Role of Reef-Building, Ecosystem Engineering Polychaetes in Shallow Water Ecosystems
Abstract
1. Introduction
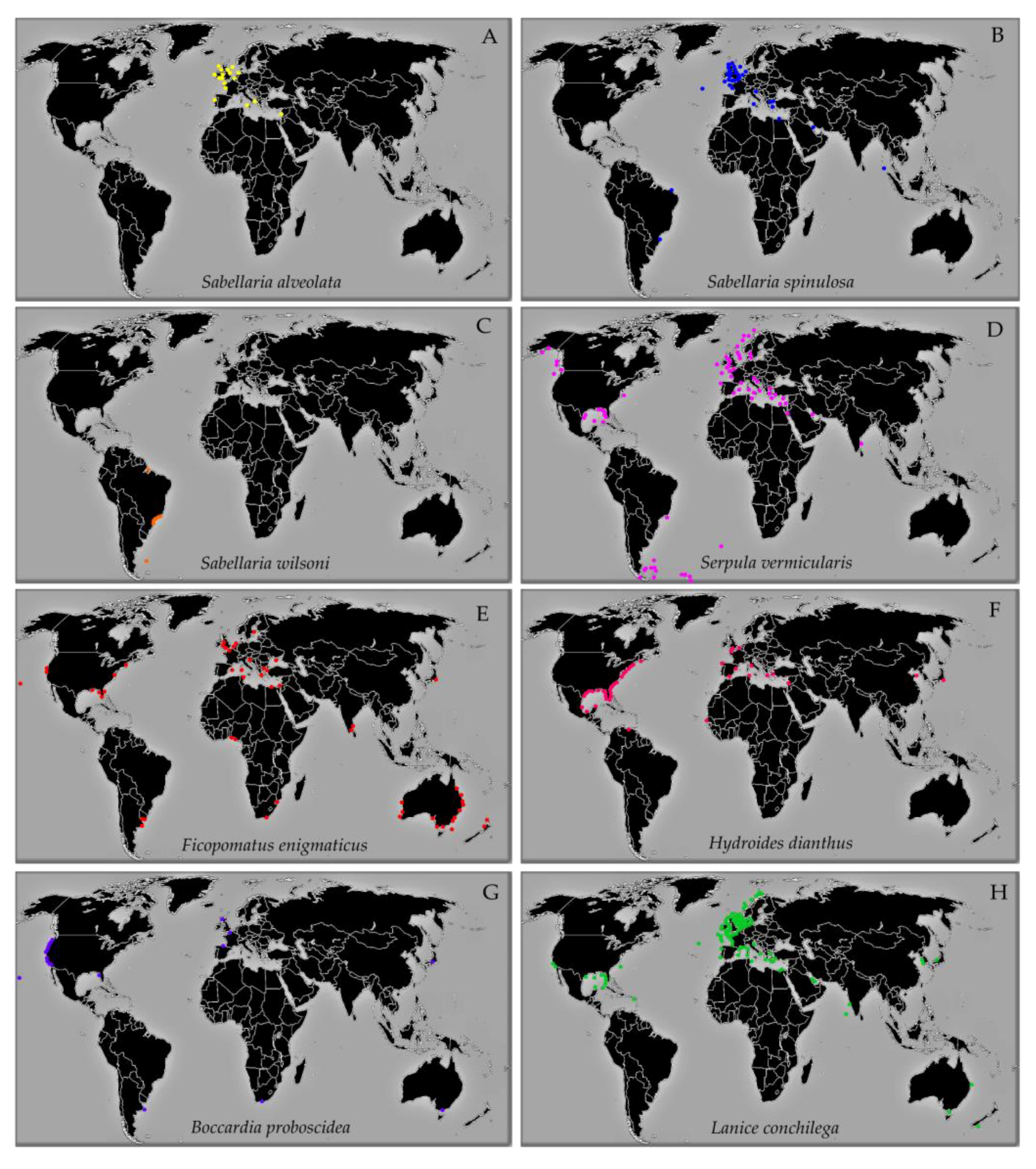
RBP as Ecosystem Engineers. Geographic Distribution, Habitat, Size, and Local Densities
2. Engineering Impact by RBP
2.1. Reefs as Living Space
2.2. Sediment Mediated Effects
2.3. Effects Mediated by Filter Feeding
2.4. Biodeposition
2.5. Extended Engineering Influences
3. Effect of the RBP on Ecosystem Services
3.1. Regulating Services
3.2. Cultural Services
3.3. Supporting Services
4. Synthesis and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Power, M.E. Ecosystem engineering by organisms: Why semantics matters Reply from M. Power. Trends Ecol. Evol. 1997, 12, 275–276. [Google Scholar] [CrossRef]
- Wright, J.P.; Jones, C.G. The Concept of Organisms as Ecosystem Engineers Ten Years On: Progress, Limitations, and Challenges. BioScience 2006, 56, 203–209. [Google Scholar] [CrossRef]
- Jones, C.; Lawton, J.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Jones, C.; Lawton, J.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Jones, C.G.; Gutiérrez, J.L.; Byers, J.E.; Crooks, J.A.; Lambrinos, J.G.; Talley, T.S. A framework for understanding physical ecosystem engineering by organisms. Oikos 2010, 119, 1862–1869. [Google Scholar] [CrossRef]
- Gutierrez, J.L.; Jones, C.G.C.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Lambrinos, J.G.; Bando, K.J. Habitat modification inhibits conspecific seedling recruitment in populations of an invasive ecosystem engineer. Biol. Invasions 2008, 10, 729–741. [Google Scholar] [CrossRef]
- Angelini, C.; Silliman, B.R. Secondary foundation species as drivers of trophic and functional diversity: Evidence from a tree–epiphyte system. Ecology 2014, 95, 185–196. [Google Scholar] [CrossRef]
- Fanjul, E.; Escapa, M.; Montemayor, D.; Addino, M.; Alvarez, M.F.; Grela, M.A.; Iribarne, O. Effect of crab bioturbation on organic matter processing in South West Atlantic intertidal sediments. J. Sea Res. 2015, 95, 206–216. [Google Scholar] [CrossRef]
- Wright, J.P.; Jones, C.G.; Flecker, A.S. An ecosystem engineer, the beaver, increases species richness at the landscape scale. Evolution 2002, 96–101. [Google Scholar] [CrossRef]
- Crooks, J. Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 2002, 2, 153–166. [Google Scholar] [CrossRef]
- van de Koppel, J.; van der Heide, T.; Altieri, A.H.; Eriksson, B.K.; Bouma, T.J.; Olff, H.; Silliman, B.R. Long-distance interactions regulate the structure and resilience of coastal ecosystems. Annu. Rev. Mar. Sci. 2015, 7, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Borthagaray, A.I.; Carranza, A. Mussels as ecosystem engineers: Their contribution to species richness in a rocky littoral community. Acta Oecol. 2007, 31, 243–250. [Google Scholar] [CrossRef]
- Wright, J.; Jones, C. Predicting effects of ecosystem engineers on patch-scale species richness from primary productivity. Ecology 2008, 85, 2071–2081. [Google Scholar] [CrossRef]
- Haussmann, N.S.; Louw, M.A.; Lewis, S.; Nicol, K.J.H.; van der Merwe, S.; le Roux, P.C. Ecosystem engineering through aardvark (Orycteropus afer) burrowing: Mechanisms and effects. Ecol. Eng. 2018, 118, 66–72. [Google Scholar] [CrossRef]
- Johnston, C.A.; Naiman, R.J. Aquatic Patch Creation in Relation to Beaver Population Trends. Ecology 1990, 71, 1617–1621. [Google Scholar] [CrossRef]
- Zhang, Q.; Zak, J.C. Effects of Gap Size on Litter Decomposition and Microbial Activity in a Subtropical Forest. Ecology 1995, 76, 2196–2204. [Google Scholar] [CrossRef]
- Froz, J.; Kalčík, J.; Cudlín, P. Accumulation of phosphorus in nests of red wood ants Formica s. str. Ann. Zool. Fenn. 2005, 42, 269–275. [Google Scholar]
- Commito, J.A.; Como, S.; Grupe, B.M.; Dow, W.E. Species diversity in the soft-bottom intertidal zone: Biogenic structure, sediment, and macrofauna across mussel bed spatial scales. J. Exp. Mar. Biol. Ecol. 2008, 366, 70–81. [Google Scholar] [CrossRef]
- Passarelli, C.; Olivier, F.; Paterson, D.M.; Meziane, T.; Hubas, C. Organisms as cooperative ecosystem engineers in intertidal flats. J. Sea Res. 2014, 92, 92–101. [Google Scholar] [CrossRef]
- Bertness, M.D.; Leonard, G.H. The role of positive interactions in communities: Lessons from intertidal habitats. Ecology 1997, 78, 1976–1989. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Iribarne, O. Role of Holocene beds of the stout razor clam Tagelus plebeius in structuring present benthic communities. Mar. Ecol. Prog. Ser. 1999, 185, 213–228. [Google Scholar] [CrossRef]
