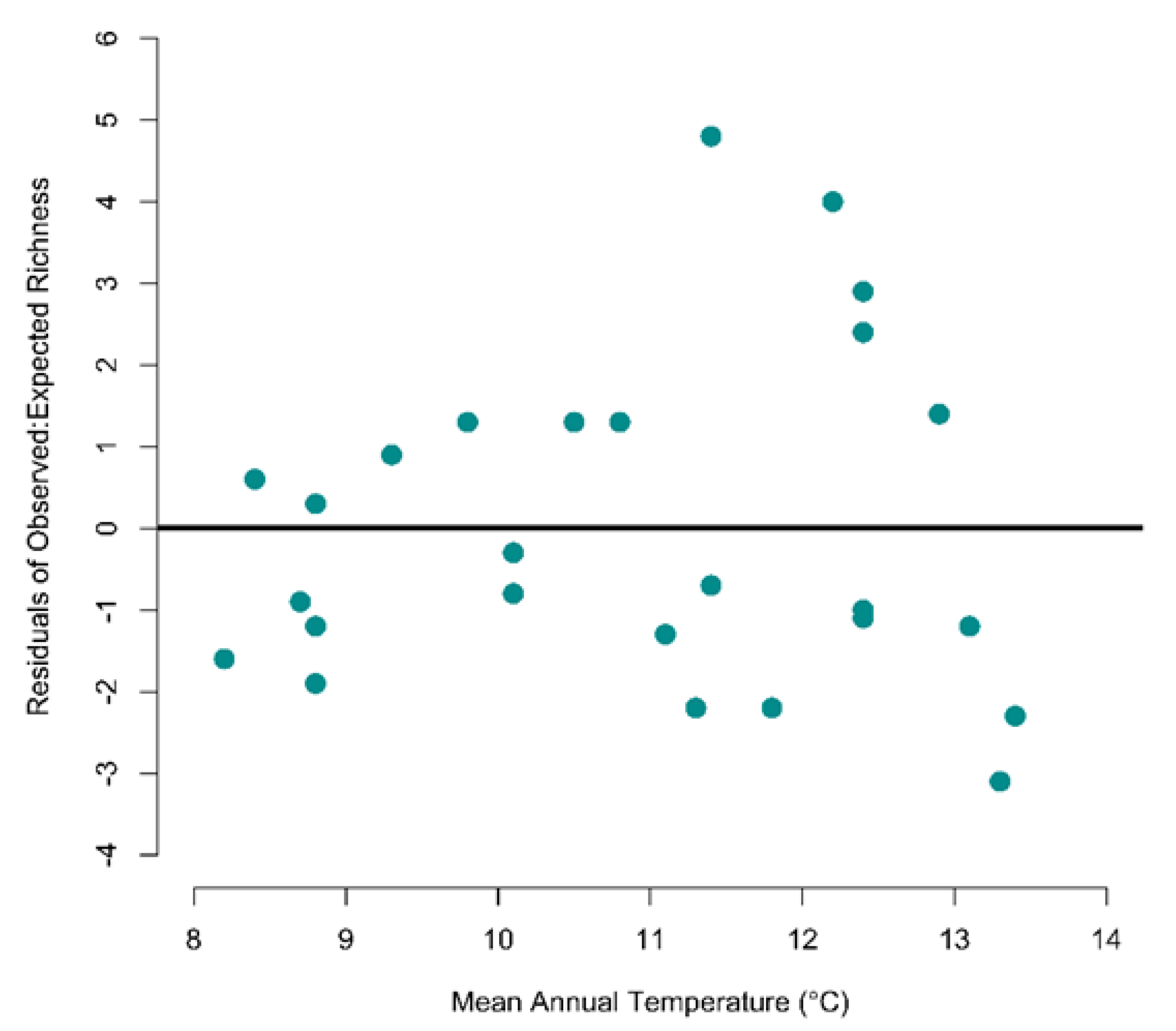
2.5. Do Species Co-Occur Less among Assemblages than Would Be Expected If Temperature Alone Limits Membership?
3. Results
3.1. Are Abundance and Species Density Related to Environmental Temperature?
3.2. Does Thermal Tolerance Predict Range Size and Species Density, as Would Be the Case If Temperature Were the Sole Driver of Species Occurrence?
