Doolia, A New Genus of Nannopodidae (Crustacea: Copepoda: Harpacticoida) from off Jeju Island, Korea
Abstract
1. Introduction
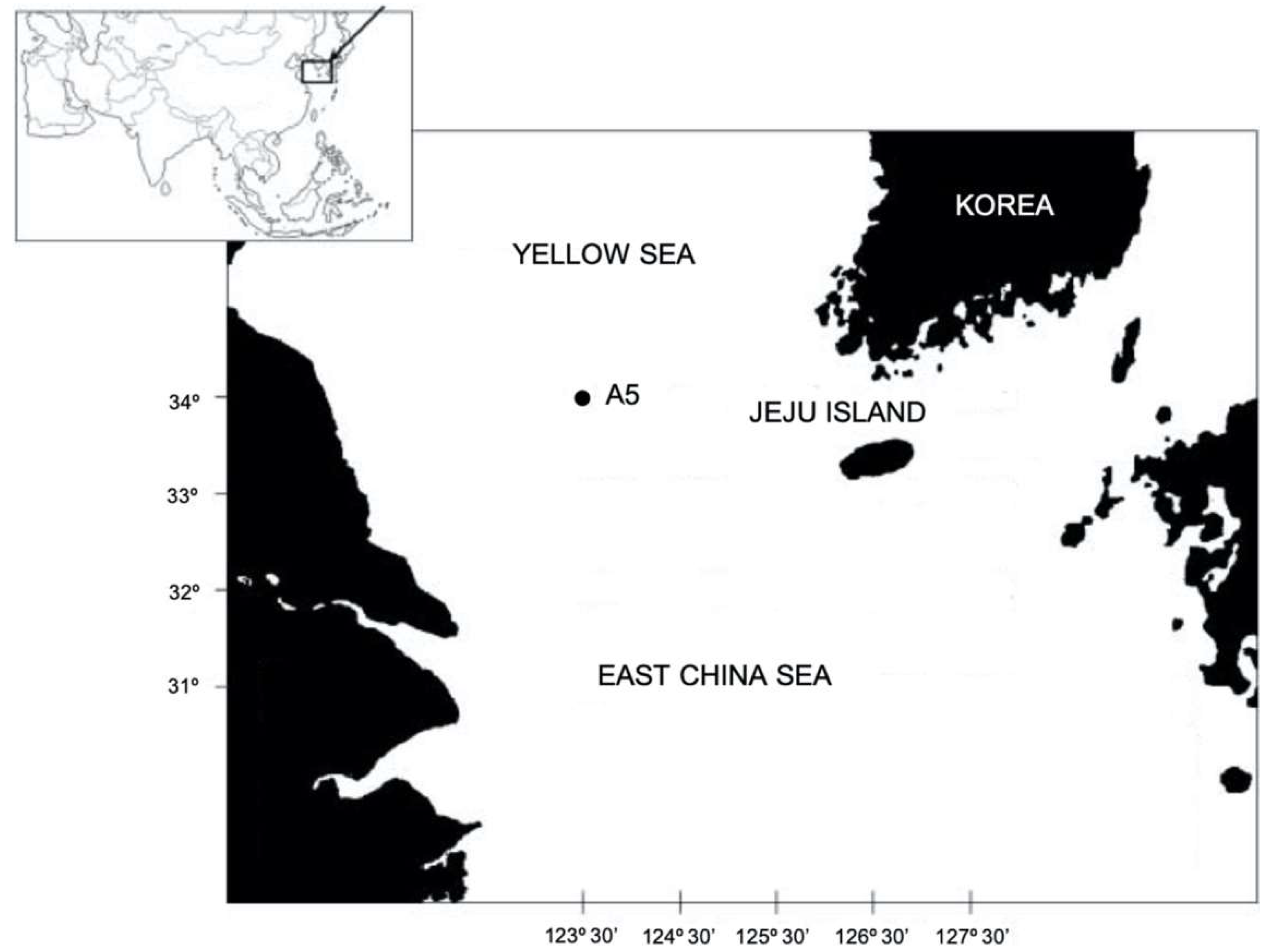
2. Materials and Methods