- Escapa, M.; Isaacch, J.P.; Daleo, P.; Iribarne, O.; Borges, M.; Santos, E.P.D.; Gagliardini, D.A.; Lasta, M. The distribution and ecological effects of the introduced Pacific oyster Crassostrea gigas (Thunberg, 1793) in Northern Patagonia. J. Shell. Res. 2004, 23, 765–772. [Google Scholar]
- Ruesink, J.L.; Lenihan, H.S.; Trimble, A.C.; Heiman, K.W.; Micheli, F.; Byers, J.E.; Kay, M.C. Introduction of non-native oysters: Ecosystem effects and restoration implications. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 643–689. [Google Scholar] [CrossRef]
- Berkenbusch, K.; Rowden, A.A. An examination of the spatial and temporal generality of the influence of ecosystem engineers on the composition of associated assemblages. Aquat. Ecol. 2007, 41, 129–147. [Google Scholar] [CrossRef]
- Chapman, N.D.; Moore, C.G.; Harries, D.B.; Lyndon, A.R. The community associated with biogenic reefs formed by the polychaete, Serpula vermicularis. J. Mar. Biol. Assoc. U. K. 2012, 92, 679–685. [Google Scholar] [CrossRef]
- Vitousek, P.M.P.M. Biological invasions and ecosystem processes: Towards an integration of population biology and ecosystem studies. Oikos 1990, 57, 7–13. [Google Scholar] [CrossRef]
- Petersen, B.; Exo, K.K.; Klaus-Michael, E.; Petersenl, B.; Exo, K.K. Predation of waders and gulls on Lanice conchilega tidal flats in the Wadden Sea. Mar. Ecol. Prog. Ser. 1999, 178, 229–240. [Google Scholar] [CrossRef]
- Bruschetti, M.; Bazterrica, C.; Luppi, T.; Iribarne, O. An invasive intertidal reef-forming polychaete affect habitat use and feeding behavior of migratory and locals birds in a SW Atlantic coastal lagoon. J. Exp. Mar. Biol. Ecol. 2009, 375, 76–83. [Google Scholar] [CrossRef]
- Duarte, C.M.; Chiscano, C.L. Seagrass biomass and production: A reassessment. Aquat. Bot. 1999, 65, 159–174. [Google Scholar] [CrossRef]
- Schwindt, E.; Iribarne, O.O.; Isla, F.I. Physical effects of an invading reef-building polychaete on an Argentinean estuarine environment. Estuar. Coast. Shelf Sci. 2004, 59, 109–120. [Google Scholar] [CrossRef]
- Jaubet, M.L.; de los Ángeles Sánchez, M.; Rivero, M.S.; Garaffo, G.V.; Vallarino, E.A.; Elías, R. Intertidal biogenic reefs built by the polychaete Boccardia proboscidea in sewage-impacted areas of Argentina, SW Atlantic. Mar. Ecol. 2011, 32, 188–197. [Google Scholar] [CrossRef]
- Dubois, S.; Commito, J.A.; Olivier, F.; Retière, C.; Retiere, C. Effects of epibionts on Sabellaria alveolata (L.) biogenic reefs and their associated fauna in the Bay of Mont Saint-Michel. Estuar. Coast. Shelf Sci. 2006, 68, 635–646. [Google Scholar] [CrossRef]
- Schwindt, E.; Bortolus, A.; Iribarne, O.O. Invasion of a reef-builder polychaete: Direct and indirect impacts on the native benthic community structure. Biol. Invasions 2001, 3, 137–149. [Google Scholar] [CrossRef]
- Zühlke, R. Polychaete tubes create ephemeral community patterns: Lanice conchilega (Pallas, 1766) associations studied over six years. J. Sea Res. 2001, 46, 261–272. [Google Scholar] [CrossRef]
- Elías, R.; Jaubet, M.L.; Llanos, E.N.; Sanchez, M.A.; Rivero, M.S.; Garaffo, G.V.; Sandrini-Neto, L. Effect of the invader Boccardia proboscidea (Polychaeta: Spionidae) on richness, diversity and structure of SW Atlantic epilithic intertidal community. Mar. Pollut. Bull. 2015, 91, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Dubois, S.F.; Desroy, N.; Fournier, J. Interplay between abiotic factors and species assemblages mediated by the ecosystem engineer Sabellaria alveolata (Annelida: Polychaeta). Estuar. Coast. Shelf Sci. 2018, 200, 1–18. [Google Scholar] [CrossRef]
- Sousa, R.; Gutiérrez, J.; Aldridge, D. Non-indigenous invasive bivalves as ecosystem engineers. Biol. Invasions 2009, 11, 2367–2385. [Google Scholar] [CrossRef]
- Dubois, S.; Barillé, L.; Cognie, B. Feeding response of the polychaete Sabellaria alveolata (Sabellariidae) to changes in seston concentration. J. Exp. Mar. Biol. Ecol. 2009, 376, 94–101. [Google Scholar] [CrossRef]
- Cugier, P.; Struski, C.; Blanchard, M.; Mazurié, J.; Pouvreau, S.; Olivier, F.; Trigui, J.R.J.R.; Thiébaut, E. Assessing the role of benthic filter feeders on phytoplankton production in a shellfish farming site: Mont Saint Michel Bay, France. J. Mar. Syst. 2010, 82, 21–34. [Google Scholar] [CrossRef]
