Population Growth and Insecticide Residues of Honey Bees in Tropical Agricultural Landscapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Site and Experiment Plot Selection
2.2. Observation of Bees in the Hives and Residue Analysis
2.3. Data Analysis
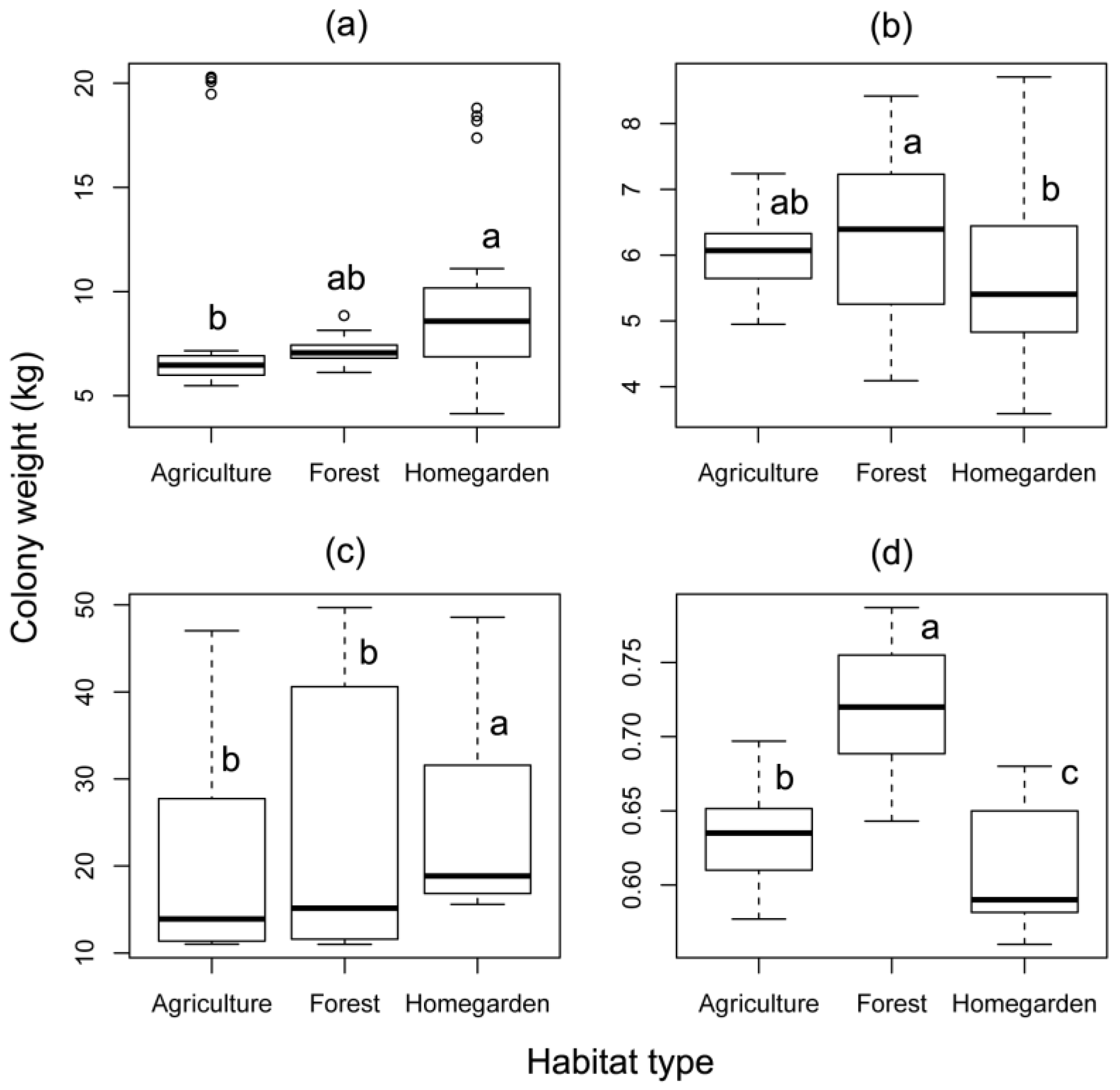
3. Results
3.1. Effect of Different Habitat Types and Season on Honey Bees
3.2. Detection Results of Insecticide Residue in Honey and Body of Honey Bees
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Fruitset of high land coffee increases with the diversity of pollinating bees. Proc. R. Soc. Lond. B 2003, 270, 955–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.; Cabirol, A.; Devaud, J.M.; Barron, A.B.; Lihoreau, M. Why bees are so vulnerable to environmental stressors? Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Kevan, P.G. Pollinators as bioindicators of the state of the environment: Species, activity and diversity. Agric. Ecosyst. Environ. 1999, 74, 373–393. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T. The Assessment Report on Pollinators, Pollination and Food Production of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES): Bonn, Germany, 2017.