Specimen Collection and Morphological Examinations
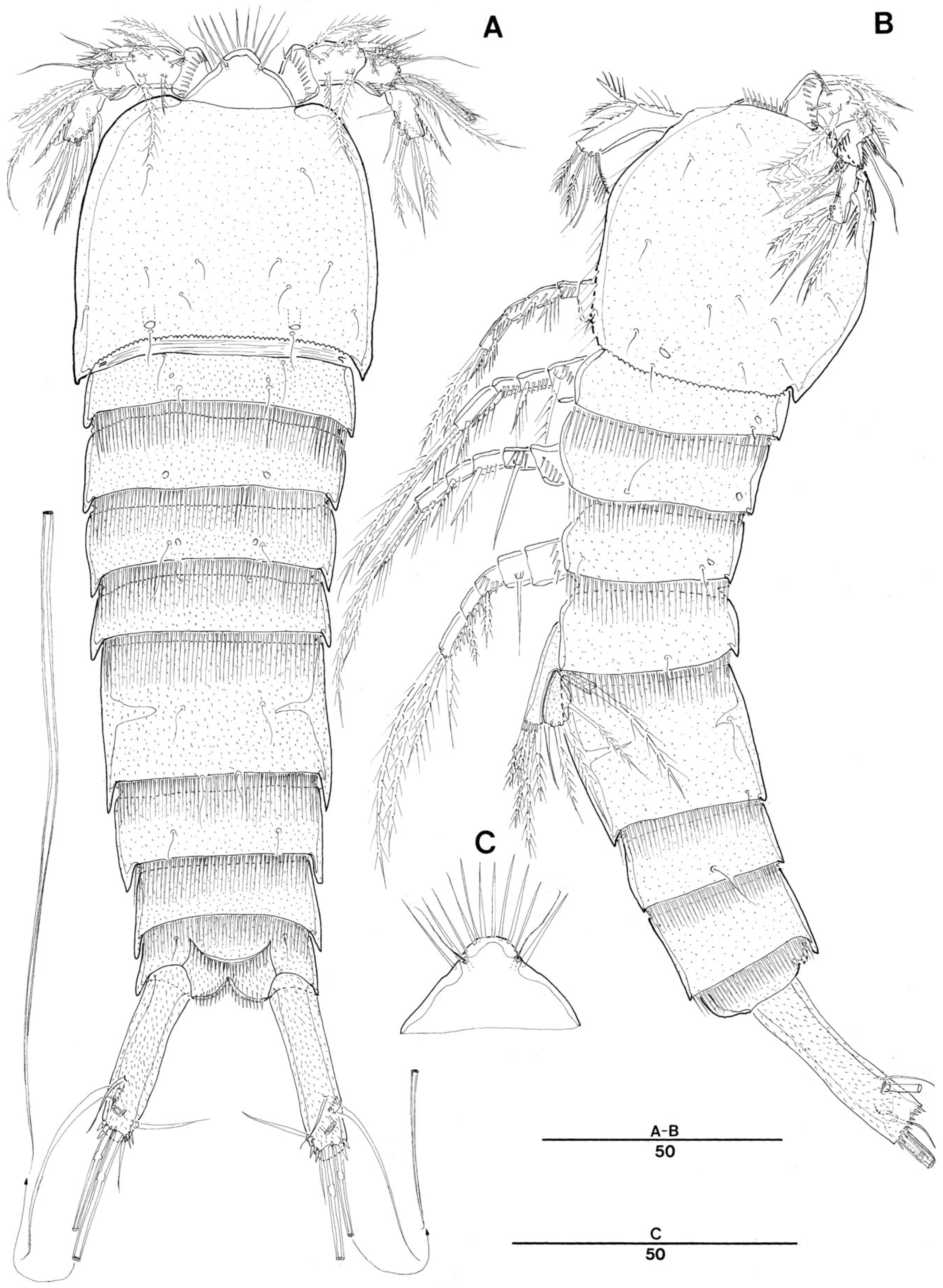
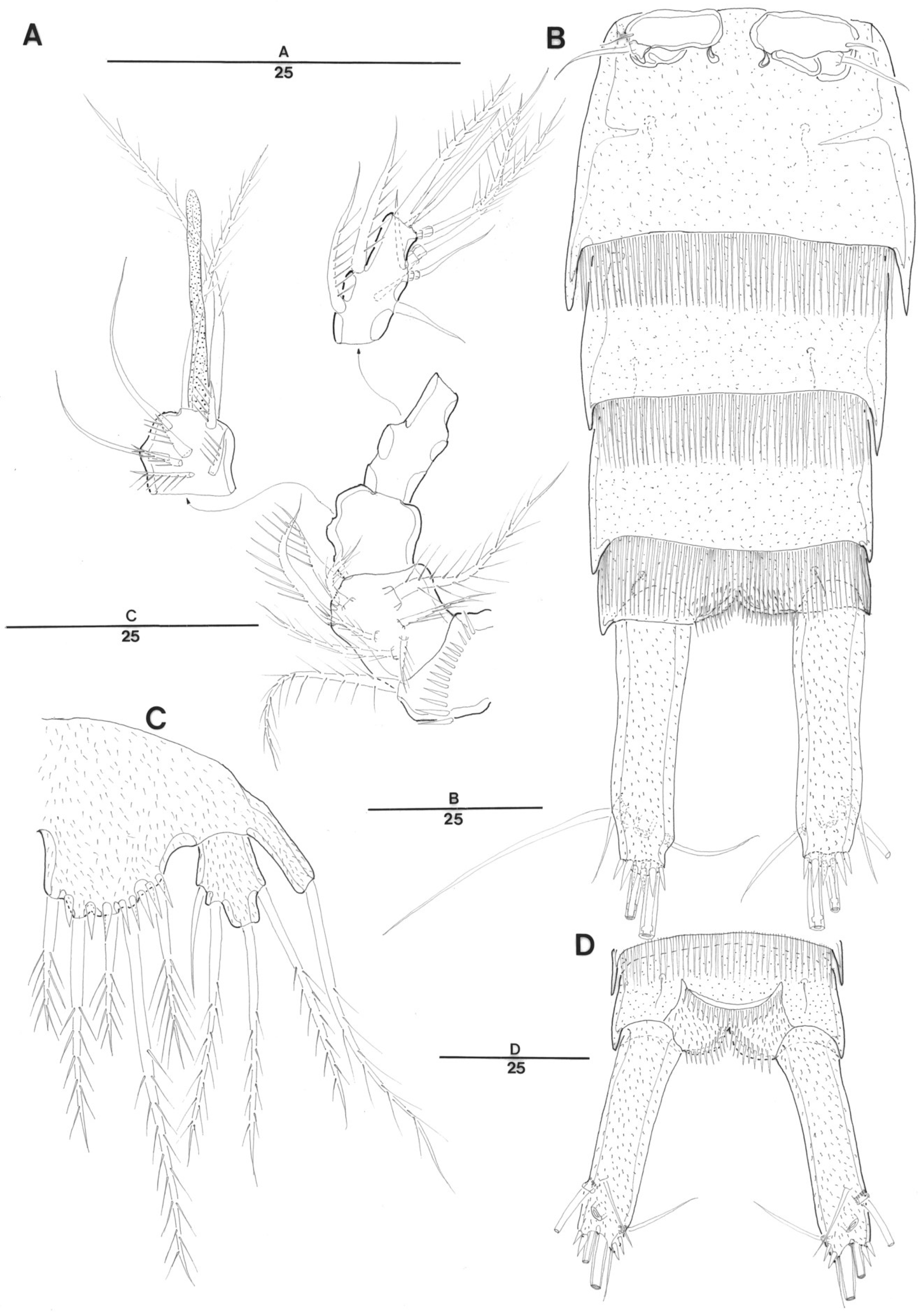
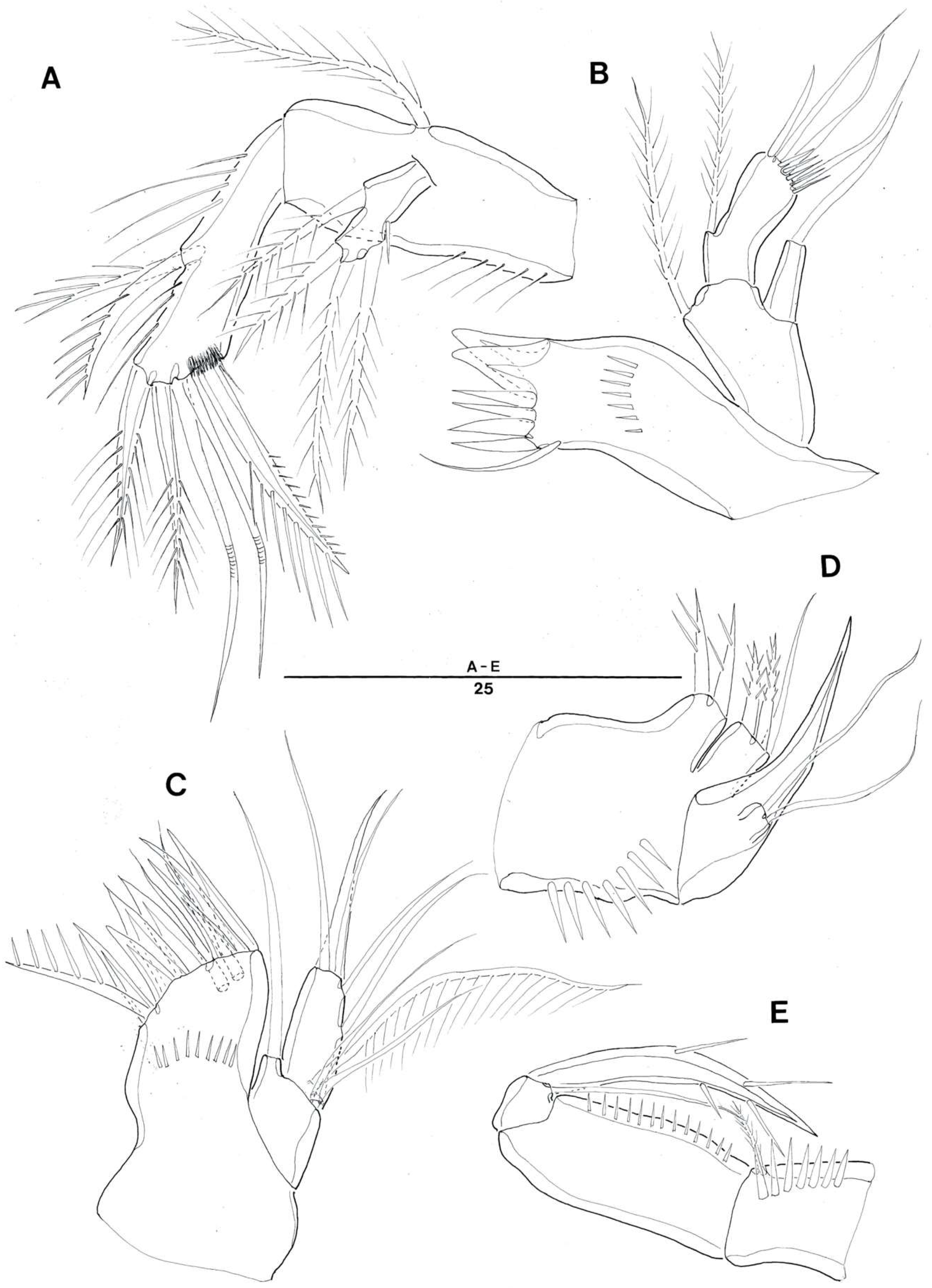
3. Systematic Account
3.1. Genus Doolia, gen. nov.