3.3. Do Species Co-Occur Less among Assemblages than Would Be Expected If Temperature Alone Limits Membership?
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- MacArthur, R.H.; Diamond, J.M.; Karr, J.R. Density compensation in island faunas. Ecology 1972, 53, 330–342. [Google Scholar] [CrossRef]
- von Humboldt, A.; Sabine, E.J. Aspects of Nature, in Different Lands and Different Climates with Scientific Elucidations, 3rd ed.; J. Murray: London, UK, 1849. [Google Scholar]
- Diez, J.; Ibáñez, I.; AJ, M.-R.; Mazer, S.; Crimmins, T.; Crimmins, M.; Bertelsen, C.; Inouye, D. Forecasting phenology: From species variability to community patterns. Ecology 2012, 15, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Fridley, J.D. Downscaling Climate over Complex Terrain: High Finescale (<1000 m) Spatial Variation of Near-Ground Temperatures in a Montane Forested Landscape (Great Smoky Mountains). J. Appl. Meteorol. Climatol. 2009, 48, 1033–1049. [Google Scholar] [CrossRef]
- McCain, C.; Colwell, R. Assessing the threat to montane biodiversity from discordant shifts in temperature and precipitation in a changing climate. Ecol. Lett. 2011, 14, 1236–1245. [Google Scholar] [CrossRef]
- Addo-Bediako, A.; Chown, S.L.; Gaston, K.J. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 2000, 267, 739–745. [Google Scholar] [CrossRef]
- Sunday, J.M.; Bates, A.E.; Dulvy, N.K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 2011, 278, 1823–1830. [Google Scholar] [CrossRef]
- Bishop, T.R.; Robertson, M.P.; Van Rensburg, B.J.; Parr, C.L. Coping with the cold: Minimum temperatures and thermal tolerances dominate the ecology of mountain ants: Thermal tolerances of mountain ants. Ecol. Entomol. 2017, 42, 105–114. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and Ecosystem Responses to Elevational Gradients: Processes, Mechanisms, and Insights for Global Change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef]
- Peters, M.K.; Hemp, A.; Appelhans, T.; Behler, C.; Classen, A.; Detsch, F.; Ensslin, A.; Ferger, S.W.; Frederiksen, S.B.; Gebert, F.; et al. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 2016, 7, 13736. [Google Scholar] [CrossRef]
- Jankowski, J.; Robinson, S.; Levey, D. Squeezed at the top: Interspecific aggression may constrain elevational ranges in tropical birds. Ecology 2010, 91, 1877–1884. [Google Scholar] [CrossRef]
- Galbreath, K.E.; Hafner, D.J.; Zamudio, K.R. When cold is better: Climate-driven elevation shifts yield complex patterns of diversification and demography in an Alpine specialist (American Pika, Ochotona princeps). Evolution 2009, 63, 2848–2863. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.R. Tropical nature and other essays. Nature 1878, 18, 140–141. [Google Scholar] [CrossRef][Green Version]
- Dobzhansky, T. Evolution in the tropics. Am. Sci. 1950, 38, 209–221. [Google Scholar]
- Fischer, A.G. Latitudinal Variations in Organic Diversity. Evolution 1960, 14, 64–81. [Google Scholar] [CrossRef]
- Schemske, D.; Mittelbach, G.; Cornell, H.; Sobel, J.; Roy, K. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 2009, 40, 245–269. [Google Scholar] [CrossRef]
- Kozak, K.; Wiens, J. Phylogeny, ecology, and the origins of climate–richness relationships. Ecology 2012, 93, S167–S181. [Google Scholar] [CrossRef]
- Graham, C.; Parra, J.; Rahbek, C. Phylogenetic structure in tropical hummingbird communities. Proc. Natl. Acad. Sci. USA 2009, 106, 19673–19678. [Google Scholar] [CrossRef] [PubMed]
- Machac, A.; Janda, M.; Dunn, R.; Sanders, N. Elevational gradients in phylogenetic structure of ant communities reveal the interplay of biotic and abiotic constraints on diversity. Ecography 2011, 34, 364–371. [Google Scholar] [CrossRef]