- Wilson, D.P. Sabellaria Colonies at Duckpool, North Cornwall, 1961–1970. J. Mar. Biol. Assoc. U. K. 1971, 51, 509–580. [Google Scholar] [CrossRef]
- Lecornu, B.; Schlund, E.; Basuyaux, O.; Cantat, O.; Dauvin, J.-C. Dynamics (from 2010–2011 to 2014) of Sabellaria alveolata reefs on the western coast of Cotentin (English Channel, France). Reg. Stud. Mar. Sci. 2016, 8, 157–169. [Google Scholar] [CrossRef]
- Bonifazi, A.; Ventura, D.; Mancini, E. Sabellaria reefs as reservoirs of preferential species: The case of Eulalia ornata Saint-Joseph, 1888 (Annelida: Phyllodocidae). Mar. Freshw. Res. 2018, 69, 1635–1640. [Google Scholar] [CrossRef]
- Stone, R.; Callaway, R.; Bull, J.C. Are biodiversity offsetting targets of ecological equivalence feasible for biogenic reef habitats? Ocean Coast. Manag. 2019, 177, 97–111. [Google Scholar] [CrossRef]
- Gravina, M.F.; Cardone, F.; Bonifazi, A.; Bertrandino, M.S.; Chimienti, G.; Longo, C.; Marzano, C.N.; Moretti, M.; Lisco, S.; Moretti, V.; et al. Sabellaria spinulosa (Polychaeta, Annelida) reefs in the Mediterranean sea: Habitat mapping, dynamics and associated fauna for conservation management. Estuar. Coast. Shelf Sci. 2018, 200, 248–257. [Google Scholar] [CrossRef]
- van der Reijden, K.J.; Koop, L.; O’Flynn, S.; Garcia, S.; Bos, O.; van Sluis, C.; Maaholm, D.J.; Herman, P.M.J.; Simons, D.G.; Olff, H.; et al. Discovery of Sabellaria spinulosa reefs in an intensively fished area of the Dutch continental shelf, North sea. J. Sea Res. 2019, 144, 85–94. [Google Scholar] [CrossRef]
- Lisco, S.; Moretti, M.; Moretti, V.; Cardone, F.; Corriero, G.; Longo, C. Sedimentological features of Sabellaria spinulosa biocontructions. Mar. Pet. Geol. 2017, 87, 203–212. [Google Scholar] [CrossRef]
- Ataide, M.B.; Venekey, V.; Filho, J.S.R.; dos Santos, P.J.P. Sandy reefs of Sabellaria wilsoni (Polychaeta: Sabellariidae) as ecosystem engineers for meiofauna in the Amazon coastal region, Brazil. Mar. Biodivers. 2014, 44, 403–413. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, T.; Wang, L. The biogenic reefs formed by the alien polychaete Hydroides dianthus (Serpulidae, Annelida) favor the polyp stage of Aurelia coerulea (Cnidaria, Scyphozoa) in a coastal artificial lake. Mar. Pollut. Bull. 2018, 129, 86–91. [Google Scholar] [CrossRef]
- Davies, B.R.; Stuart, V.; de Villiers, M. The filtration activity of a serpulid polychaete population (Ficopomatus enigmaticus (Fauvel) and its effects on water quality in a coastal marina. Estuar. Coast. Shelf Sci. 1989, 29, 613–620. [Google Scholar] [CrossRef]
- Bruschetti, M.; Luppi, T.; Fanjul, E.; Rosenthal, A.; Iribarne, O. Grazing effect of the invasive reef-forming polychaete Ficopomatus enigmaticus (Fauvel) on phytoplankton biomass in a SW Atlantic coastal lagoon. J. Exp. Mar. Biol. Ecol. 2008, 354, 212–219. [Google Scholar] [CrossRef]
- Pan, J.; Marcoval, M.A. Top-down effects of an exotic serpulid polychaete on natural plankton assemblage of estuarine and brackish systems in the SW Atlantic. J. Coast. Res. 2014, 30, 1226–1235. [Google Scholar] [CrossRef]
- Heiman, K.; Vidargas, N.; Micheli, F. Non-native habitat as home for non-native species: Comparison of communities associated with invasive tubeworm and native oyster reefs. Aquat. Biol. 2008, 2, 47–56. [Google Scholar] [CrossRef]
- Bazterrica, M.C.; Botto, F.; Iribarne, O.O. Effects of an invasive reef-building polychaete on the biomass and composition of estuarine macroalgal assemblages. Biol. Invasions 2012, 14, 765–777. [Google Scholar] [CrossRef]
- Bruschetti, M.; Bazterrica, C.; Fanjul, E.; Luppi, T.; Iribarne, O.; Fanjul, M.E.; Luppi, T.; Iribarne, O. Effect of biodeposition of an invasive polychaete on organic matter content and productivity of the sediment in a coastal lagoon. J. Sea Res. 2011, 66, 20–28. [Google Scholar] [CrossRef]
- Garaffo, G.V.; Jaubet, M.L.; Sánchez, M.D.L.Á.; Rivero, M.S.; Vallarino, E.A.; Elías, R. Sewage-induced polychaete reefs in a SW Atlantic shore: Rapid response to small-scale disturbance. Mar. Ecol. 2012, 33, 272–279. [Google Scholar] [CrossRef]
