Abstract
The ecology of an epibiont may depend not only on the dynamics of its biogenic habitat but also on microclimate variation generated within aggregations of its host, a process called physical ecosystem engineering. This study explored variation in the abundance and demography of Membranipora, a suspension-feeding bryozoan, within forests of giant kelp (Macrocystis pyrifera) off the coast of Santa Barbara, California, USA. First, we assessed differences in Membranipora abundance between the edge and interior of kelp forests. The occurrence of Membranipora on kelp blades and its percent cover on occupied blades were higher along forest edges than interiors. Second, we conducted observational studies and field experiments to understand spatial variation in substrate longevity, colony mortality, larval recruitment, and colony growth rates. A higher density of recruits and colonies occurred along forest edges than interiors, suggesting kelp acts like a sieve, whereby larvae settle to edge blades first. Moreover, growth rates along the edge were up to 45% higher than forest interiors. Reduced current speeds, combined with feeding by exterior colonies, may have lowered the uptake of suspended food particles by interior colonies. These results suggest that variation in Membranipora abundance is due in part to differences in colony growth between forest edges and interiors, and not solely the result of recruitment limitation. Our results highlight the importance of ecosystem engineers in influencing the ecological dynamics of epiphytic flora and fauna in marine systems.
1. Introduction
Biogenic habitats increase structural complexity and provide numerous other species with refuge from predation and sites for recruitment and growth [1,2,3,4,5,6,7]. The surfaces of many biogenic structures also serve as a substrate for a variety of epibionts, including plants (e.g., lichens, bromeliads, algae) and animals (e.g., polychaetes, bryozoans, hydroids). Because epibionts require physical support, their demography (i.e., colonization and life span) is linked to the availability and dynamics of the living substrate on which they reside [1,8,9]. In addition to the direct provision of habitat, structure-forming taxa may alter ambient environmental conditions and mediate delivery of resources, a phenomenon described as ecosystem engineering (sensu [10]). Ecosystem engineering by host biogenic habitats may have important consequences for the abundance and demography of epibionts [11,12,13], especially in coastal and marine systems [1,14,15].
Ecosystem engineers are likely to have the greatest influence on ambient environmental conditions and associated species when they occur in large aggregations [10,16]. Such is the case with giant kelp (Macrocystis pyrifera), which forms dense underwater forests along many temperate coasts, including the western shores of North and South America, and sections of South Africa, New Zealand, and Australia [2,17,18]. Giant kelp forests provide habitat for hundreds of species of nearshore fishes and invertebrates [19,20,21,22], including epiphytic suspension-feeders that live on kelp blades [1]. In addition to its vital role as a biogenic habitat, giant kelp exerts a strong influence on environmental conditions, by diminishing light levels (reviewed in [2,22]) and modifying water flow [23,24,25,26], which in turn can influence associated species [27,28,29]. Because the ecology of suspension feeders is closely related to the fluid environment in which they live, the dampening effect of kelp on flow may have consequences for the growth and abundance of epibionts that live on kelp and feed on suspended particles [30]. The distribution of epiphytic suspension feeders within kelp forests may also be affected by spatial variation in mortality via predation and substrate longevity. Predators may be distributed in a nonrandom way within kelp forests. Additionally, spatial patterns in nutrient and light availability [31] and the physiological condition of kelp [32] could lead to predictable variation in the loss of kelp blades.
This study is about the distribution and demography of one of the most abundant epibionts in temperate reef systems, the genus of encrusting bryozoan, Membranipora. Understanding the factors that influence this cosmopolitan bryozoan is important. Membranipora can lead to large decreases in the cover of laminarian kelps [33,34,35,36], alter subtial community composition [34,37,38], have both negative and positive effects on its algal host ([39] and references therein), serve as prey for higher trophic levels [1], and provide an important filtering function in nearshore waters [40,41]. Several studies have documented temperature-mediated outbreaks of Membranipora in the North Atlantic, where, as an invasive species, it has led to defoliation of kelps [33,34,35]. A few studies have explored variation in Membranipora growth rates in modified habitats [42,43] and understory kelp [30]. One study looked at spatial variation in the recruitment of Membranipora in giant kelp forests [1]. However, despite the many papers on the interactions between Membranipora and kelps, to our knowledge, none have examined spatial variation in Membranipora growth rates (i.e., clonal reproduction) within aggregations of its most ubiquitous host, giant kelp (Macrocystis pyrifera).
To understand the extent to which the abundance and demography of Membranipora vary within giant kelp forests, we conducted a series of observational studies and field experiments at several sites in Santa Barbara, CA, USA. First, we estimated the occurrence and percent cover of colonies on kelp blades collected from the interior and along the edge of three kelp forests. We then assessed four demographic explanations for the observed spatial patterns: Differences in (1) loss of the attachment substrate (kelp blades), (2) colony mortality on the substrate, (3) larval recruitment, and (4) clonal reproduction (i.e., colony specific growth rate). The results of our study indicate that within-forest differences in Membranipora abundance are in part due to differences in the growth rates of colonies between the edge and interior and not solely the result of recruitment limitation to forest interiors, a pattern observed in previous studies [1]. Our results also indicate some disparity among sites in the magnitude of the difference in Membranipora growth and recruitment between forest edges and interiors. This finding in turn motivated us to explore relationships between structural attributes (e.g., density and size) of kelp forests and Membranipora demography. The results of this study provide insights into the consequences of ecosystem engineering for epibionts in coastal and marine systems.
2. Materials and Methods
2.1. Description of Study Sites and Species
In southern California, species in the genus, Membranipora, form encrusting colonies on blades of the giant kelp, Macrocystis pyrifera. Several studies suggest that Membranipora spp. along the west coast of North America (i.e., M. membranacea, M. villosa, and M. serrilamella) are alternative morphologies of the same species [44,45,46]. We identified specimens in this study as Membranipora serrilamella because of the characteristic crypocyst (inner extension of zooid wall) in our samples ([45] and references therein). However, because the species we studied may be functionally indistinct from those described as Membranipora membranacea and Membranipora villosa, we refer to it in this paper as simply Membranipora. Like all bryozoans, Membranipora is a suspension-feeder that relies on water flow to deliver food particles (e.g., phytoplankton). Colonies consist of semi-autonomous units (zooids) that are capable of feeding and reproducing. Clonal reproduction results in the addition of new zooids via budding and increases in colony area. Zooids are also simultaneous hermaphrodites, and following internal self- or crossed-fertilization (from sister zooids or nearby colonies [47]), they broadcast zygotes, which develop into feeding larvae that spend 2 to 4 weeks in offshore waters [44].