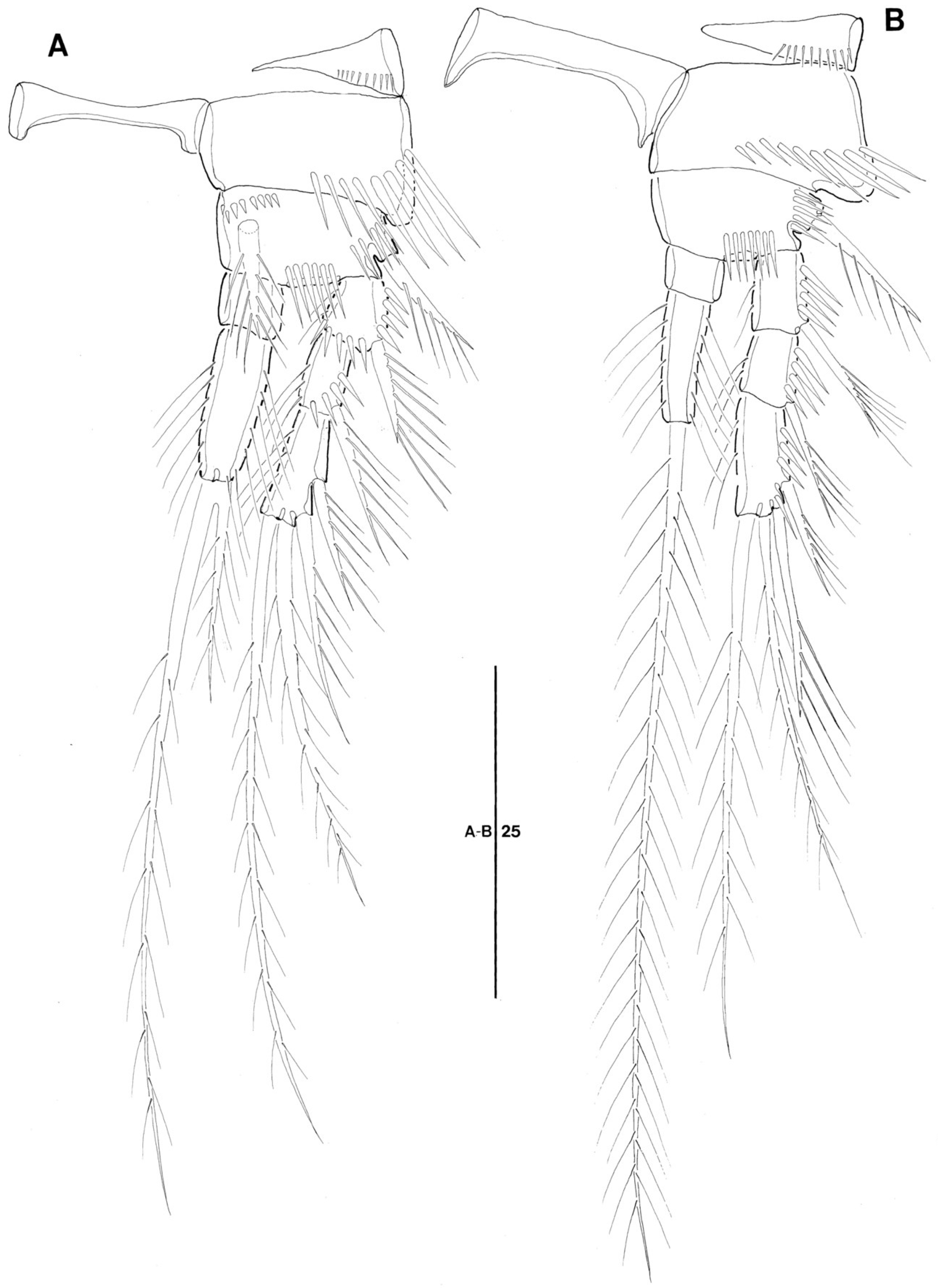
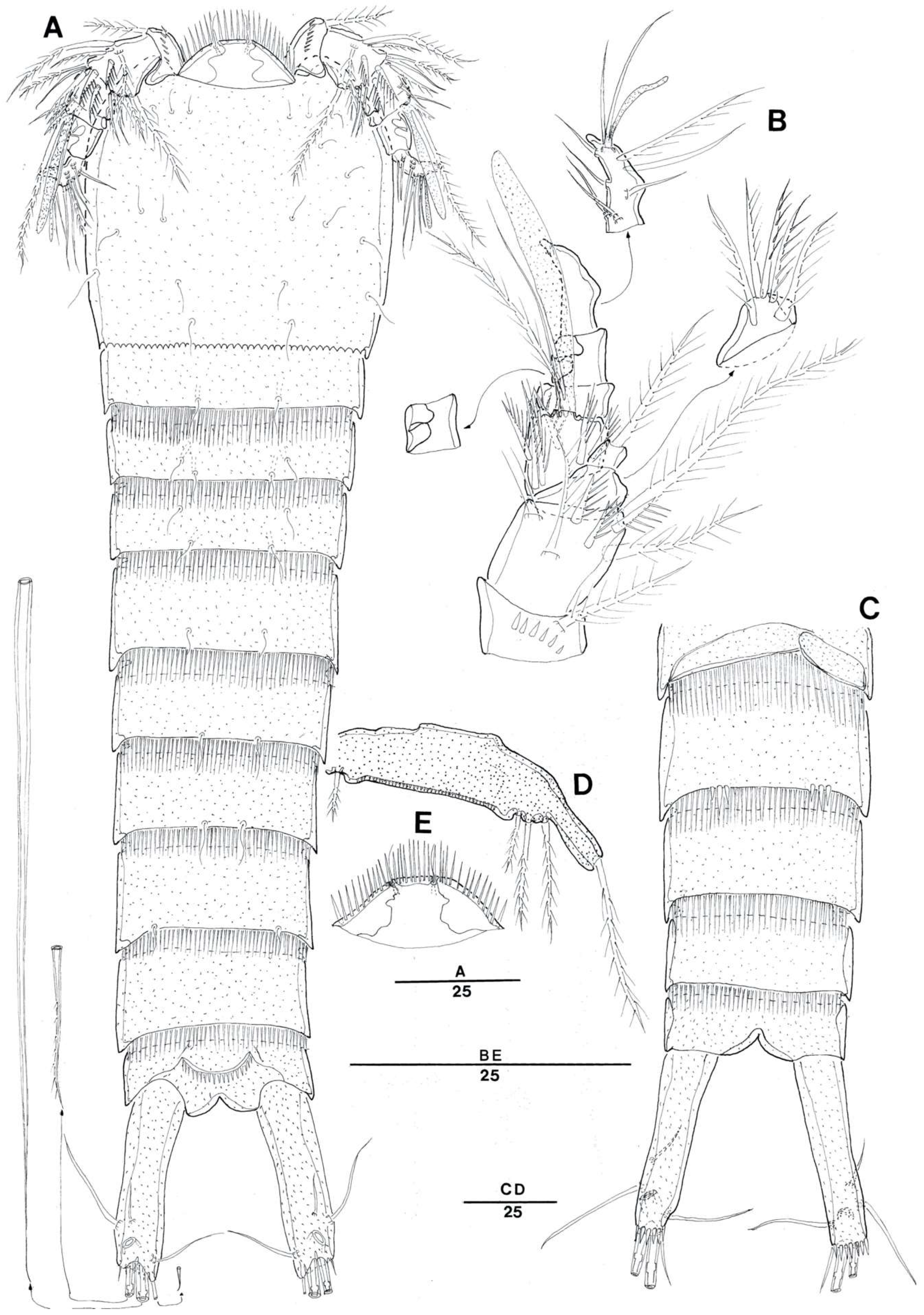
3.2. Doolia ara, sp. nov.
- Exopod Endopod
- P1 0.0.022 0.020
- P2 0.0.022 0.010
- P3 0.0.022 0.010
- P4 0.0.022 0.010
4. Discussion
- Exopods of P2–P4 three-segmented … 2Exopods of P2–P4 two-segmented … 4
- Rostrum bell-shaped, anterior margin with multiple rows of long setules; P1 endopod distinctly shorter than exopod; P2 endopod two-segmented … 3Rostrum triangular, without setular ornamentations; P1 endopod extending beyond distal margin of exp-3; P2 endopod one-segmented with one apical seta … Pontopolites T.Scott, 1894
- Antennule ♀ five-segmented; P2 enp-2 with 2–3 setae; P3 endopod ♂ with apophysis … Nannopus Brady, 1880Antennule ♀ five-segmented; P2 enp-2 with one seta; P3 endopod ♂ with no modification … Doolia gen. nov.