- Jaubet, M.L.; Garaffo, G.V.; Sánchez, M.A.; Elías, R. Reef-forming polychaetes outcompetes ecosystem engineering mussels. Mar. Pollut. Bull. 2013, 71, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.; Desroy, N.; Dubois, S.F.; Fournier, J.; Frost, M.; Godet, L.; Hendrick, V.J.; Rabaut, M. Ephemeral bio-engineers or reef-building polychaetes: How stable are aggregations of the tube worm Lanice conchilega (Pallas, 1766)? Integr. Comp. Biol. 2010, 50, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Rabaut, M.; Van de Moortel, L.; Vincx, M.; Degraer, S. Biogenic reefs as structuring factor in Pleuronectes platessa (Plaice) nursery. J. Sea Res. 2010, 64, 102–106. [Google Scholar] [CrossRef]
- Alves, R.M.S.; Vanaverbeke, J.; Bouma, T.J.; Guarini, J.-M.; Vincx, M.; Van Colen, C. Effects of temporal fluctuation in population processes of intertidal Lanice conchilega (Pallas, 1766) aggregations on its ecosystem engineering. Estuar. Coast. Shelf Sci. 2017, 188, 88–98. [Google Scholar] [CrossRef]
- Denis, L.; Desroy, N.; Ropert, M. Ambient flow velocity and resulting clearance rates of the terebellid polychaete Lanice conchilega (Pallas, 1766). J. Sea Res. 2007, 58, 209–219. [Google Scholar] [CrossRef][Green Version]
- Schwindt, E.; De Francesco, C.G.; Iribarne, O.O. Individual and reef growth of the invasive reef-building polychaete Ficopomatus Enigmaticus in a south-western Atlantic coastal lagoon. J. Mar. Biol. Assoc. U. K. 2004, 84, 987–993. [Google Scholar] [CrossRef]
- McQuaid, K.A.; Griffiths, C.L. Alien reef-building polychaete drives long-term changes in invertebrate biomass and diversity in a small, urban estuary. Estuar. Coast. Shelf Sci. 2014, 138, 101–106. [Google Scholar] [CrossRef]
- Van Hoey, G.; Guilini, K.; Rabaut, M.; Vincx, M.; Degraer, S. Ecological implications of the presence of the tube-building polychaete Lanice conchilega on soft-bottom benthic ecosystems. Mar. Biol. 2008, 154, 1009–1019. [Google Scholar] [CrossRef]
- Chouikh, N.; Gillet, P.; Langston, W.J.; Cheggour, M.; Maarouf, A.; Mouabad, A. first investigation of the composition and spatial distribution of polychaete feeding guilds from essaouira protected coastal area (Atlantic coast of Morocco). Appl. Ecol. Environ. Res. 2019, 17, 3231–3249. [Google Scholar] [CrossRef]
- Desroy, N.; Dubois, S.F.; Fournier, J.; Ricquiers, L.; Mao, P.L.; Guerin, L.; Gerla, D.; Rougerie, M.; Legendre, A. The conservation status of Sabellaria alveolata (L.) (Polychaeta: Sabellariidae) reefs in the bay of Mont-Saint-Michel. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 462–471. [Google Scholar] [CrossRef]
- Hove, H.T.; Weerdenburg, J. A generic revision of the brackish-water serpulid Ficopomatus. ReVision 1978, 96–120. [Google Scholar]
- Obenat, S.S.M.; Pezzani, S.E.S. Life cycle and population structure of the polychaete Ficopomatus enigmaticus (Serpulidae) in Mar Chiquita coastal lagoon, Argentina. Estuaries 1994, 17, 263. [Google Scholar] [CrossRef]
- Porta, B.L.; Nicoletti, L. Sabellaria alveolata (Linnaeus) reefs in the central Tyrrhenian sea (Italy) and associated polychaete fauna. Zoosymposia 2009, 2, 527–536. [Google Scholar]
- Dubois, S.; Retière, C.; Olivier, F. Biodiversity associated with Sabellaria alveolata (Polychaeta: Sabellariidae) reefs: Effects of human disturbances. J. Mar. Biol. Assoc. U. K. 2002, 82, 817–826. [Google Scholar] [CrossRef]
- Gruet, Y. Spatio-temporal changes of sabellarian reefs built by the sedentary polychaete Sabellaria alveolata (Linné). Mar. Ecol. 1986, 7, 303–319. [Google Scholar] [CrossRef]
- Bonifazi, A.; Lezzi, M.; Ventura, D.; Lisco, S.; Cardone, F.; Gravina, M.F. Macrofaunal biodiversity associated with different developmental phases of a threatened Mediterranean Sabellaria alveolata (Linnaeus, 1767) reef. Mar. Environ. Res. 2019, 145, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A. Sabellaria alveolata Honeycomb worm. In Marine Life Information Network: Biology and Sensitivity Key Information Reviews; Tyler-Walters, H., Hiscock, K., Eds.; Marine Biological Association of the United Kingdom: Plymouth, UK, 2008; pp. 1–14. [Google Scholar]
- Aviz, D.; Silva, R.F.D.; Filho, J.S.R. Sabellaria wilsoni (Polychaeta: Sabellariidae): An ecosystem engineer and promoter of zoobenthos diversity in the Brazilian Amazon coast. J. Mar. Biol. Assoc. U. K. 2019, 99, 1099–1109. [Google Scholar] [CrossRef]
- Moore, C.G.; Bates, C.R.; Mair, J.M.; Saunders, G.R.; Harries, D.B.; Lyndon, A.R. Mapping serpulid worm reefs (Polychaeta: Serpulidae) for conservation management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 226–236. [Google Scholar] [CrossRef]