Giant kelp individuals (hereafter referred to as plants) consist of vine-like fronds (often totaling more than 100) that extend from a common holdfast vertically through the water column and form a canopy at the ocean surface. The density of fronds and plants can vary dramatically between kelp forests, as can forest extent [48,49,50]. Along the kelp fronds, hundreds of thin, wide blades provide the primary substrate for epibionts, such as Membranipora. Longevity of blades is a few months at most, as the fronds from which they extend generally live for 1 to 4 months [51,52].
This study was conducted at three sites off the coast of Santa Barbara, California, USA: Arroyo Quemado (AQUE, 34° 27.897′ N, 120° 07.179′ W), Mohawk (MOHK, 34° 23.639′ N, 119° 43.750′ W), and Carpinteria (CARP, 34° 23.409′ N, 119° 32.394′ W). We chose to work at these sites because the Santa Barbara Coastal Long-term Ecological Research Project (SBC LTER) surveys the kelp annually, providing us with additional information about the extent and dynamics of the forests [48,49,50]. The kelp forests occur at a 3 to 12 m depth on low relief bedrock reefs whose dimensions range from about 300 m (Mohawk) to 1500 m (Arroyo Quemado) to 2000 m (Carpinteria) in length (alongshore dimension) and approximately 200 to 400 m in width (cross-shore dimension). We worked in the interior and along the edge of the three forests. The interior locations were in approximately the center of the forests, surrounded by kelp, and ≥100 m from the edge locations. The edge locations were alongshore on the “upstream” side of the three forests, such that the prevailing current tended to encounter the edge locations first.
2.2. Distribution and Abundance of Membranipora in Kelp Forests
To understand the spatial variation in the distribution of Membranipora, we assessed the abundance of the encrusting bryozoan on kelp blades collected from the interior and edge of the three forests in August 2007. Fifty blades were collected haphazardly over a distance of approximately 40 m within the interior and along the edge of each forest (for a total of 300 blades). Blades were collected from the mid-water portion of kelp plants at depths of 3 to 6 m from the surface. Because the population size of encrusting bryozoans results from two distinct processes (larval recruitment and clonal reproduction), we used two metrics to assess abundance: (1) Occurrence of one or more Membranipora colonies on each kelp blade and (2) the percent cover of Membranipora on occupied blades. Percent cover was determined at 50 uniformly distributed points in a 10 cm × 5 cm quadrat placed in the center of each blade.
To examine the influence of location within the forest (i.e., edge vs. interior) on Membranipora distribution and abundance, we used generalized linear mixed-effects models. Using mixed-effects models allowed us to focus our inference on the influence of location within a forest. We included site as a predictor and took into account variation in the effects of edge vs. interior between sites. We constructed models for both response variables (occurrence of colonies on a blade and percent cover on occupied blades). We fitted three models with (1) a random intercept for site, (2) a random intercept for site and the fixed effect of location within the forest, and (3) a random intercept for site, the fixed effect of location within the forest, and a random slope for site. We fit all models using the lme4 package in the R program [53,54]. Because the response variables were binary (occurrence) and percent cover data, we used a logit link with family set to binomial distribution. We selected the best-fit random effects structure for each model using Akaike’s information criterion [55], fitting models with restricted maximum likelihood and keeping the fixed effects constant across models [56]. We also tested for significance in the best-fit mixed-effects model using likelihood ratio (LR) tests. The LR tests were implemented in R by comparing full and reduced models as recommended by [57].
2.3. Colony and Blade Mortality Within a Kelp Forest
Differences in Membranipora abundance within a kelp forest may result from differences in the longevity of kelp blades [51,52] or colony mortality on surviving blades. To investigate spatial variation in these two sources of mortality, we tagged 80 kelp blades on five plants along the edge and within the interior of the forest at CARP on 8 August 2006. One colony on each blade was tagged with two small holes (~1 cm) punched on either side of the colony. After 12 days, we measured blade mortality as the fraction of kelp blades that were lost from the interior and edge locations. Colony mortality was estimated on surviving kelp blades. Tagged colonies were found either almost entirely intact or had completely disappeared from the blade so the status (dead or alive) was easily distinguished. Separate two-way contingency table analyses on the frequency of lost blades and colonies were used to test for within-forest differences in colony and blade mortality.
2.4. Larval Recruitment in Kelp Forests
Spatial variation in Membranipora abundance may also result from differences in larval recruitment. To determine whether larval recruitment varies between the interior and edge of kelp forests, we counted and measured colonies on the 300 kelp blades collected in August 2007 (see above). Three metrics were used to assess spatial variation in larval recruitment: (1) Occurrence of one or more recruits on a kelp blade, (2) density of recruits on recruit-occupied blades, and (3) density of all colonies irrespective of size or age. The first two metrics represented “snap shots” of larval recruitment, in comparison to the third, which represented a “time integrated” measure of the minimum level of recruitment that occurred over the life span of the kelp blade, as each colony originated from a different larva. Density also incorporated colony mortality between the time of initial recruitment and the sampling date. Our primary interest for this study was the relative difference between blades along the edge versus inside rather than the overall magnitude of the colony density.
For each blade, we measured the diameter (i.e., longest length) of the 10 colonies proximal to the kelp nematocyst and used these diameters to estimate colony area. Recruits were identified as colonies <12 mm2 in size, and were <2 weeks old, according to [44]. We determined variation in the colony density by counting all colonies on each of the 300 kelp blades and measuring the area of each blade (assuming an elliptical shape). Colony density was calculated using only blades occupied by ≥1 colony of any size or age (MOHK N = 82, AQUE N = 73, CARP N = 44). We estimated the density of recruits on recruit-occupied blades as the product of colony density and the fraction of the 10 proximal colonies on each blade that were recruits.
Like the previous analyses, we used generalized linear mixed effects models. We took into account variation in the effect of within-forest location (edge vs. interior) between sites. We constructed models for the three response variables (occurrence of recruits on blades, density of recruits, and density of all colonies). We fitted three models in R with a random intercept for site (models 1, 2, and 3), random slope for site (model 3), and the effect of location within the forest (models 2 and 3). For the binary data (recruit occurrence), we used a logit link with family set to binomial distribution. For the other two response variables (recruit and colony density), we log transformed densities and used an identity link and the Gaussian family. See Section 2.2. Distribution and abundance of Membranipora in Kelp Forests for a complete description of the mixed effects models and analysis.