- Antennary exopod one-segmented; P1 exopod one-segmented … Talpacoxa Corgosinho, 2012Antennary exopod absent, or represented by a seta; P1 exopod two- or, three-segmented … 5
- Antennule ♀ four-segmented; P1 endopod prehensile, distinctly longer than exopod … 6Antennule ♀ five-segmented; P1 endopod not prehensile, distinctly shorter than exopod … 7
- P2–P4 with outer spinous projection on coxa; P5 exopod and baseoendopod fused in ♀, forming a single plate with eight setae/spines … Acuticoxa Huys & Kihara, 2010P2–P4 without coxal processes; P5 biramous in ♀ … Laophontisochra George, 2002
- Rostrum with setula ornamentation around apex: antennule ♀ with aesthetasc on segment three; distal exopod segment of P1 with innermost seta much longer than inner distal spine and penicillate apically … Huntemannia Poppe, 1884Rostrum without setula ornamentation around apex: antennule ♀ with aesthetasc on segment four; distal exopod segment of P1 with innermost seta much shorter than inner distal spine and penicillate at tip … Rosacletodes Wells, 1985
Funding
Acknowledgments
Conflicts of Interest
References
- Por, F.D. A Re-evaluation of the family Cletodidae Sars, Lang (Copepoda, Harpacticoida). Syllogeus 1986, 58, 420–425. [Google Scholar]
- Lang, K. Die familie der Cletodidae Sars, 1909. Zool. Jahrb. Abt. Anat. Ontog. Tiere 1936, 68, 446–480. [Google Scholar]
- Huys, R. Unresolved cases of type fixation, synonymy and homonymy in harpacticoid copepod nomenclature (Crustacea: Copepoda). Zootaxa 2009, 2183, 1–99. [Google Scholar] [CrossRef]
- Huys, R.; Kihara, T.C. Systematics and phylogeny of Cristacoxidae (Copepoda, Harpacticoida): A review. Zootaxa 2010, 2568, 1–38. [Google Scholar] [CrossRef]
- Nam, E.-J.; Lee, W. Two new species of the genus Heteropsyllus (Crustacea, Copepoda, Harpacticoida) from Jeju Island, Korea and Devon, England. J. Nat. Hist. 2006, 40, 1719–1745. [Google Scholar] [CrossRef]
- Huys, R.; Gee, J.M.; Moore, C.G.; Hamond, R. Marine and Brackish Water Harpacticoid copepods. Part 1. In Synopses of the British Fauna (New Series); Field Studies Council: Shrewsbury, UK, 1996; pp. 4–19. [Google Scholar]
- Vakati, V.; Lee, W. Five new species of the genus Nannopus (Copepoda: Harpacticoida: Nannopodidae) from intertidal mudflats of the Korean West Coast (Yellow Sea). Zootaxa 2017, 4360, 001–066. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Rho, H.S.; Kim, W. A new species of Huntemannia (Copepoda: Harpacticoida: Huntemanniidae) from the Yellow Sea, Korea. Zootaxa 2007, 1616, 37–48. [Google Scholar] [CrossRef]
- Karanovic, T.; Cho, J.-L. Second members of the harpacticoid genera Pontopolites and Pseudoleptomesochra (Crustacea, Copepoda) are new species from Korean marine interstitial. Mar. Biodivers. 2018, 48, 367–393. [Google Scholar] [CrossRef]
- Pallares, R.E. Copépodos harpacticoides marinos de Tierra del Fuego (Argentina). IV. Bahía Tethis. Contrib. Cient. Centr. Investig. Biol. Mar. Buenos Aires 1982, 186, 3–39. [Google Scholar]
- Björnberg, T. Three new species of benthonic Harpacticoida (Copepoda, Crustacea) from São Sebastião Channel. Nauplius 2014, 22, 75–90. [Google Scholar] [CrossRef]
- George, K.H. New phylogenetic aspects of the Cristacoxidae Huys (Copepoda, Harpacticoida), including the description of a new genus from the Magellan region. Vie Milieu 2002, 52, 31–41. [Google Scholar]
- Corgosinho, P.H.C. Tapacoxa brandini get. et sp. nov. a new Nannopodidae Brady, 1880 (Copepoda: Harpacticoida) from submersed sands of Pontal do Sul (Paraná, Brazil). J. Nat. Hist. 2012, 46, 2865–2879. [Google Scholar] [CrossRef]