- Read, G.; Gordon, D. Adventive occurrence of the fouling serpulid Ficopomatus enigmaticus (Polychaeta) in New Zealand. N. Z. J. Mar. Freshw. Res. 1991, 25, 269–273. [Google Scholar] [CrossRef]
- Asakura, A. Recent introductions of marine benthos into Tokyo Bay (Review): Process of invasion into an urban ecosystem with discussion on the factors inducing their successful introduction. J. Nat. Hist. Mus. Inst. Chiba 1992, 2, 1–14. [Google Scholar]
- Thorp, C.H. Population variation in Ficopomatus enigmaticus (Fauvel) (Polychaeta, Serpulidae) in a brackish water millpond at Emsworth, West Sussex, U.K. Mém Mus Natl Hist Nat Sér Zool 1994, 162, 585–591. [Google Scholar]
- Bianchi, C.N.; Morri, C. Ficopomatus ’reefs in the Po river delta (Northern Adriatic): Their constructional dynamics, biology, and influence on the brackish-water biota. Mar. Ecol. 1996, 17, 51–66. [Google Scholar] [CrossRef]
- Borthagaray, A.I.; Clemente, J.M.; Boccardi, L.; Brugnoli, E. Impacto potencial de invasión de Ficopomatus enigmaticus (Fauvel) (Polychaeta: Serpulidae) en la Laguna de Rocha, Uruguay. Pan-Am. J. 2006, 1, 57–65. [Google Scholar]
- Link, H.; Nishi, E.; Tanaka, K.; Bastida-Zavala, R.; Kupriyanova, E.; Yamakita, T. Hydroides dianthus (Polychaeta: Serpulidae), an alien species introduced into Tokyo Bay, Japan. Mar. Biodivers. Rec. 2009, 1–6. [Google Scholar] [CrossRef]
- Toonen, R.J.; Pawlik, J.R. Foundations of gregariousness: A dispersal polymorphism among the planktonic larvae of a marine invertebrate. Evolution 2001, 55, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Hartman, O. Some contributions to the biology and life history of Spionidae from California. Allan Hancock Pac. Exped. 1941, 7, 289–323. [Google Scholar]
- Bailey-Brock, J.H. A new record of the polychaete Boccardia proboscidea (Family Spionidae), imported to Hawai’i with oysters! Pac. Sci. 2000, 54, 4. [Google Scholar]
- Sato-Okoshi, W. Polydorid species (Polychaeta: Spionidae) in Japan, with descriptions of morphology, ecology and burrow structure. 2. Non-boring species. J. Mar. Biol. Assoc. U. K. 2000, 80, 443–456. [Google Scholar] [CrossRef]
- Hewitt, C.L.; Campbell, M.L.; Thresher, R.E.; Martin, R.B.; Boyd, S.; Cohen, B.F.; Currie, D.R.; Gomon, M.F.; Keough, M.J.; Lewis, J.A.; et al. Introduced and cryptogenic species in port Phillip bay, Victoria, Australia. Mar. Biol. 2004, 144, 183–202. [Google Scholar]
- Martínez, J.; Adarraga, I.; López, E. New data of the genus Boccardia Carazzi, 1893 (Polychaeta: Spionidae) for the Iberian peninsula and the Atlantic ocean. Boletin Inst. Espanol Oceanogr. 2006, 22, 53–64. [Google Scholar]
- Simon, C.A.; Worsfold, T.M.; Lange, L.; Sterley, J. The genus Boccardia (Polychaeta: Spionidae) associated with mollusc shells on the south coast of South Africa. J. Mar. Biol. Assoc. U. K. 2010, 90, 585–598. [Google Scholar] [CrossRef]
- Becherucci, M.E.; Jaubet, M.L.; Saracho Bottero, M.A.; Llanos, E.N.; Elías, R.; Garaffo, G.V. Rapid sewage pollution assessment by means of the coverage of epilithic taxa in a coastal area in the SW Atlantic. Sci. Total Environ. 2018, 628–629, 826–834. [Google Scholar] [CrossRef]
- Rabaut, M.; Vincx, M.; Degraer, S. Do Lanice conchilega (sandmason) aggregations classify as reefs? Quantifying habitat modifying effects. Helgol. Mar. Res. 2009, 63, 37–46. [Google Scholar] [CrossRef]
- De Smet, B.; D’Hondt, A.S.; Verhelst, P.; Fournier, J.; Godet, L.; Desroy, N.; Rabaut, M.; Vincx, M.; Vanaverbeke, J. Biogenic reefs affect multiple components of intertidal soft-bottom benthic assemblages: The Lanice conchilega case study. Estuar. Coast. Shelf Sci. 2015, 152, 44–55. [Google Scholar] [CrossRef]
- Dodd, J.; Baxter, L.; Hughes, D.J. Mapping Serpula vermicularis (Polychaeta: Serpulidae) aggregations in Loch Teacuis, western Scotland, a new record. Mar. Biol. Res. 2009, 5, 200–205. [Google Scholar] [CrossRef]
- Bazterrica, M.C.; Alvarez, M.F.; Bruschetti, C.M.; Hidalgo, F.J.; Fanjul, M.E.; Iribarne, O.; Botto, F. Factors controlling macroalgae assemblages in a Southwest Atlantic coastal lagoon modified by an invading reef forming polychaete. J. Exp. Mar. Biol. Ecol. 2013, 443, 169–177. [Google Scholar] [CrossRef]
