Interannual Variation of Benthic Macroinvertebrate Communities at Long-Term Monitoring Sites Impacted by Human Activities: Implications for Bioassessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
3.1. Interannual Variability of the Biological Assemblage
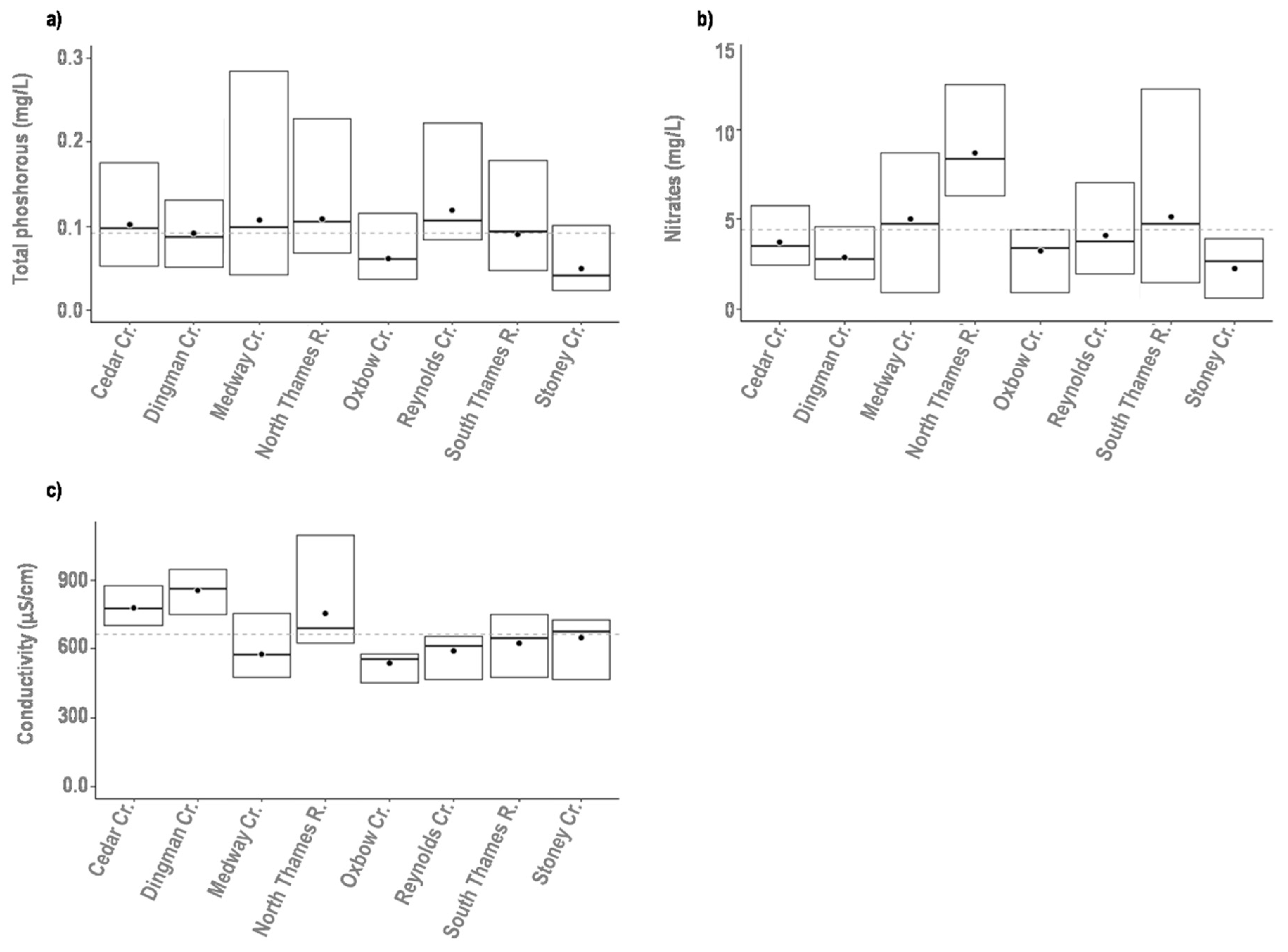
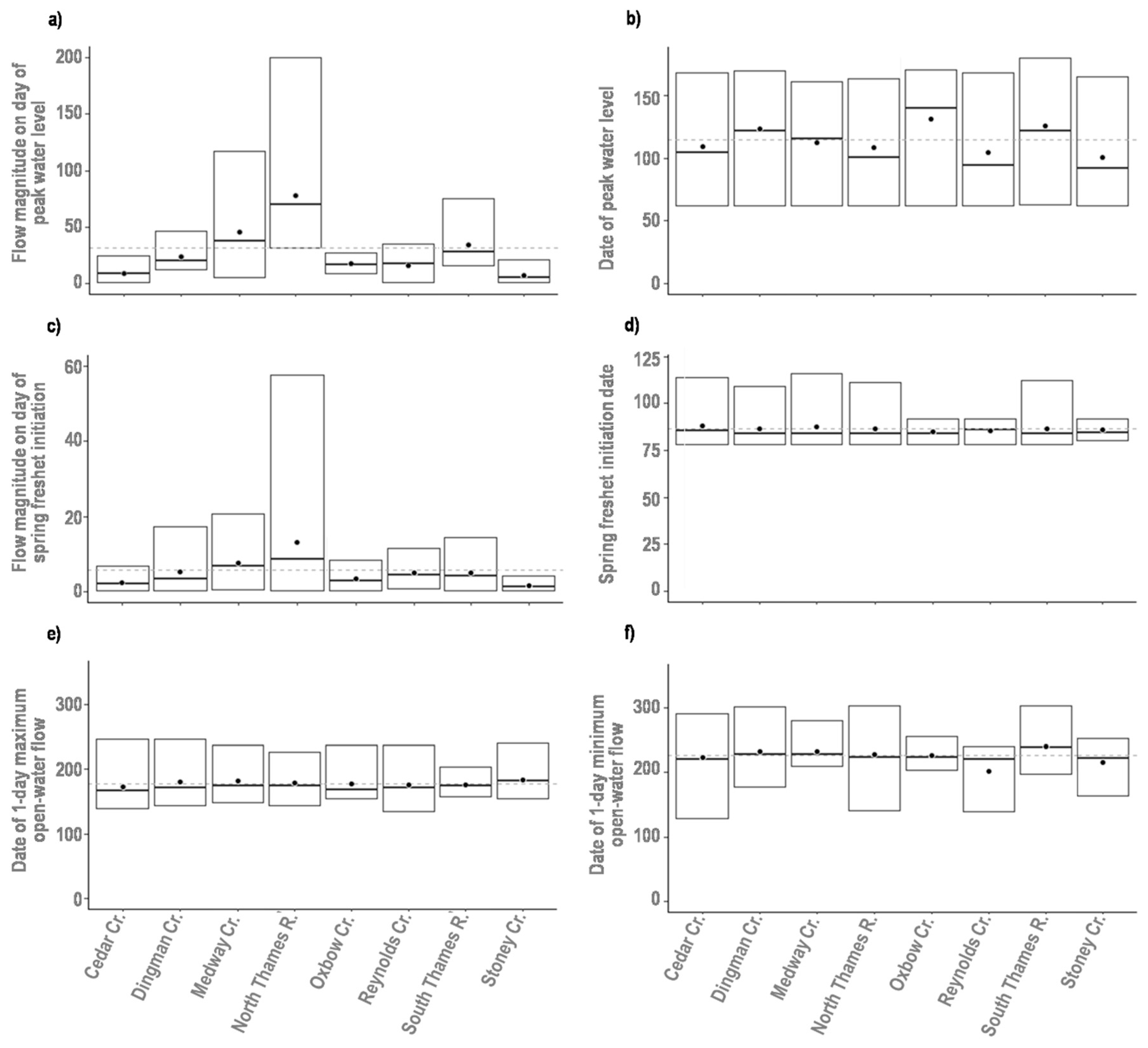
3.2. Assessment of Environmental Variability
3.3. Environment to Biota Associations
4. Discussion
4.1. Temporal Variations in Benthic Macroinvertebrate Communities
4.2. Implications for Biomonitoring Using Benthic Macroinvertebrates
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hering, D.; Johnson, R.K.; Kramm, S.; Shmutz, S.; Szoszkiewicz, K.; Verdonschot, P.F.M. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw. Biol. 2006, 51, 1757–1785. [Google Scholar] [CrossRef]
- Bonada, N.; Prat, N.; Resh, V.H.; Statzner, B. Developments in aquatic insect biomonitoring: A comparative analysis of recent approaches. Annu. Rev. Entomol. 2006, 51, 495–523. [Google Scholar] [CrossRef] [PubMed]
- Buss, D.F.; Carlisle, D.M.; Chon, T.-S.; Culp, J.; Harding, J.S.; Keizer-Vlek, H.E.; Robinson, W.A.; Strachan, S.; Thirion, C.; Hughes, R.M. Stream biomonitoring using macroinvertebrates around the globe: A comparison of large-scale programs. Environ. Monit. Assess. 2014, 187, 4132. [Google Scholar] [CrossRef] [PubMed]
- Clements, W.H.; Carlisle, D.M.; Lazorchak, J.M.; Johnson, P.C. Heavy metals structure benthic communities in Colorado mountain streams. Ecol. Appl. 2000, 10, 626–638. [Google Scholar] [CrossRef]
- Walsh, C.J. Biological indicators of stream health using macroinvertebrate assemblage composition: A comparison of sensitivity to an urban gradient. Mar. Freshw. Res. 2006, 57, 37–47. [Google Scholar] [CrossRef]
- Wang, L.Z.; Robertson, D.M.; Garrison, P.J. Linkages between nutrients and assemblages of macroinvertebrates and fish in wadeable streams: Implication to nutrient criteria development. Environ. Manag. 2007, 39, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.C.; Norris, R.H.; Reynoldson, T.B. Bioassessment of Freshwater Ecosystems. In Bioassessment of Freshwater Ecosystems: Using the Reference Condition Approach; Springer US: Boston, MA, USA, 2004; pp. 1–15. [Google Scholar] [CrossRef]