- Losos, J. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 2008, 11, 995–1003. [Google Scholar] [CrossRef]
- Helmuth, B. Climate Change and Latitudinal Patterns of Intertidal Thermal Stress. Science 2002, 298, 1015–1017. [Google Scholar] [CrossRef]
- Sinclair, B.; Scott, M.; Klok, C. Determinants of terrestrial arthropod community composition at Cape Hallett, Antarctica. Antarct. Sci. 2006, 18, 303–312. [Google Scholar] [CrossRef]
- Buckley, L.; Rodda, G.; Jetz, W. Thermal and energetic constraints on ectotherm abundance: A global test using lizards. Ecology 2008, 89, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Gotelli, N. Spatial and temporal niche partitioning in grassland ants. Oecologia 2001, 126, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Parr, C. Dominant ants can control assemblage species richness in a South African savanna. J. Anim. Ecol. 2008, 77, 1191–1198. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. TREE 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Stuble, K.L.; Rodriguez-Cabal, M.A.; McCormick, G.L.; Jurić, I.; Dunn, R.R.; Sanders, N.J. Tradeoffs, competition, and coexistence in eastern deciduous forest ant communities. Oecologia 2013, 171, 981–992. [Google Scholar] [CrossRef]
- Cerda, X.; Retana, J.; Cros, S. Thermal Disruption of Transitive Hierarchies in Mediterranean Ant Communities. J. Anim. Ecol. 1997, 66, 363. [Google Scholar] [CrossRef]
- Diamond, S.E.; Sorger, D.M.; Hulcr, J.; Pelini, S.L.; Toro, I.D.; Hirsch, C.; Oberg, E.; Dunn, R.R. Who likes it hot? A global analysis of the climatic, ecological, and evolutionary determinants of warming tolerance in ants. Glob. Chang. Biol. 2012, 18, 448–456. [Google Scholar] [CrossRef]
- Kaspari, M.; Clay, N.A.; Lucas, J.; Yanoviak, S.P.; Kay, A. Thermal adaptation generates a diversity of thermal limits in a rainforest ant community. Glob. Chang. Biol. 2015, 21, 1092–1102. [Google Scholar] [CrossRef]
- Chick, L.D.; Perez, A.; Diamond, S.E. Social dimensions of physiological responses to global climate change: What we can learn from ants (Hymenoptera: Formicidae). Myrmecol. News 2017, 25, 29–40. [Google Scholar]
- Geraghty, M.J.; Dunn, R.R.; Sanders, N.J. Body size, colony size, and range size in ants (Hymenoptera: Formicidae): Are patterns along elevational and latitudinal gradients consistent with Bergmann’s rule. Myrmecol. News 2007, 10, 51–58. [Google Scholar]
- Warren, R.J., II; Chick, L. Upward ant distribution shift corresponds with minimum, not maximum, temperature tolerance. Glob. Chang. Biol. 2013, 19, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.E.; Chick, L.D. Thermal specialist ant species have restricted, equatorial geographic ranges: Implications for climate change vulnerability and risk of extinction. Ecography 2018. [Google Scholar] [CrossRef]
- Lessard, J.; Fordyce, J.; Gotelli, N.; Sanders, N. Invasive ants alter the phylogenetic structure of ant communities. Ecology 2009, 90, 2664–2669. [Google Scholar] [CrossRef]
- Stuble, K.L.; Pelini, S.L.; Diamond, S.E.; Fowler, D.A.; Dunn, R.R.; Sanders, N.J. Foraging by forest ants under experimental climatic warming: A test at two sites. Ecol. Evol. 2013, 3, 482–491. [Google Scholar] [CrossRef]
- Kaspari, M. A primer on ant ecology. In Ants—Standard Methods for Measuring and Monitoring Biodiversity; Smithsonian Institution Press: Washington, DC, USA; London, UK, 2000; pp. 9–24. [Google Scholar]
- Bujan, J.; Roeder, K.A.; de Beurs, K.; Weiser, M.D.; Kaspari, M. Thermal diversity of North American ant communities: Cold tolerance but not heat tolerance tracks ecosystem temperature. Glob. Ecol. Biogeogr. 2020, 29, 1486–1494. [Google Scholar] [CrossRef]