- Luppi, T.A.; Bas, C.C. The role of the invasive polychaete Ficopomatus enigmaticus Fauvel 1923 (Polychaeta: Serpulidae) reefs in the recruitment of Cyrtograpsus angulatus Dana 1851 (Brachyura: Grapsidae), in the Mar Chiquita coastal lagoon, Argentin. Cienc. Mar. 2002, 28, 319–330. [Google Scholar] [CrossRef]
- Obenat, S.; Spivak, E.; Garrido, L. Life history and reproductive biology of the invasive amphipod Melita palmata (Amphipoda: Melitidae) in the Mar Chiquita coastal lagoon, Argentina. J. Mar. Biol. Assoc. U. K. 2006, 86, 1381. [Google Scholar] [CrossRef]
- Rabaut, M.; Guilini, K.; Van Hoey, G.; Vincx, M.; Degraer, S. A bio-engineered soft-bottom environment: The impact of Lanice conchilega on the benthic species-specific densities and community structure. Estuar. Coast. Shelf Sci. 2007, 75, 525–536. [Google Scholar] [CrossRef]
- Badano, E.E.I.; Villarroel, E.; Bustamante, R.R.O.; Marquet, P.A.; Cavieres, L.A. Ecosystem engineering facilitates invasions by exotic plants in high-Andean ecosystems. J. Ecol. 2007, 95, 682–688. [Google Scholar] [CrossRef]
- Llanos, E.N.; Becherucci, M.E.; Garaffo, G.V.; Vallarino, E.A. A shift of ecosystem engineers during the succession of an intertidal benthic community associated with natural and anthropogenic disturbances. Reg. Stud. Mar. Sci. 2019, 31, 100754. [Google Scholar] [CrossRef]
- Bouma, T.J.; De Vries, M.B.; Herman, P.M.J. Comparing ecosystem engineering efficiency of two plant species with contrasting growth strategies. Ecology 2010, 91, 2696–2704. [Google Scholar] [CrossRef]
- Newell, R.I.E.; Koch, E.W. Modeling seagrass density and distribution in response to changes in turbidity stemming from bivalve filtration and seagrass sediment stabilization. Estuaries 2004, 27, 793–806. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Byers, J.E.; Arkema, K.K.; Berkenbusch, K.; Commito, J.A.; Duarte, C.M.; Hacker, S.D.; Lambrinos, J.G.; Hendriks, I.E.; et al. 7.04—Physical ecosystem engineers and the functioning of estuaries and coasts. In Treatise on estuarine and coastal science; Wolanski, E., McLusky, D., Eds.; Academic Press: Waltham, MA, USA, 2011; pp. 53–81. [Google Scholar]
- Bailey-Brock, J.H. Sediment trapping by Chaetopterid polychaetes on a Hawaiian fringing reef. J. Mar. Res. 1979, 37, 643–656. [Google Scholar]
- Murray, J.M.H.; Meadows, A.; Meadows, P.S. Biogeomorphological implications of microscale interactions between sediment geotechnics and marine benthos: A review. Geomorphology 2002, 47, 15–30. [Google Scholar] [CrossRef]
- Braeckman, U.; Rabaut, M.; Vanaverbeke, J.; Degraer, S.; Vincx, M. Protecting the commons: The use of subtidal ecosystem engineers in marine management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 275–286. [Google Scholar] [CrossRef]
- Friedrichs, M.; Graf, G.; Springer, B. Skimming flow induced over a simulated polychaete tube lawn at low population densities. Mar. Ecol. Prog. Ser. 2000, 192, 219–228. [Google Scholar] [CrossRef]
- Toupoint, N.; Godet, L.; Fournier, J.; Retière, C.; Olivier, F. Does Manila clam cultivation affect habitats of the engineer species Lanice conchilega (Pallas, 1766)? Mar. Pollut. Bull. 2008, 56, 1429–1438. [Google Scholar] [CrossRef]
- Dame, R.; Spurrier, J.; Wolaver, T. Carbon, nitrogen and phosphorus processing by an oyster reef. Mar. Ecol. Prog. Ser. 1989, 54, 249–256. [Google Scholar] [CrossRef]
- Zhu, B.; Fitzgerald, D.G.; Mayer, C.M.; Rudstam, L.G.; Mills, E.L. Alteration of ecosystem function by zebra mussels in Oneida lake: Impacts on submerged macrophytes. Ecosystems 2006, 9, 1017–1028. [Google Scholar] [CrossRef]
- Jordana, E.; Charles, F.; Grémare, A.; Amouroux, J.-M.; Chrétiennot-Dinet, M.-J. Food sources, ingestion and absorption in the suspension-feeding polychaete, Ditrupa arietina (O.F. Müller). J. Exp. Mar. Biol. Ecol. 2001, 266, 219–236. [Google Scholar] [CrossRef]
- Dubois, S.; Barillé, L.; Retière, C. Efficiency of particle retention and clearance rate in the polychaete Sabellaria alveolata L. Comptes Rendus Biol. 2003, 326, 413–421. [Google Scholar] [CrossRef]
- Riisgård, H. Efficiency of particle retention and filtration rate in 6 species of Northeast American bivalves. Mar. Ecol. Prog. Ser. 1988, 45, 217–223. [Google Scholar] [CrossRef]
- Riisgård, H. On measurement of filtration rate in bivalves-the stony road to reliable data: Review and interpretation. Mar. Ecol. Prog. Ser. 2001, 211, 275–291. [Google Scholar] [CrossRef]
- Dame, R.; Prins, T. Bivalve carrying capacity in coastal ecosystems. Aquat. Ecol. 1997, 31, 409–421. [Google Scholar] [CrossRef]