- Linke, S.; Bailey, R.C.; Schwindt, J. Temporal variability of stream bioassessments using benthic macroinvertebrates. Freshw. Biol. 1999, 42, 575–584. [Google Scholar] [CrossRef]
- Leunda, P.M.; Oscoz, J.; Miranda, R.; Ariño, A.H. Longitudinal and seasonal variation of the benthic macroinvertebrate community and biotic indices in an undisturbed Pyrenean river. Ecol. Indic. 2009, 9, 52–63. [Google Scholar] [CrossRef]
- Robinson, C.T.; Minshall, G.W.; Royer, T.V. Inter-annual patterns in macroinvertebrate communities of wilderness streams in Idaho, USA. Hydrobiologia 2000, 421, 187–198. [Google Scholar] [CrossRef]
- Scarsbrook, M.R. Persistence and stability of lotic invertebrate communities in New Zealand. Freshw. Biol. 2002, 47, 417–431. [Google Scholar] [CrossRef]
- Milner, A.M.; Conn, S.C.; Brown, L.E. Persistence and stability of macroinvertebrate communities in streams of Denali National Park, Alaska: Implications for biological monitoring. Freshw. Biol. 2006, 51, 373–387. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Connell, J.H.; Sousa, W.P. On the evidence needed to judge ecological stability or persistence. Am. Nat. 1983, 121, 789–824. [Google Scholar] [CrossRef]
- Meffe, G.K.; Minckley, W.L. Persistence and stability of fish and invertebrate assemblages in a repeatedly disturbed Sonoran Desert stream. Am. Midl. Nat. 1987, 117, 177–191. [Google Scholar] [CrossRef]
- Townsend, C.R.; Hildrew, A.G.; Schofield, K. Persistence of stream invertebrate communities in relation to environmental variability. J. Anim. Ecol. 1987, 56, 597–613. [Google Scholar] [CrossRef]
- Bradley, D.C.; Ormerod, S.J. Community persistence among stream invertebrates tracks the North Atlantic Oscillation. J. Anim. Ecol. 2001, 70, 987–996. [Google Scholar] [CrossRef]