- Dunn, R.R.; Guenard, B.S.; Weiser, M.D.; Sanders, N.J. Geographic gradients. In Ant Ecology; Lach, L., Parr, C.L., Abbott, K.L., Eds.; Oxford University Press: New York, NY, USA, 2010; pp. 38–58. [Google Scholar]
- Guisan, A.; Rahbek, C. SESAM—A new framework integrating macroecological and species distribution models for predicting spatio-temporal patterns of species assemblages. J. Biogeogr. 2011, 38, 1433–1444. [Google Scholar] [CrossRef]
- Fordham, D.; Mellin, C.; Russell, B.; Akçakaya, R.; Bradshaw, C.; ME, A.-L.; Shepherd, S.; Brook, B. Population dynamics can be more important than physiological limits for determining range shifts under climate change. Glob. Chang. Biol. 2013, 19, 3224–3237. [Google Scholar] [CrossRef]
- Cerdá, X.; Arnan, X.; Retana, J. Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology? Myrmecol. News 2013, 18, 131–147. [Google Scholar]
- Bestelmeyer, B.T. The trade-off between thermal tolerance and behavioural dominance in a subtropical South American ant community. J. Anim. Ecol. 2000, 69, 998–1009. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Mccabe, D.J. Species Co-occurence: A Meta-analysis of J.M. Diamond’s Assembly Rules Model. Ecology 2002, 83, 6. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Cazelles, K.; Gravel, D. Co-occurrence is not evidence of ecological interactions. Ecol. Lett. 2020, 23, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Kaspari, M.; O’Donnell, S.; Kercher, J. Energy, density, and constraints to species richness: Ant assemblages along a productivity gradient. Am. Nat. 2000, 155, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Longino, J.; Coddington, J.; Colwell, R.K. The ant fauna of a tropical rain forest: Estimating species richness three different ways. Ecology 2002, 83, 689–702. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ellison, A.M.; Dunn, R.R.; Sanders, N.J. Counting ants (Hymenoptera: Formicidae): Biodiversity sampling and statistical analysis for myrmecologists. Myrmecol. News 2011, 15, 13–19. [Google Scholar]
- Gotelli, N.J.; Colwell, R.K. Estimating species richness. Biol. Divers. Front. Meas. Assess. 2011, 12, 39–54. [Google Scholar]
- Lessard, J.; Dunn, R.; Parker, C.; Sanders, N. Rarity and diversity in forest ant assemblages of Great Smoky Mountains National Park. Southeast. Nat. 2007, 6, 215–228. [Google Scholar] [CrossRef]
- Sanders, N.J.; Lessard, J.-P.; Fitzpatrick, M.C.; Dunn, R.R. Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Glob. Ecol. Biogeogr. 2007, 16, 640–649. [Google Scholar] [CrossRef]
- Lutterschmidt, W.; Hutchison, V. The critical thermal maximum: Data to support the onset of spasms as the definitive end point. Can. J. Zool. 1997, 75, 1553–1560. [Google Scholar] [CrossRef]
- Lighton, J.R.; Turner, R.J. Thermolimit respirometry: An objective assessment of critical thermal maxima in two sympatric desert harvester ants, Pogonomyrmex rugosus and P. californicus. J. Exp. Biol. 2004, 207, 1903–1913. [Google Scholar] [CrossRef]
- Hijmans, R.; Cameron, S.; Parra, J.; Jones, P.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. A J. R. Meteorol. Soc. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Rohde, K.; Heap, M.; Heap, D. Rapoport’s Rule Does Not Apply to Marine Teleosts and Cannot Explain Latitudinal Gradients in Species Richness. Am. Nat. 1993, 142, 1–16. [Google Scholar] [CrossRef]
- Sanders, N. Elevational gradients in ant species richness: Area, geometry, and Rapoport’s rule. Ecography 2002, 25, 25–32. [Google Scholar] [CrossRef]