2.5. Clonal Reproduction in Kelp Forests
Variation in Membranipora abundance may be due to differences in clonal reproduction (i.e., colony growth) via budding of new zooids. To explore differences in colony growth rates between the edge and interiors of kelp forests, we conducted an observational study and a transplant experiment. The purpose of the observational study was to ask whether growth rates of naturally occurring colonies differed between the edge and interior of a kelp forest. Increases in the area (estimated by measuring the length of the longest diameter) of 80 tagged colonies located on the edge and inside of the forest at CARP were assessed on four dates in August 2006 (8, 10, 14, and 22 August). The shape of the relationship between the colony area and time indicated exponential clonal reproduction (i.e., exponential colony growth [58]). The specific colony growth rate (d−1) was calculated as ln(N/N0)/T, with N = colony area on the final sampling date, N0 = initial area, and T = time (days). The mean rate of clonal reproduction was calculated on a per plant basis using the colonies on surviving blades (N = 28 on 5 inside plants, N = 25 on 5 edge plants). The influence of location within the forest on growth (or clonal reproduction) was tested using one-way ANOVA. The data met the assumptions of normality and homogeneity of variances.
Results from the observational study motivated a transplant experiment in August 2007 to determine if location within the forest influenced clonal reproduction. At each site, we collected 120 kelp blades occupied by young colonies from throughout the forest. We transplanted 60 blades to two experimental fronds within the interior of the forest and 60 blades to two experimental fronds along the edge of each forest. One colony (4 to 10 mm diameter) was measured on each kelp blade and then marked using holes punched in the kelp blade (see [40] for a description of experimental fronds and transplant methods).
Colonies on transplanted blades were allowed to reproduce clonally for approximately 1 week (AQUE = 8, MOHK = 5, and CARP = 7 days). On the last day of the experiment, we measured the longest diameter of the colony and estimated the colony specific growth rate (d−1) as described above. To examine the influence of location within the forest (i.e., edge vs. interior) on the growth rate, we used linear mixed-effects models. As in the previous analyses, using mixed-effects models allowed us to focus our inference on the influence of location within a forest. We included site as a predictor and took into account variation in the effects of edge vs. interior between sites. We fitted three models with (1) a random intercept for site, (2) a random intercept for site and the fixed effect of location within the forest, and (3) a random intercept for site, the fixed effect of location within the forest, and a random slope for site. We fit all models using the lme4 package in the R program [53,54]. The data met the assumptions of normality and homogeneity of variance. See Section 2.2. Distribution and abundance of Membranipora in Kelp Forests for a complete description of the mixed effects models and analysis.
2.6. Relationships Between Kelp Forest Structure and Membranipora Demography
Lastly, we used log-linear regression to explore whether differences in the rates of larval recruitment and clonal reproduction between the interior and the edge of kelp forests were related to two structural attributes of forests: Frond density and forest size. We used data on the density of all plants and fronds in permanent transects (40 m × 2 m) at each site (MOHK = 2, AQUE = 6, CARP = 6) during July 2007 [21,48,49]. The area of each kelp forest was quantified in April 2007 (and also for CARP in 2006) using high spatial resolution, multi-spectral imagery of the surface canopy of giant kelp from the Satellite Pour l’Observation de la Terre (SPOT) 5 sensor [50]. We also used data on colony density and growth rates from this study, on colony density from [1], and on forest size from [59]. We estimated forest size as plant abundance, and kelp density as the number of fronds per m−2. The response variables were the differences in clonal reproduction (colony specific growth rate) and recruitment (colony density) between the edge and interior of the kelp forests.
3. Results
3.1. Distribution and Abundance of Membranipora in Kelp Forests
The abundance of Membranipora was consistently greater along the edge of kelp forests than the interior. Out of the 300 blades collected, Membranipora occurred on 135 blades from the edge and 64 blades from the interior of the three forests. The median percent cover of Membranipora on occupied blades from the edge of the forests was 20 times that of the interior (10 vs. 0.5). In addition, the difference between the edge and interior abundance varied by site, for both metrics. The model selection procedure showed that the data for occurrence and percent cover were best explained by models with a random intercept for site, random slope for site, and location within the forest as a fixed effect (Table S1). The difference between the edge and interior in the occurrence and percent cover were generally the greatest for CARP and lowest for MOHK (Figure 1a,b). After accounting for the significance of random differences among sites, the fixed effects part of the final model indicates that the occurrence and percent cover of Membranipora were significantly greater along the edge versus the interior of the kelp forests (Table S2).
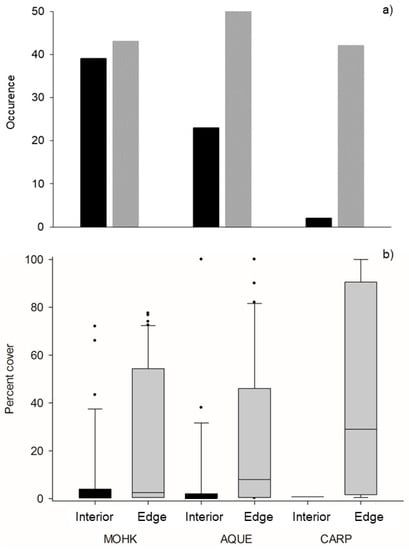
Figure 1.
(a) Occurrence of Membranipora on kelp blades and (b) percent cover of Membranipora on occupied blades. Grey bars indicate edge locations and black bars indicate interior locations at the three sites. Box plots show the median (central line), 25th and 75th percentiles (bottom and top of bars), and 5th and 95th percentiles (whiskers).
3.2. Colony and Blade Mortality Within a Kelp Forest
Loss of blades from along the edge of the forest at CARP was nearly twice that of the interior (11 versus 6 lost out of 40), but not significantly different (Pearson χ2 = 1.9, df = 1, p > 0.2) during the 12 day duration of our study. This finding is a conservative measure of the potential for substrate longevity to explain within-forest variation in Membranipora abundance because it would tend to reduce spatial differences (i.e., greater loss along the edge where the observed abundance was higher). Similarly, colony loss on surviving blades (3 of 29 on edge; 5 of 34 inside) was not influenced by location within the forest at CARP (Pearson χ2 = 0.3, df = 1, p > 0.6).
3.3. Larval Recruitment in Kelp Forests
Patterns of larval recruitment varied within forests and among sites. Occurrence of Membranipora recruits on kelp blades was no better explained by a model including a random intercept for site and the effect of location within the forest than a model with a random intercept for site only (Table S3). The greatest number of recruit occupied blades occurred at MOHK (68), followed by AQUE (61), and CARP (11, Figure 2a). This result suggests that the presence of recruits is less influenced by location within the forest and more affected by site-to-site variation in the availability of Membranipora larvae.
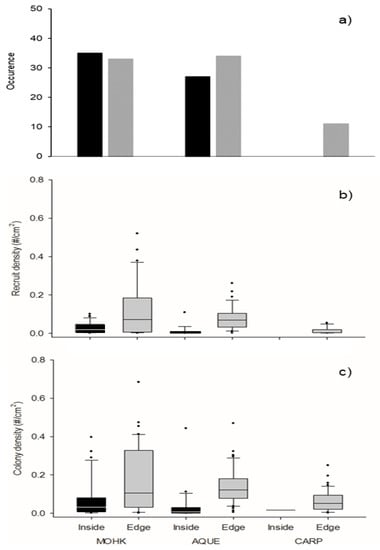
Figure 2.