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemuller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D. Parallel declines in pollinators and insect pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Liow, L.H.; Sodhi, N.S.; Elmqvist, T. Bee diversity along a disturbance gradient in tropical lowland forests of south-east Asia. J. Appl. Ecol. 2001, 38, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Parui, A.K.; Chatterjee, S.; Dutta, A.; Chakraborty, P.; Roberts, S.; Smith, B. Scale dependent drivers of wild bee diversity in tropical heterogeneous agricultural landscapes. Ecol. Evol. 2016, 6, 6983–6992. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Vanbergen, A.J. The insect pollinators initiative threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.-F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Rundlof, M.; Andersson, G.K.; Bommarco, R.; Fries, I.; Hederstrom, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Scott-Dupree, C.D.; Sultan, M.; McFarlane, A.D.; Brewer, L. A large-scale field study examining effects of exposure to clothianidin seed-treated canola on honey bee colony health, development, and overwintering success. PeerJ 2014, 2, e652. [Google Scholar] [CrossRef] [PubMed]
- Widiatmaka, W.; Ambarwulan, W. Sudarsono Spatial multi-criteria decision making for delineating agricultural land in Jakarta metropolitan area’s hinterland: Case study of Bogor regency, West Java. AGRIVITA J. Agric. Sci. 2016, 38, 105–115. [Google Scholar]
- Hansen, G.E. Indonesia’s Green Revolution: The Abandonment of a Non-Market Strategy toward Change. Asian Surv. 1972, 12, 932–946. [Google Scholar] [CrossRef]
- Mariyono, J.; Kompas, T.; Grafton, R.Q. Shifting from Green Revolution to environmentally sound policies: technological change in Indonesian rice agriculture. J. Asia Pac. Econ. 2010, 15, 128–147. [Google Scholar] [CrossRef]
- El Hassani, A.K.; Dacher, M.; Gauthier, M.; Armengaud, C. Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera). Pharmacol. Biochem. Behav. 2005, 82, 30–39. [Google Scholar] [CrossRef]
- Koch, H.; Weißer, P. Exposure of honey bees during pesticide application under field conditions. Apidologie 1997, 28, 439–447. [Google Scholar] [CrossRef] [Green Version]
- BPS-Statistics Indonesia. Statistics of Forestry Production 2017; BPS-Statistics Indonesia: Jakarta, Indonesia, 2017.
- Abou-Shaara, H.F.; Al-Ghamdi, A.A.; Mohamed, A.A. Honey bee colonies performance enhance by newly modified beehives. J. Apic. Sci. 2013, 57, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Human, H.; Brodschneider, R.; Dietemann, V.; Dively, G.; Ellis, J.D.; Forsgren, E.; Fries, I.; Hatjina, F.; Hu, F.-L.; Jaffé, R.; et al. Miscellaneous standard methods for Apis mellifera research. J. Apic. Res. 2013, 52, 1–53. [Google Scholar] [CrossRef] [Green Version]
- Calatayud-Vernich, P.; Calatayud, F.; Simo, E.; Pico, Y. Efficiency of QuEChERS approach for determining 52 pesticide residues in honey and honey bees. MethodsX 2016, 3, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Komsta, L. mblm: Median-Based Linear Models, R package version 0.12.1; 2019. Available online: https://CRAN.R-project.org/package=mblm (accessed on 14 November 2019).
- Wu, P.; Axmacher, J.C.; Song, X.; Zhang, X.; Xu, H.; Chen, C.; Yu, Z.; Liu, Y. Effects of plant diversity, vegetation composition, and habitat type on different functional trait groups of wild bees in rural Beijing. J. Insect Sci. 2018, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaluza, B.F.; Wallace, H.M.; Heard, T.A.; Minden, V.; Klein, A.; Leonhardt, S.D. Social bees are fitter in more biodiverse environments. Sci. Rep. 2018, 8, 12353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danner, N.; Keller, A.; Hartel, S.; Steffan-Dewenter, I. Honey bee foraging ecology: Season but not landscape diversity shapes the amount and diversity of collected pollen. PLoS ONE 2017, 12, e0183716. [Google Scholar] [CrossRef] [Green Version]
- Simone-Finstrom, M.; Li-Byarlay, H.; Huang, M.H.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 2016, 6, 32023. [Google Scholar] [CrossRef]