- Bruschetti, C.M.; Addino, M.; Luppi, T.; Iribarne, O. Effects of nutrient enrichment and grazing by an invasive filter feeder on phytoplankton biomass in a South West Atlantic coastal lagoon. Biol. Invasions 2018, 20, 2245–2256. [Google Scholar] [CrossRef]
- Collin, A.; Dubois, S.; Ramambason, C.; Etienne, S. Very high-resolution mapping of emerging biogenic reefs using airborne optical imagery and neural network: The honeycomb worm (Sabellaria alveolata) case study. Int. J. Remote Sens. 2018, 39, 5660–5675. [Google Scholar] [CrossRef]
- Stutes, A.; Cebrian, J.; Corcoran, A. Effects of nutrient enrichment and shading on sediment primary production and metabolism in eutrophic estuaries. Mar. Ecol. Prog. Ser. 2006, 312, 29–43. [Google Scholar] [CrossRef]
- Pratt, D.R.; Pilditch, C.A.; Lohrer, A.M.; Thrush, S.F. The effects of short-term increases in turbidity on sandflat microphytobenthic productivity and nutrient fluxes. J. Sea Res. 2014, 92, 170–177. [Google Scholar] [CrossRef]
- Forster, R.; Créach, V.; Sabbe, K.; Vyverman, W.; Stal, L. Biodiversity–Ecosystem function relationship in microphytobenthic diatoms of the Westerschelde estuary. Mar. Ecol. Prog. Ser. 2006, 311, 191–201. [Google Scholar] [CrossRef]
- Moncreiff, C.A.; Sullivan, M.J.; Daehnick, A.E. Primary production dynamics in seagrass beds of Mississippi Sound: The contributions of seagrass, epiphytic algae, sand microflora, and phytoplankton. Mar. Ecol. Prog. Ser. 1992, 87, 161–171. [Google Scholar] [CrossRef]
- MacIntyre, H.; Geider, R.; Miller, D. Microphytobenthos: The ecological role of the “secret garden” of unvegetated, shallow-water marine habitats. I. Distribution, abundance and primary production. Estuaries Coasts 1996, 19. [Google Scholar] [CrossRef]
- Botto, F.; Valiela, I.; Iribarne, O.; Martinetto, P.; Alberti, J. Impact of burrowing crabs on C and N sources, control, and transformations in sediments and food webs of SW Atlantic estuaries. Mar. Ecol. Prog. Ser. 2005, 293, 155–164. [Google Scholar] [CrossRef]
- Martinetto, P.; Valinas, M.; Palomo, G.; Iribarne, O.; Valiñas, M.; Palomo, G.; Iribarne, O. Negative interactions between two SW Atlantic intertidal crabs in soft-bottom habitats. Mar. Biol. 2007, 151, 1479–1490. [Google Scholar] [CrossRef]
- Wotton, R.S.; Malmqvist, B. Feces in aquatic ecosystems feeding animals transform organic matter into fecal pellets, which sink or are transported horizontally by currents; these fluxes relocate organic matter in aquatic ecosystems. BioScience 2001, 51, 537–544. [Google Scholar] [CrossRef]
- Kooijman, S. Pseudo-faeces production in bivalves. J. Sea Res. 2006, 56, 103–106. [Google Scholar] [CrossRef]
- Giles, H.; Pilditch, C. Effects of diet on sinking rates and erosion thresholds of mussel Perna canaliculus biodeposits. Mar. Ecol. Prog. Ser. 2004, 282, 205–219. [Google Scholar] [CrossRef]
- Kautsky, N.; Evans, S. Role of biodeposition by Mytilus edulis in the circulation of matter and nutrients in a Baltic coastal ecosystem. Mar. Ecol. Prog. Ser. 1987, 38, 201–212. [Google Scholar] [CrossRef]
- Dubois, S.; Barillé, L.; Cognie, B.; Beninger, P.P.G. Particle capture and processing mechanisms in Sabellaria alveolata (Polychaeta: Sabellariidae). Mar. Ecol. Prog. Ser. 2005, 301, 159–171. [Google Scholar] [CrossRef]
- Turner, R.K.; Georgiou, S.; Fisher, B. Valuing Ecosystem Services: The Case of Multi-Functional Wetlands; Routledge: London, UK, 2008; ISBN 978-1-84407-615-4. [Google Scholar]
- Gutierrez, J.L. Modification of habitat quality by non-native species Impact of Biological Invasions on Ecosystem Services. In Impact of Biological Invasions on Ecosystem Services; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 33–47. [Google Scholar]
- Masselink, G.; Russell, P. Impacts of climate change on coastal erosion. MCCIP Sci. Rev. 2013, 71–86. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Ross Robertson, D.; Kirtley, D.W. Roles for worms in reef-building. Coral Reefs 1998, 17, 120. [Google Scholar] [CrossRef]
- Grabowski, J.H.; Brumbaugh, R.D.; Conrad, R.F.; Keeler, A.G.; Opaluch, J.J.; Peterson, C.H.; Piehler, M.F.; Powers, S.P.; Smyth, A.R. Economic valuation of ecosystem services provided by oyster reefs. BioScience 2012, 62, 900–909. [Google Scholar] [CrossRef]