(a) Occurrence of Membranipora recruits on kelp blades, (b) density of Membranipora recruits on occupied blades, and (c) density of all Membranipora colonies on kelp blades. Grey bars indicate edge locations and black bars indicate interior locations at the three sites. Box plots show the median (central line), 25th and 75th percentiles (bottom and top of bars), and 5th and 95th percentiles (whiskers).
The density of recruits on recruit-occupied blades was better explained by a model including a random intercept for site and the effect of location within the forest than one including only a random intercept for site (Table S3). We did not test a model including the random slope for site as there were no recruit-occupied blades in the interior of CARP (Figure 2a). After accounting for random differences in recruit density between sites, the analysis of fixed effects in the final model indicates the density of recruits was significantly higher along the edge than the interior of kelp forests (Table S4; Figure 2b).
Lastly, for colony density, a model including a random intercept for site, random slope for site, and effect of location within the forest was superior to the two more parsimonious models (Table S3). The median colony density was consistently higher along the edge of the forests (ranging from 0.05 to 0.1) than in the interiors (ranging from 0.01 to 0.03). However, we were not able to assess the significance of location within the forest for the most complicated model, as it would not converge due to a low sample size (there were only two blades occupied by colonies within the interior of the forest at CARP (Figure 1a)). According to model 2, the next best performing model (Table S3), a significantly higher density of colonies occurred along the edge of the forests than the interior (Table S4, Figure 2c).
3.4. Clonal Reproduction in Kelp Forests
Clonal reproduction was higher along the edge than the interior of kelp forests. After two weeks, tagged colonies along the edge of the forest at CARP grew to a size that was nearly five times greater than that of interior colonies (Figure 3a). Their rate of clonal reproduction (2.5 d−1) was more than 20% greater (log difference) than that of interior ones (2.0 d−1; t = 2.7, df = 8, p < 0.03). The average rate of clonal reproduction of transplanted colonies to MOHK, CARP, and AQUE was also greater along forest edges than the interiors (Figure 3b). A model including the fixed effect of location within the forest (i.e., edge versus interior), a random intercept for site, and a random slope for site (model 3) was far superior to a model including just a random intercept for site (model 1, Table S5). However, because the more complex model was only marginally superior to model 2 (fixed effect of edge vs. interior and a random intercept for site, Table S5) and the most complex model resulted in correlation estimates of nearly −1 (which can lead to singularity issues), we decided to report significance for model 2 (Table S6). Furthermore, significant results for edge vs. inside of the forest were qualitatively the same for model 3 as model 2. According to model 2, colonies along the edge of the forests had higher growth rates than those within the interior, after taking into account variation in the random variables (Table S6, Figure 3b).
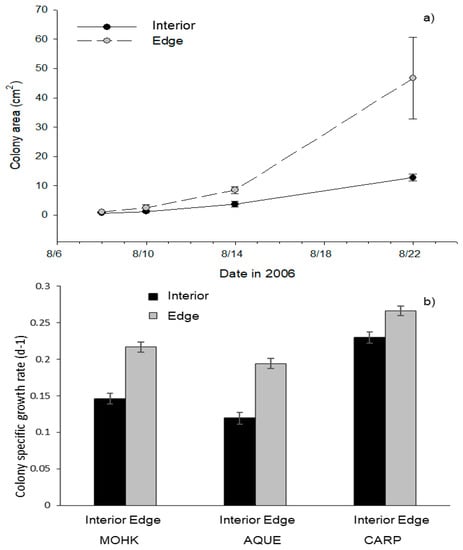
Figure 3.
Clonal reproduction (measured as colony specific growth rate, d−1) of Membranipora within and among kelp forests. (a) The area of colonies on naturally occurring kelp blades in the interior and along the edge of CARP during August 2006 (mean ± SE). (b) The average colony specific growth rates (mean ± SE) for colonies transplanted to the interior (black bars) and along the edge (grey bars) of each forest (MOHK interior (N = 60), MOHK edge (N = 59), AQUE interior (N = 55), AQUE edge (N = 58), CARP interior (N = 52), and CARP edge (N = 53)).
3.5. Relationships Between Kelp Forest Structure and Membranipora Demography
Differences between the edge and interior of kelp forests in colony density were higher in larger forests. The relationship between relative colony density and forest size (area x plant density) is decelerating (Figure 4a; y = −0.7 + 0.2ln(x); F1,4 = 56.4, p < 0.005) and maintains approximately the same quantitative shape even when the largest forest was removed from the analysis (Figure 4a inset; y = –1.2 + 0.2ln(x); F1,3 = 347, p < 0.003). Relative differences in colony density between the interior and edge were also related to forest area and the decelerating relationship was qualitatively similar (y = –2.1 + 0.3ln(x); F1,4 = 71.4, p < 0.004). Differences between the edge and interior of kelp forests in clonal reproduction increased with the density of fronds, rather than forest size. The relationship also appears to be decelerating (Figure 4b; y = –0.02 + 2.0ln(x); F1,3 = 20.0, p < 0.05). However, its shape should be interpreted with caution given the limited number of data points (N = 4).
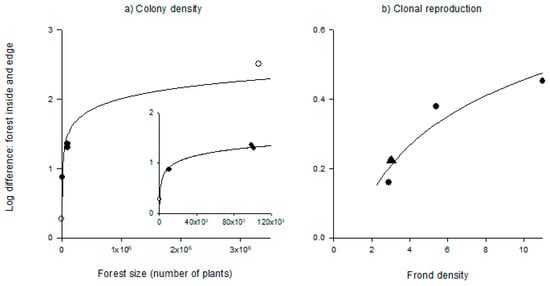
Figure 4.
Relative difference between the forest edge and interior in (a) colony density (i.e., a time integrated measure of larval recruitment) with respect to forest size, and (b) clonal reproduction in relation to frond density. Closed circles are experimental data from the three forests in Santa Barbara in 2007 (this study), open circles are data from the forests in San Diego (Bernstein and Jung 1979, Dayton 1984), and the triangle is the observational data from CARP in 2006 (this study). The inset in (a) includes the same data without the largest forest.