- Stone, L.; Roberts, A. The checkerboard score and species distributions. Oecologia 1990, 85, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Sanders, N.J.; Lessard, J.-P.; Dunn, R.R. Great Smoky Mountain Ant Community Composition 2020, 91888 Bytes; Zenodo: Genève, Switzerland, 2020. [Google Scholar]
- Kaspari, M.; Bujan, J.; Roeder, K.A. Species energy and Thermal Performance Theory predict 20-yr changes in ant community abundance and richness. Ecology 2019, 100, 7. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Hawkins, B.; Albuquerque, F.; Araújo, M.; Beck, J.; Bini, L.; Cabrero-Sañudo, F.J.; Castro-Parga, I.; Diniz-Filho, J.A.F.; Ferrer-Castán, D.; Field, R.; et al. A global evaluation of metabolic theory as an explanation for terrestrial species richness gradients. Ecology 2007, 88, 1877–1888. [Google Scholar] [CrossRef]
- McCain, C.M.; Sanders, N.J. Metabolic theory and elevational diversity of vertebrate ectotherms. Ecology 2010, 91, 601–609. [Google Scholar] [CrossRef]
- Lessard, J.-P.; Sackett, T.E.; Reynolds, W.N.; Fowler, D.A.; Sanders, N.J. Determinants of the detrital arthropod community structure: The effects of temperature and resources along an environmental gradient. Oikos 2011, 120, 333–343. [Google Scholar] [CrossRef]
- McGlinn, D.J.; Engel, T.; Blowes, S.A.; Gotelli, N.J.; Knight, T.M.; McGill, B.J.; Sanders, N.; Chase, J.M. A multiscale framework for disentangling the roles of evenness, density, and aggregation on diversity gradients. Ecology 2020, e03233. [Google Scholar] [CrossRef]
- Gifford, M.E.; Kozak, K.H. Islands in the sky or squeezed at the top? Ecological causes of elevational range limits in montane salamanders. Ecography 2012, 35, 193–203. [Google Scholar] [CrossRef]
- Sunday, J.M.; Bates, A.E.; Dulvy, N.K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2012, 2, 686–690. [Google Scholar] [CrossRef]
- Andersen, A. Regulation of “momentary” diversity by dominant species in exceptionally rich ant communities of the Australian seasonal tropics. Am. Nat. 1992, 140, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.L.; Sinclair, B.J.; Andersen, A.N.; Gaston, K.J.; Chown, S.L. Constraint and competition in assemblages: A cross-continental and modeling approach for ants. Am. Nat. 2005, 165, 481–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The Causes and Consequences of Ant Invasions. Annu. Rev. Ecol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Gotelli, N.; Arnett, A. Biogeographic effects of red fire ant invasion. Ecol. Lett. 2000, 3, 257–261. [Google Scholar] [CrossRef]
- Sanders, N.; Gotelli, N.; Heller, N.; Gordon, D. Community disassembly by an invasive species. Proc. Natl. Acad. Sci. USA 2003, 100, 2474–2477. [Google Scholar] [CrossRef]
- Cros, S.; Cerdá, X.; Retana, J. Spatial and temporal variations in the activity patterns of Mediterranean ant communities. Ecoscience 1997, 4, 269–278. [Google Scholar] [CrossRef]
- Sanders, N.; Gordon, D. Resource-dependent interactions and the organization of desert ant communities. Ecology 2003, 84, 1024–1031. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Graves, G.R.; Rahbek, C. Macroecological signals of species interactions in the Danish avifauna. Proc. Natl. Acad. Sci. USA 2010, 107, 5030–5035. [Google Scholar] [CrossRef]
- MacArthur, R.; Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P. Assembly rules, null models, and trait dispersion: New questions from old patterns. Oikos 1995, 74, 159–164. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating extinction rish from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.; Entsminger, G. EcoSim: Null Models Software for Ecology. Available online: https://zenodo.org/record/16504#.X8Sjqdr7RPY (accessed on 1 October 2020).