- Alper, A.; Sak, S.; Metin, O. First record of the family Rhizotrichidae (Copepoda, Harpacticoida) from Turkey with description of a new species. Mar. Biodivers. 2018, 48, 357–365. [Google Scholar] [CrossRef]
- Nam, E.J.; Lee, W. A new species of the genus Rhizothrix (Copepoda: Harpacticoida: Rhizotrichidae) from Korean waters. Proc. Biol. Soc. Wash. 2005, 118, 692–705. [Google Scholar] [CrossRef]
- Vakati, V.; Eyun, S.-I.; Lee, W. Unraveling the intricate biodiversity of the benthic harpacticoid genus Nannopus (Copepoda, Harpacticoida, Nannopodidae) in Korean waters. Mol. Phylogenet. Evol. 2019, 130, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Karanovic, T.; Kim, K.; Lee, W. Concordance between molecular and morphology-based phylogenies of Korean Enhydrosoma (Copepoda: Harpacticoida: Cletodidae) highlights important synapomorphies and homoplasies in this genus globally. Zootaxa 2015, 3990, 451–496. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.; Nikitin, M.A.; Ivanenko, V.N.; Lee, W. A new minute ectosymbiotic harpacticoid copepod living on the sea cucumber Eupentacta fraudatrix in the East/Japan Sea. PeerJ 2018, 6, e4979. [Google Scholar] [CrossRef] [PubMed]

| Character\Species | Doolia gen. nov. | Nannopus Brady, 1880 | Huntemannia Poppe, 1884 | Pontopolites T.Scott, 1894 | Rosacletodes Wells, 1985 | Laophontisochra George, 2002 | Acuticoxa Huys & Kihara, 2010 | Talpacoxa Corgosinho, 2012 |
|---|---|---|---|---|---|---|---|---|
| Rostrum | defined | fused to cephalothorax | defined | defined | defined | defined | defined | defined |
| Rostral spinules | present | present | present | absent | absent | present | absent | absent |
| A1 segment no., female | 4 | 5 | 5 | 6 | 5 | 4 | 4 | 5 |
| A2 exopodal segment | 4 setae | 3–4 setae | 4 setae | 2-segmented; 1, 120 | 3 setae | 1 segmented or, 1 seta | absent, or 1 minute seta | 3 setae |
| Mandibular exopod | 1 segment | absent | absent | 1 segment | fused, 1 seta | fused, 1 seta | fused, 1 seta | 1 segment |
| Maxilliped syncoxa | 1 seta | 1 seta | 1 seta | no seta | 1 seta | no seta | no seta | no seta |
| P1 exp:enp segment | 3:2 | 3:1-2 | 3:1 | 3:2 | 2:1 | 2:2 | 2:2 | 1:2 |
| P2 exp:enp segment | 3:2 | 3:2 | 1–2:1 | 3:1 | 2:0 | 2:1 | 1-2:1 | 2:1 |
| P3 exp:enp segment | 3:2 | 3:1-2 | 1–2:1 | 3:1 | 1:0 | 2:1 | 1-2:1 | 2:1 |
| P4 exp:enp segment | 3:2 | 3:1 | 1–2:1 | 3:1 | 1:0 | 2:1 (1:1 in male) | 1:1 | 2:1 |
| P3 endopod in male | no modification | with apophysis | with additional seta | with apophysis | with apophysis | no modification | unknown | with apophysis |
| P5 exp:enp seta in female | 4:5 | 4–5:3–4 | 5:4 | fused to 1 plate with 10 setae | 5:6 | 4:1–3 | fused to 1 plate with 8 setae | 5:3 |
| P5 exp:enp seta in male | 3:0 | 4–5:3–4 | 4:4 | 1 plate with 8 setae | 5:4 | 4:2–4 | unknown | 4:2 |
| P6 seta F:M | 2:0 | 1:2–3 | 1:3 | 2:2 | 0:3 | 3:3 | 2: unknown | 0:3 |
| No. of valid species | 1 | 11 | 5 | 2 | 1 | 2 | 2 | 1 |
| Reference | present study | [7] | [8] | [9] | [10] | [11,12] | [4] | [13] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W. Doolia, A New Genus of Nannopodidae (Crustacea: Copepoda: Harpacticoida) from off Jeju Island, Korea. Diversity 2020, 12, 3. https://doi.org/10.3390/d12010003
Lee W. Doolia, A New Genus of Nannopodidae (Crustacea: Copepoda: Harpacticoida) from off Jeju Island, Korea. Diversity. 2020; 12(1):3. https://doi.org/10.3390/d12010003
Chicago/Turabian StyleLee, Wonchoel. 2020. "Doolia, A New Genus of Nannopodidae (Crustacea: Copepoda: Harpacticoida) from off Jeju Island, Korea" Diversity 12, no. 1: 3. https://doi.org/10.3390/d12010003
APA StyleLee, W. (2020). Doolia, A New Genus of Nannopodidae (Crustacea: Copepoda: Harpacticoida) from off Jeju Island, Korea. Diversity, 12(1), 3. https://doi.org/10.3390/d12010003