4. Discussion
Most studies of the relationships between Membranipora and kelps have examined the negative effects of this epiphytic bryozoan on its host [33,34,35,37,38,39], but see [60,61]. We focused instead on variation in bryozoan abundance and demography within aggregations of giant kelp. Our goal was to understand how forests not only provide Membranipora with a substrate but also influence its recruitment and growth. We found that the occurrence and percent cover of Membranipora differed between the edge and interior of kelp forests and that the within forest disparity among these metrics varied with site. We did not find evidence that within forest differences in the abundance of Membranipora could be explained by variation in colony mortality or substrate longevity. Instead, we found that recruitment and clonal reproduction differed between the edge and interior of kelp forests. Reduced growth rates of Membranipora within kelp forests suggest an inhibitory effect of giant kelp on this encrusting bryozoan that has not been documented before.
Our finding of a higher density of recruits along forest edges (Figure 2b,c) agrees with previous research on Membranipora and giant kelp [1], as well as recruitment patterns of other invertebrate taxa within aggregations of macrophytes (e.g., seagrass and bivalves [62]). Within- and among-forest differences in colony and recruit density suggest that kelp acts as a sieve, whereby larvae returning to the forest from offshore settle out onto edge blades first [1]. This process results in a lower concentration of larvae in the waters flushed to the interior of the forest and the potential for higher interior densities of colonies at smaller forests with less available space for colonization. The sieve effect of kelp is one possible explanation for the relationship between forest size (i.e., availability of substrate for recruitment) and differences in recruitment to the edge and inside of forests in Southern California that emerged when we combined our results with data from [1,59] (Figure 4a). Our results suggest that as forest size increases, differences in colony density between the edge and inside appear to increase before saturating at large forest sizes. Variation in the larval supply [44] may explain our result that a model with site alone performed better at estimating variation in the occurrence of Membranipora on kelp blades than a model including location within the forest.
In addition to recruitment, our results show that spatial variation in Membranipora abundance within forests is also the result of large differences in colony specific growth rates. Colonies that occurred along forest edges were three to more than four times the size of those in the interior after 6 days and two weeks of measurement, respectively. Colonies transplanted to the edge of forests grew at rates 15% to 45% faster than interior colonies. The specific growth rates we observed are within the same magnitude of those observed in the field in previous studies [40,63,64]. However, the patterns in relation to giant kelp are in contrast to [30], which found no consistent differences in the growth of Membranipora colonies located in and around forests of understory kelp (see below). The wealth of papers examining variation in growth rates of invertebrates between the edge and interior of seagrass meadows suggests a variety of potential mechanisms that may underlie variation in the growth rates of suspension-feeders within other biogenic habitats, such as kelp. These mechanisms include dampened flows and the interaction between flow speed and predation (reviewed in [3,7,65]).
The well-documented dampening effect of Macrocystis pyrifera on water flow [23,24,25,26] may affect a wide variety of forest residents, including bryozoans. Yet, to our knowledge, few studies have explored the potential influence of kelp-dampened flows on planktivores in giant kelp forests [27,29]. This is surprising given the importance of flow for rocky subtidal communities (reviewed in [66]) and in stark contrast to the wealth of studies exploring flow-mediated effects of seagrasses on associated fauna (reviewed in [65]). Our observations of reduced clonal reproduction in forest interiors likely resulted from limited food resources. The availability of particles for sessile suspension-feeders depends on both their population density and rates of particle delivery [67]. High densities of suspension feeders can deplete food levels [68,69], while high ambient current speeds counteract depletion by increasing the flux of food particles available to Membranipora [40,64,67]. Reduced rates of colony growth within forests, compared to along the edge, suggest that kelp-dampened interior flows may have been insufficient to fully compensate for a reduction in suspended food. Among site variation in differences in growth rates between edge and interior colonies may be the result of differences in forest density (Figure 4b), as current speeds are expected to decay more rapidly over shorter distances in denser forests [23].
Unfortunately, we were unable to directly test the hypothesis that kelp-dampened interior flows were insufficient to replenish suspended food particles necessary for growth. However, continuous data on water flow from the interior and edge of MOHK during the transplant experiment do reveal insights into the potential mechanisms that may underlie the difference in growth rates that we observed (Appendix A, Figure 5). The distribution of current speeds along the edge of the MOHK forest was significantly different from the distribution of current speeds within the interior of the forest (Figure 5a vs. Figure 5b; Kolmogorov–Smirnov two sample test: D = 0.636, df = 1, p < 0.0001). Inspection of the data indicate that a far higher frequency of slow flows occurred within the interior of the forest than along the edge, where the current speeds were more variable and generally faster. A greater frequency of optimal flow speeds for feeding and growth occurred along the edge of the forest at MOHK (Figure 5 this paper and Figure 1 in [40]). These data suggest that a reduction in the delivery of particles to interior colonies due to dampened currents by giant kelp and feeding by exterior colonies may have resulted in reduced growth rates of inside colonies relative to edge colonies.
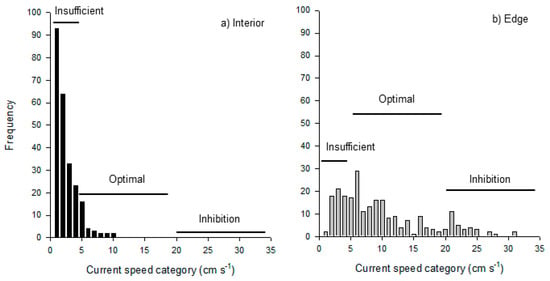
Figure 5.
Current speeds occurring in the (a) interior and (b) along the edge of the kelp forest at MOHK during the growth experiment. Data are a frequency of 30 min periods in which the current speed was 0 to 35 cm s−1 (N = 242 periods). Insufficient, optimal, and inhibition labels are based on Figure 1 in Arkema 2009.
Our results imply that forest edges are superior for clonal reproduction compared to the interior of the forest, but this may not always be the case. For instance, extremely fast speeds—which have been shown to negatively affect the feeding structures of encrusting bryozoans by causing hydrodynamic drag [40,64,70]—are more likely to occur along the edge of a kelp forest (Figure 5; [23,24]). Consequently, at times or locations with fast water flow, the dampening effect of kelp may facilitate, rather than inhibit, Membranipora feeding success and clonal reproduction. This may have led to the inconsistent influence of the forest edge versus interior on Membranipora growth observed by [30]. In their study, a positive effect of kelp on clonal reproduction occurred at a site with ambient current speeds that would have inhibited Membranipora feeding (24 cm s−1) and a negative effect of kelp occurred where ambient speeds (16 cm s−1) would have been ideal [30,40,70]. The general pattern that organisms exhibit positive associations in stressful conditions and negative interactions in more benign conditions [5,6,71] may hold true for ecosystem engineers and their epibionts in ocean forests as well as on land [11,12,13].