- Robertson, L.M.; Edlin, J.S.; Edwards, J.D. Investigating the importance of altitude and weather conditions for the production of toxic honey in New Zealand. N. Z. J. Crop Hortic. Sci. 2010, 38, 87–100. [Google Scholar] [CrossRef]
- Bargańska, Ż.; Ślebioda, M.; Namieśnik, J. Pesticide residues levels in honey from apiaries located of Northern Poland. Food Control 2013, 31, 196–201. [Google Scholar] [CrossRef]
- Porrini, C.; Sabatini, A.G.; Girotti, S.; Fini, F.; Monaco, L.; Celli, G.; Bortolotti, L.; Ghini, S. The death of honey bees and environmental pollution by pesticides: The honey bees as biological indicators. Bull. Insectol. 2003, 56, 147–152. [Google Scholar]


| Area | Agriculture | Forest | Home Garden |
|---|---|---|---|
| Bogor |  |  |  |
| Description | Dominated by rice, maize, and cucumber with frequent pesticide application | Tree plants, dominated by Paraserienthes falcataria or Hevea braziliensis | Habitat surrounding housing area, dominated by fruit trees and flowering plants |
| Malang |  |  |  |
| Description | Highland agroecosystem, dominated by vegetable crops with high insecticide application | Tree plants, dominated by pine (Pinus merkusii) | Vegetation surrounding housing area, dominated by fruit trees and flowering plants |
| Variable | Bee | Region | Season (df = 1) | Habitat Type (df = 2) | Observation Time (df = 3) | |||
|---|---|---|---|---|---|---|---|---|
| χ2 | p-Value | χ2 | p-Value | χ2 | p-Value | |||
| Forager abundance | Apis cerana | Malang | 0.960 | 0.327 | 22.448 | <0.001 | 4.430 | 0.219 |
| Bogor | 0.006 | 0.937 | 3.804 | 0.149 | 0.239 | 0.971 | ||
| Apis mellifera | Malang | 0.323 | 0.570 | 18.543 | <0.001 | 0.106 | 0.991 | |
| Tetragonula laeviceps | Bogor | 0.051 | 0.822 | 17.106 | <0.001 | 1.159 | 0.763 | |
| Colony weight | A. cerana | Malang | 2.144 | 0.143 | 8.737 | 0.013 | 0.581 | 0.901 |
| Bogor | 4.202 | 0.040 | 4.425 | 0.109 | 3.383 | 0.336 | ||
| A. mellifera | Malang | 18.365 | <0.001 | 9.092 | 0.011 | 0.056 | 0.997 | |
| T. laeviceps | Bogor | 0.011 | 0.915 | 45.094 | <0.001 | 1.615 | 0.656 | |
| Parameter | A. cerana | A. mellifera | T. laeviceps | |||||
|---|---|---|---|---|---|---|---|---|
| Malang | Bogor | Malang | Bogor | |||||
| Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | |
| Forager abundance | ||||||||
| (Intercept) | 89.000 | <0.001 | 530.500 | <0.001 | 783.750 | <0.001 | 105.500 | <0.001 |
| Observation | −0.639 | 0.001 | −0.342 | 0.567 | 1.700 | 0.637 | 0.339 | 0.160 |
| Colony weight | ||||||||
| (Intercept) | 7.095 | <0.001 | 5.738 | <0.001 | 15.915 | <0.001 | 0.646 | <0.001 |
| Observation | −0.005 | 0.167 | 0.013 | 0.001 | 0.014 | 0.966 | 0.000 | 0.033 |
| Species | Product | Habitat | Residue (µg/kg) | |
|---|---|---|---|---|
| Bogor | Malang | |||
| A. mellifera | Honey | Agriculture | - | - |
| Forest | 4.4 | - | ||
| Home garden | - | - | ||
| Bee body | Agriculture | - | - | |
| Forest | - | - | ||
| Home garden | - | - | ||
| A. cerana | Honey | Agriculture | - | - |
| Forest | - | 0.5 | ||
| Home garden | - | - | ||
| Bee body | Agriculture | - | - | |
| Forest | 3.1 | - | ||
| Home garden | 11.2 | - | ||
| T. laeviceps | Honey | Agriculture | - | - |
| Forest | - | - | ||
| Home garden | - | - | ||
| Bee body | Agriculture | - | - | |
| Forest | - | - | ||
| Home garden | - | 2.9 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchori, D.; Rizali, A.; Priawandiputra, W.; Sartiami, D.; Johannis, M. Population Growth and Insecticide Residues of Honey Bees in Tropical Agricultural Landscapes. Diversity 2020, 12, 1. https://doi.org/10.3390/d12010001
Buchori D, Rizali A, Priawandiputra W, Sartiami D, Johannis M. Population Growth and Insecticide Residues of Honey Bees in Tropical Agricultural Landscapes. Diversity. 2020; 12(1):1. https://doi.org/10.3390/d12010001
Chicago/Turabian StyleBuchori, Damayanti, Akhmad Rizali, Windra Priawandiputra, Dewi Sartiami, and Midzon Johannis. 2020. "Population Growth and Insecticide Residues of Honey Bees in Tropical Agricultural Landscapes" Diversity 12, no. 1: 1. https://doi.org/10.3390/d12010001
APA StyleBuchori, D., Rizali, A., Priawandiputra, W., Sartiami, D., & Johannis, M. (2020). Population Growth and Insecticide Residues of Honey Bees in Tropical Agricultural Landscapes. Diversity, 12(1), 1. https://doi.org/10.3390/d12010001