- Gracia, A.; Rangel-Buitrago, N.; Oakley, J.A.; Williams, A.T. Use of ecosystems in coastal erosion management. Ocean Coast. Manag. 2018, 156, 277–289. [Google Scholar] [CrossRef]
- Fenchel, T. Marine Plankton Food Chains. Annu. Rev. Ecol. Syst. 1988, 19, 19–38. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Zhang, T.; Liu, S.; Zhang, S.; Liu, Q.; Xiang, J.; Zhang, F. Influence of filtering and biodeposition by the cultured scallop Chlamys farreri on benthic-pelagic coupling in a eutrophic bay in China. Mar. Ecol. Prog. Ser. 2006, 317, 127–141. [Google Scholar] [CrossRef][Green Version]

| Species | Depth/Area | Activity | Effect | References | Study Site |
|---|---|---|---|---|---|
| Sabellaria alveolata (Sabellidae) | Intertidal and subtidal (0–10 m) | Filter-feeding activity | Control of primary production | [39,40] | Mont-Saint-Michel (France) |
| Build reefs and provide complex l habitat | Increase infaunal abundance and biodiversity | [33,41,42,43,44] | Mont-Saint-Michel (France) Central Tyrrhenian Sea (Italy) English Channel, (France) Bristol Channel (Wales, UK) North Cornwall (England) | ||
| Sabellaria spinulosa (Sabellidae) | Low intertidal zone and subtidal (0–10 m) | Form tubes with terrigenous particles and can build reefs in subtidal areas | Increase surface heterogeneity of the bottom and enlarges landscape complexity | [45,46] | Mediterranean Sea; Adriatic coast (Italy) |
| Acts as a physical barrier for storm waves and as a storage of sandy sediments, mitigating coastal erosion | [47] | Adriatic Sea (Italy) | |||
| Sabellaria wilsoni (Sabellidae) | Coastal intertidal, shallow estuaries and continental shelf (0–100 m) | Build reefs and provide substrate | Increase density, richness and diversity of fauna, and sediment organic matter | [48] | Amazon coastal region (Brazil). |
| Serpula vermicularis (Serpulidae) | Intertidal to sublittoral zone (0–20 m) | Increase of substratum complexity forming small reefs | Increase diversity and abundance of infauna | [26] | Loch Creran (Scotland) |
| Hydroides dianthus (Serpulidae) | Shallow estuaries | Create substrate | Provide benthic habitat suitable for the settlement of jellyfish polyps | [49] | Northern Yellow Sea (China) |
| Ficopomatus enigmaticus (Serpulidae) | Intertidal and subtidal of shallow estuaries, ports and marinas (0–4 m) | Filter-feeding activity | Control of phytoplankton biomass and turbidity | [50,51,52] | Mar Chiquita lagoon (Argentina), Zandvlei (South Africa). |
| Supply of substrate | Provide benthic habits suitable for the settlement of amphipods, crabs, oysters, gastropods, polychaetes and macro-algae | [34,53,54] | Mar Chiquita lagoon (Argentina), Californian estuary, Elkhorn Slough (EEUU). | ||
| Augments the substrate and the number of prey for shorebirds | [29] | Mar Chiquita lagoon (Argentina). | |||
| Biodeposition: a portion of the material filtrated is rejected to the water as feces or pseudofeces | Increase sedimentary organic matter | [55] | Mar Chiquita lagoon (Argentina) | ||
| Boccardia proboscidea (Spionidae) | Intertidal area. Sandy beaches and stony rocks of consolidate loess (0–1m) | Build ephemeral biogenic reefs in sewage areas | Excludes all sessile fauna and flora in rocky inter-tidal communities | [36,56,57] | Mar del Plata (Argentina) |
| Lanice conchilega (Terebellidae) | From lower intertidal of estuaries and coastal marine (0–1 m) to bathypelagic areas (1900 m) | Build biogenic emergent reefs | Increase refuges for fishes and benthic biodiversity | [58,59,60] | German Wadden sea, Gower peninsula (South Wales), Boulogne-sur-Mer (France) |
| Filter-feeding activity | Control of phytoplankton biomass | [40,61] | Mont-Saint-Michel (France) |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruschetti, M. Role of Reef-Building, Ecosystem Engineering Polychaetes in Shallow Water Ecosystems. Diversity 2019, 11, 168. https://doi.org/10.3390/d11090168
Bruschetti M. Role of Reef-Building, Ecosystem Engineering Polychaetes in Shallow Water Ecosystems. Diversity. 2019; 11(9):168. https://doi.org/10.3390/d11090168
Chicago/Turabian StyleBruschetti, Martín. 2019. "Role of Reef-Building, Ecosystem Engineering Polychaetes in Shallow Water Ecosystems" Diversity 11, no. 9: 168. https://doi.org/10.3390/d11090168
APA StyleBruschetti, M. (2019). Role of Reef-Building, Ecosystem Engineering Polychaetes in Shallow Water Ecosystems. Diversity, 11(9), 168. https://doi.org/10.3390/d11090168