Inferring mechanisms for the patterns in the growth rates found in our study is limited by only having corresponding flow data for the interior and edge of the forest at one site. Our analyses of the relationship between kelp density and abundance and Membranipora growth and recruitment, respectively, were also limited by the small number of sites at which we worked. Future work could assess growth along forest edges and interiors at more sites with simultaneous collection of data on current speeds. Other areas of inquiry include potential differences in Membranipora abundance and demography between offshore and nearshore edges of a forest, which differ in flow regime [24] and kelp physiological condition [32], and variation within the interior, which could depend on forest patchiness and configuration [50]. In addition, future work could begin earlier in the summer and track Membranipora growth over the multiple month life span of kelp blades [52]. Finally, it would be interesting to explore interannual variability in growth rates of Membranipora within the interior and along the edge of forests, as giant kelp forests are highly dynamic [59,72] and Membranipora is a perennial species with fast growth rates. Whereas for terrestrial forests, it could take decades to understand temporal relationships between ecosystem engineers and epiphytes, in kelp forests, change happens so quickly that consequences of the kelp forest structure for associated species assemblages can emerge over several years [21,22,28,49].
The results of this study demonstrate differences in Membranipora abundance between the edge and interior of giant kelp forests, an important ecosystem engineer in temperate waters. Our findings suggest that these differences are driven not only by a lower recruitment to forest interiors but also by lower colony specific growth rates. Lowered growth rates within forest interiors may be the result of a reduced delivery of food due to feeding by exterior colonies and dampened and diverted flow caused by the ecosystem engineering of giant kelp. Furthermore, we found relationships between structural attributes of kelp and Membranipora demography. Such nonlinear relationships between structure-forming organisms and associated species occur in other systems and can be important for ocean management [73]. Differences in recruitment between the edge and inside of forests appear to be greater in larger forests and differences in growth are greater in denser forests. Together these results highlight the importance of ecosystem engineers in influencing the ecological dynamics of epibionts in marine systems. Flow-mediated relationships between giant kelp and epiphytic suspension-feeders also likely extend to other marine organisms whose growth rates depend on current regimes. These relationships could have implications for the role that suspension feeders in kelp forests play in filtering nearshore waters.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/11/8/120/s1, Table S1: Model selection results for occurrence and percent cover of Membranipora on kelp blades, Table S2: Parameter estimates for final models of occurrence and percent cover, Table S3: Model selection results for occurrence of recruits, density of recruits, and density of colonies on kelp blades, Table S4: Parameter estimates for final models of recruit density and colony density, Table S5: Model selection results for colony specific growth rate, Table S6: Parameter estimates for final model of colony specific growth rate.
Author Contributions
K.K.A. conceptualized the research, developed the methodology, led the field investigations, and drafted the original version of the manuscript. K.K.A. and J.F.S. conducted the model selection and statistical analyses. J.F.S. assisted with the field investigation and reviewed and edited the manuscript.
Funding
This research was funded by the National Science Foundation in support of the Santa Barbara Coastal Long Term Ecological Research Program (OCE-9982105, OCE-0620276), the University of California Marine Council’s Coastal Environmental Quality Initiative, and the long-time generosity of Bruce and Susan Worster.
Acknowledgments
We are especially grateful to S. Honig, S. Heidelberger, and C. Santschi for their endless energy and enthusiasm in the field and S. Harrer and C. Nelson for support with all diving operations. Thank you to Nick Tolimieri and Kristin Marshall for discussing statistical analyses. Special thanks to D. Reed, S. Holbrook, R. Schmitt, and M. Brzezinski for advice and support throughout the project.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
Current speeds inside and along the edge of the forest at MOHK were measured during the August 2007 transplant experiment using bottom-mounted Acoustic Doppler Current Profilers (ADCPs; 600 and 1200 kHz, RD Instruments). These collected a 1 to 4 min burst of 1 Hz velocity data every 2 to 8 min in 0.5 m vertical bins. Data from all three axes were used to calculate current speed so that the mean speed was independent of the direction of flow. The raw time series were smoothed temporally by block-averaging over 15 min segments and the mean current speed was calculated for each 30 min period. Data from depths of 4 to 6 m were averaged so that each flow datum represented the speed for the vertical location where the Membranipora colonies were transplanted.
References
- Bernstein, B.B.; Jung, N. Selective Pressures and Coevolution in a Kelp Canopy Community in Southern California. Ecol. Monogr. 1979, 49, 335–355. [Google Scholar] [CrossRef]
- Dayton, P.K. Ecology of Kelp Communities. Annu. Rev. Ecol. Syst. 1985, 16, 215–245. [Google Scholar] [CrossRef]
- Irlandi, E.A.; Peterson, C.H. Modification of animal habitat by large plants: Mechanisms by which seagrasses influence clam growth. Oecologia 1991, 87, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Hixon, M.A.; Beets, J.P. Predation, Prey Refuges, and the Structure of Coral-Reef Fish Assemblages. Ecol. Monogr. 1993, 63, 77–101. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and Negative Effects of Organisms as Physical Ecosystem Engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Crain, C.M.; Bertness, M.D. Community Impacts of a Tussock Sedge: Is Ecosystem Engineering Important in Benign Habitats? Ecology 2005, 86, 2695–2704. [Google Scholar] [CrossRef]