- Gotelli, N. Null model analysis of species co-occurrence patterns. Ecology 2000, 81, 2606–2621. [Google Scholar] [CrossRef]

| Elevation (m) | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | 379 | 388 | 403 | 440 | 462 | 511 | 520 | 625 | 656 | 688 | 719 | 770 | 786 | 900 | 941 | 941 | 988 | 1087 | 1231 | 1300 | 1342 | 1419 | 1456 | 1530 | 1651 | 1658 | 1707 | 1742 | 1828 |
| Aphaenogaster rudis | |||||||||||||||||||||||||||||
| Myrmecina americana | |||||||||||||||||||||||||||||
| Lasius alienus | |||||||||||||||||||||||||||||
| Stigmatomma pallipes | |||||||||||||||||||||||||||||
| Ponera pennsylvanica | |||||||||||||||||||||||||||||
| Stenamma diecki | |||||||||||||||||||||||||||||
| Stenamma meridionalis | |||||||||||||||||||||||||||||
| Myrmica punctiventris | |||||||||||||||||||||||||||||
| Stenamma schmitti | |||||||||||||||||||||||||||||
| Nylanderia faisonensis | |||||||||||||||||||||||||||||
| Temnothorax curvispinosus | |||||||||||||||||||||||||||||
| Strumigenys ohioensis | |||||||||||||||||||||||||||||
| Stenamma brevicorne | |||||||||||||||||||||||||||||
| Stenamma impar | |||||||||||||||||||||||||||||
| Brachymyrmex depilis | |||||||||||||||||||||||||||||
| Proceratium silaceum | |||||||||||||||||||||||||||||
| Camponotus chromaiodes | |||||||||||||||||||||||||||||
| Prenolepis imparis | |||||||||||||||||||||||||||||
| Strumigenys clypeata | |||||||||||||||||||||||||||||
| Strumigenys rostratum | |||||||||||||||||||||||||||||
| Temnothorax longispinosus | |||||||||||||||||||||||||||||
| Aphaenogster fulva | |||||||||||||||||||||||||||||
| Formica subsericea | |||||||||||||||||||||||||||||
| Stenamma sp. A | |||||||||||||||||||||||||||||
| Aphaenogaster carolonensis | |||||||||||||||||||||||||||||
| Camponotus americanus | |||||||||||||||||||||||||||||
| Camponotus subbarbatus | |||||||||||||||||||||||||||||
| Myrmica pinetorum | |||||||||||||||||||||||||||||
| Lasius umbratus | |||||||||||||||||||||||||||||
| Monomorium minimum | |||||||||||||||||||||||||||||
| Myrmica spatulata | |||||||||||||||||||||||||||||
| Strumigenys ornata | |||||||||||||||||||||||||||||
| Tapinoma sessile | |||||||||||||||||||||||||||||
| Camponotus pennsylvanicus | |||||||||||||||||||||||||||||
| Crematogaster minutissima | |||||||||||||||||||||||||||||
| Myrmica latifrons | |||||||||||||||||||||||||||||
| Pheidole bicarinata | |||||||||||||||||||||||||||||
| Solenopsis molesta | |||||||||||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chick, L.D.; Lessard, J.-P.; Dunn, R.R.; Sanders, N.J. The Coupled Influence of Thermal Physiology and Biotic Interactions on the Distribution and Density of Ant Species along an Elevational Gradient. Diversity 2020, 12, 456. https://doi.org/10.3390/d12120456
Chick LD, Lessard J-P, Dunn RR, Sanders NJ. The Coupled Influence of Thermal Physiology and Biotic Interactions on the Distribution and Density of Ant Species along an Elevational Gradient. Diversity. 2020; 12(12):456. https://doi.org/10.3390/d12120456
Chicago/Turabian StyleChick, Lacy D., Jean-Philippe Lessard, Robert R. Dunn, and Nathan J. Sanders. 2020. "The Coupled Influence of Thermal Physiology and Biotic Interactions on the Distribution and Density of Ant Species along an Elevational Gradient" Diversity 12, no. 12: 456. https://doi.org/10.3390/d12120456
APA StyleChick, L. D., Lessard, J.-P., Dunn, R. R., & Sanders, N. J. (2020). The Coupled Influence of Thermal Physiology and Biotic Interactions on the Distribution and Density of Ant Species along an Elevational Gradient. Diversity, 12(12), 456. https://doi.org/10.3390/d12120456