- Boström, C.; Jackson, E.L.; Simenstad, C.A. Seagrass landscapes and their effects on associated fauna: A review. Estuar. Coast. Shelf Sci. 2006, 68, 383–403. [Google Scholar] [CrossRef]
- Winkler, M.; Hülber, K.; Hietz, P. Population dynamics of epiphytic bromeliads: Life strategies and the role of host branches. Basic Appl. Ecol. 2007, 8, 183–196. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Orth, R.J.; Duarte, C. (Eds.) Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; ISBN 978-1-4020-2942-4. [Google Scholar]
- Jones, C.; Lawton, J.H.; Shachak, M. Organisms as Ecosystem Engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Pearson, L.C. Influence of Temperature and Humidity on Distribution of Lichens in a Minnesota Bog. Ecology 1969, 50, 740–746. [Google Scholar] [CrossRef]
- Esseen, P.-A.; Renhorn, K.-E. Edge Effects on an Epiphytic Lichen in Fragmented Forests. Conserv. Biol. 1998, 12, 1307–1317. [Google Scholar] [CrossRef]
- Coote, L.; Smith, G.; Kelly, D.; O’Donoghue, S.; Dowding, P.; Iremonger, S.; Mitchell, F. Epiphytes of Sitka spruce (Picea sitchensis) plantations in Ireland and the effects of open spaces. Biodivers. Conserv. 2008, 17, 953–968. [Google Scholar] [CrossRef]
- Saunders, J.E.; Attrill, M.J.; Shaw, S.M.; Rowden, A.A. Spatial variability in the epiphytic algal assemblages of Zostera marina seagrass beds. Mar. Ecol. Prog. Ser. 2003, 249, 107–115. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Lavery, P.S.; van Keulen, M. Epiphytes of Seagrasses. In Seagrasses: Biology, Ecologyand Conservation; Springer: Dordrecht, The Netherlands, 2007; pp. 441–461. [Google Scholar]
- Gutiérrez, J.L.; Jones, C.G.; Byers, J.E.; Arkema, K.K.; Berkenbusch, K.; Commito, J.A.; Duarte, C.M.; Hacker, S.D.; Lambrinos, J.G.; Hendriks, I.E.; et al. Physical Ecosystem Engineers and the Functioning of Estuaries and Coasts. In Treatise on Estuarine and Coastal Science; Elsevier: Amsterdam, The Netherlands, 2011; pp. 53–81. ISBN 978-0-08-087885-0. [Google Scholar]
- Graham, M.H.; Vásquez, J.A.; Buschmann, A.H. Global ecology of the giant kelp Macrocystis: From ecotypes to ecosystems. Oceanogr. Mar. Biol. 2007, 45, 39–88. [Google Scholar]
- Krumhansl, K.A.; Okamoto, D.K.; Rassweiler, A.; Novak, M.; Bolton, J.J.; Cavanaugh, K.C.; Connell, S.D.; Johnson, C.R.; Konar, B.; Ling, S.D.; et al. Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. USA 2016, 113, 13785–13790. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Schiel, R. The ecology of giant kelp forests in California: A community profile. US Fish Wildl. Serv. Biol. Rep. 1985, 85. [Google Scholar]
- Graham, M.H. Effects of Local Deforestation on the Diversity and Structure of Southern California Giant Kelp Forest Food Webs. Ecosystems 2004, 7, 341–357. [Google Scholar] [CrossRef]
- Miller, R.J.; Lafferty, K.D.; Lamy, T.; Kui, L.; Rassweiler, A.; Reed, D.C. Giant kelp, Macrocystis pyrifera, increases faunal diversity through physical engineering. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172571. [Google Scholar] [CrossRef] [PubMed]
- Teagle, H.; Hawkins, S.J.; Moore, P.J.; Smale, D.A. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Biol. Ecol. 2017, 492, 81–98. [Google Scholar] [CrossRef]
- Jackson, G.A.; Winant, C.D. Effect of a kelp forest on coastal currents. Cont. Shelf Res. 1983, 2, 75–80. [Google Scholar] [CrossRef]
- Gaylord, B.; Rosman, J.H.; Reed, D.C.; Koseff, J.R.; Fram, J.; MacIntyre, S.; Arkema, K.; McDonald, C.; Brzezinski, M.A.; Largier, J.L.; et al. Spatial patterns of flow and their modification within and around a giant kelp forest. Limnol. Oceanogr. 2007, 52, 1838–1852. [Google Scholar] [CrossRef]
- Gaylord, B.; Nickols, K.J.; Jurgens, L. Roles of transport and mixing processes in kelp forest ecology. J. Exp. Biol. 2012, 215, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Rosman, J.H.; Koseff, J.R.; Monismith, S.G.; Grover, J. A field investigation into the effects of a kelp forest (Macrocystis pyrifera) on coastal hydrodynamics and transport. J. Geophys. Res. Oceans 2007, 112. [Google Scholar] [CrossRef]
- Bray, R. Influence of water currents and zooplankton densities on daily foraging movements of blacksmith, Chromis punctipinnis, a planktivorous reef fish. Fish. Bull. 1980, 78, 829–841. [Google Scholar]
- Arkema, K.K.; Reed, D.C.; Schroeter, S.C. Direct and indirect effects of giant kelp determine benthic community structure and dynamics. Ecology 2009, 90, 3126–3137. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Page, H.M.; Reed, D.C. Trophic versus structural effects of a marine foundation species, giant kelp (Macrocystis pyrifera). Oecologia 2015, 179, 1199–1209. [Google Scholar] [CrossRef]
- Eckman, J.E.; Duggins, D.O. Life and death beneath macrophyte canopies: Effects of understory kelps on growth rates and survival of marine, benthic suspension feeders. Oecologia 1991, 87, 473–487. [Google Scholar] [CrossRef]
- Stewart, H.; Fram, J.; Reed, D.; Williams, S.; Brzezinski, M.; Macintyre, S.; Gaylord, B. Differences in growth, morphology and tissue carbon and nitrogen of Macrocystis pyrifera within and at the outer edge of a giant kelp forest in California, USA. Mar. Ecol. Prog. Ser. 2009, 375, 101–112. [Google Scholar] [CrossRef]
- Bell, T.W.; Cavanaugh, K.C.; Siegel, D.A. Remote monitoring of giant kelp biomass and physiological condition: An evaluation of the potential for the Hyperspectral Infrared Imager (HyspIRI) mission. Remote Sens. Environ. 2015, 167, 218–228. [Google Scholar] [CrossRef]
- Saunders, M.; Metaxas, A. High recruitment of the introduced bryozoan Membranipora membranacea is associated with kelp bed defoliation in Nova Scotia, Canada. Mar. Ecol. Prog. Ser. 2008, 369, 139–151. [Google Scholar] [CrossRef]
- Scheibling, R.E.; Gagnon, P. Temperature-mediated outbreak dynamics of the invasive bryozoan Membranipora membranacea in Nova Scotian kelp beds. Mar. Ecol. Prog. Ser. 2009, 390, 1–13. [Google Scholar] [CrossRef]
- Krumhansl, K.A.; Lee, J.M.; Scheibling, R.E. Grazing damage and encrustation by an invasive bryozoan reduce the ability of kelps to withstand breakage by waves. J. Exp. Mar. Biol. Ecol. 2011, 407, 12–18. [Google Scholar] [CrossRef]
- Dixon, J.; Schroeter, S.C.; Kastendiek, J. Effects of the Encrusting Bryozoan, Membranipora membranacea, on the Loss of Blades and Fronds by the Giant Kelp, Macrocystis pyrifera (Laminariales)1. J. Phycol. 1981, 17, 341–345. [Google Scholar] [CrossRef]
- Watanabe, S.; Scheibling, R.E.; Metaxas, A. Contrasting patterns of spread in interacting invasive species: Membranipora membranacea and Codium fragile off Nova Scotia. Biol. Invasions 2010, 12, 2329–2342. [Google Scholar] [CrossRef]
- Levin, P.S.; Coyer, J.A.; Petrik, R.; Good, T.P. Community-Wide Effects of Nonindigenous Species on Temperate Rocky Reefs. Ecology 2002, 83, 3182–3193. [Google Scholar] [CrossRef]
- Hepburn, C.D.; Hurd, C.L.; Frew, R.D. Colony Structure and Seasonal Differences in Light and Nitrogen Modify the Impact of Sessile Epifauna on the Giant Kelp Macrocystis pyrifera (L.) C Agardh. Hydrobiologia 2006, 560, 373–384. [Google Scholar] [CrossRef]
- Arkema, K.K. Flow-mediated feeding in the field: Consequences for the performance and abundance of a sessile marine invertebrate. Mar. Ecol. Prog. Ser. 2009, 388, 207–220. [Google Scholar] [CrossRef]
- Arkema, K.K. Consequences of Kelp Forest Structure and Dynamics for Epiphytes and Understory Communities. Ph.D. Thesis, University of California, Santa Barbara, CA, USA, 2008. [Google Scholar]
- Marzinelli, E.M.; Underwood, A.J.; Coleman, R.A. Modified Habitats Influence Kelp Epibiota via Direct and Indirect Effects. PLoS ONE 2011, 6, e21936. [Google Scholar] [CrossRef]
- Marzinelli, E.M.; Underwood, A.J.; Coleman, R.A. Modified habitats change ecological processes affecting a non-indigenous epibiont. Mar. Ecol. Prog. Ser. 2012, 446, 119–129. [Google Scholar] [CrossRef][Green Version]
- Yoshioka, P.M. Role of Planktonic and Benthic Factors in the Population Dynamics of the Bryozoan Membranipora membranacea. Ecology 1982, 63, 457–468. [Google Scholar] [CrossRef]
- Schwaninger, H.R. Population structure of the widely dispersing marine bryozoan Membranipora membranacea (Cheilostomata): Implications for population history, biogeography, and taxonomy. Mar. Biol. 1999, 135, 411–423. [Google Scholar] [CrossRef]
- Dick, M.H.; Grischenko, A.V.; Mawatari, S.F. Intertidal Bryozoa (Cheilostomata) of Ketchikan, Alaska. J. Nat. Hist. 2005, 39, 3687–3784. [Google Scholar] [CrossRef]
- Temkin, M.H. Gamete spawning and fertilization in the gymnolaemate bryozoan Membranipora membranacea. Biol. Bull. 1994, 187, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.C.; Rassweiler, A.; Arkema, K.K. Biomass rather than growth rate determines variation in net primary production by giant kelp. Ecology 2008, 89, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.C.; Rassweiler, A.R.; Miller, R.J.; Page, H.M.; Holbrook, S.J. The value of a broad temporal and spatial perspective in understanding dynamics of kelp forest ecosystems. Mar. Freshw. Res. 2016. [Google Scholar] [CrossRef]
- Cavanaugh, K.; Siegel, D.; Kinlan, B.; Reed, D. Scaling giant kelp field measurements to regional scales using satellite observations. Mar. Ecol. Prog. Ser. 2010, 403, 13–27. [Google Scholar] [CrossRef]
- Rodriguez, G.E.; Rassweiler, A.; Reed, D.C.; Holbrook, S.J. The importance of progressive senescence in the biomass dynamics of giant kelp (Macrocystis pyrifera). Ecology 2013, 94, 1848–1858. [Google Scholar] [CrossRef]
- Rodriguez, G.E.; Reed, D.C.; Holbrook, S.J. Blade life span, structural investment, and nutrient allocation in giant kelp. Oecologia 2016, 182, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach; Springer-Verlag: New York, NY, USA, 1998; ISBN 978-1-4757-2917-7. [Google Scholar]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Hermansen, P.; Larsen, P.S.; Riisgård, H.U. Colony growth rate of encrusting marine bryozoans (Electra pilosa and Celleporella hyalina). J. Exp. Mar. Biol. Ecol. 2001, 263, 1–23. [Google Scholar] [CrossRef]
- Dayton, P.K.; Tegner, M.J. Catastrophic storms, el nino, and patch stability in a southern california kelp community. Science 1984, 224, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, C.D.; Hurd, C.L. Conditional mutualism between the giant kelp Macrocystis pyrifera and colonial epifauna. Mar. Ecol. Prog. Ser. 2005, 302, 37–48. [Google Scholar] [CrossRef]
- Hepburn, C.; Frew, R.; Hurd, C. Uptake and transport of nitrogen derived from sessile epifauna in the giant kelp Macrocystis pyrifera. Aquat. Biol. 2012, 14, 121–128. [Google Scholar] [CrossRef]
- Bologna, P.; Heck, K. Impacts of Seagrass Habitat Architecture on Bivalve Settlement. Estuaries Coasts 2012, 23, 449–457. [Google Scholar] [CrossRef]
- Saunders, M.; Metaxas, A. Effects of temperature, size, and food on the growth of Membranipora membranacea in laboratory and field studies. Mar. Biol. 2009, 156, 2267–2276. [Google Scholar] [CrossRef]
- Pratt, M.C. Living where the flow is right: How flow affects feeding in bryozoans. Integr. Comp. Biol. 2008, 48, 808–822. [Google Scholar] [CrossRef]
- Williams, S.L.; Heck, K.L. Seagrass community ecology. In Marine Community Ecology; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 317–337. [Google Scholar]
- Witman, J.D.; Dayton, P.K. Rocky subtidal communities. In Marine Community Ecology; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 339–366. [Google Scholar]
- Wildish, D.; Kristmanson, D. Benthic Suspension Feeders and Flow; Cambridge University Press: Cambridge, UK, 2005; ISBN 978-0-521-02347-4. [Google Scholar]
- Buss, L.W.; Jackson, J.B.C. Planktonic food availability and suspension-feeder abundance: Evidence of in situ depletion. J. Exp. Mar. Biol. Ecol. 1981, 49, 151–161. [Google Scholar] [CrossRef]
- Peterson, C.H.; Black, R. Resource depletion by active suspension feeders on tidal fiats: Influence of local density and tidal elevation1. Limnol. Oceanogr. 1987, 32, 143–166. [Google Scholar] [CrossRef]
- Eckman, J.E.; Duggins, D.O. Effects of Flow Speed on Growth of Benthic Suspension Feeders. Biol. Bull. 1993, 185, 28–41. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Iribarne, O.O. Conditional responses of organisms to habitat structure: An example from intertidal mudflats. Oecologia 2004, 139, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.C.; Rassweiler, A.; Carr, M.H.; Cavanaugh, K.C.; Malone, D.P.; Siegel, D.A. Wave disturbance overwhelms top-down and bottom-up control of primary production in California kelp forests. Ecology 2011, 92, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Samhouri, J.F.; Levin, P.S.; Ainsworth, C.H. Identifying Thresholds for Ecosystem-Based Management. PLoS ONE 2010, 5, e8907. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).