- Collier, K.J. Temporal patterns in the stability, persistence and condition of stream macroinvertebrate communities: relationships with catchment land-use and regional climate. Freshw. Biol. 2008, 53, 603–616. [Google Scholar] [CrossRef]
- Pace, G.; Bonada, N.; Prat, N. Long-term effects of climatic–hydrological drivers on macroinvertebrate richness and composition in two Mediterranean streams. Freshw. Biol. 2013, 58, 1313–1328. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Q.; Jiang, W.; Qu, X. Inter-annual patterns in the stability and persistence of stream macroinvertebrate communities: relationship with water physicochemical parameters. J. Freshw. Ecol. 2013, 28, 79–90. [Google Scholar] [CrossRef]
- Quinlan, C.; Maaskant, K. 2017 Upper Thames River Watershed Report Cards; Upper Thames River Conservation Authority: London, ON, Canada, 2017. [Google Scholar]
- Maaskant, K.; Quinlan, C.; Taylor, I. The Upper Thames River Watershed Report Cards 2001; Upper Thames River Conservation Authority: London, ON, Canada, 2001. [Google Scholar]
- Maaskant, K.; Quinlan, C. 2007 Upper Thames River Watershed Report Cards; Upper Thames River Conservation Authority: London, ON, Canada, 2007. [Google Scholar]
- Maaskant, K.; Quinlan, C. 2012 Upper Thames River Watershed Report Cards; Upper Thames River Conservation Authority: London, ON, Canada, 2012. [Google Scholar]
- Berger, W.H.; Parker, F.L. Diversity of Planktonic Foraminifera in Deep-Sea Sediments. Science 1970, 168, 1345. [Google Scholar] [CrossRef]
- Armanini, D.G.; Horrigan, N.; Monk, W.A.; Peters, D.L.; Baird, D.J. Development of a benthic macroinvertebrate flow sensitivity index for Canadian rivers. River Res. Appl. 2011, 27, 723–737. [Google Scholar] [CrossRef]
- Hering, D.; Moog, O.; Sandin, L.; Verdonschot, P.F.M. Overview and application of the AQEM assessment system. Hydrobiologia 2004, 516, 1–20. [Google Scholar] [CrossRef]
- Hilsenhoff, W.L. Rapid field assessment of organic pollution with a family-level biotic Index. J. N. Am. Benthol. Soc. 1988, 7, 65–68. [Google Scholar] [CrossRef]
- Ontario Provincial Water Quality Monitoring Network (PWQMN) database. Available online: http://www.ontario.ca/environment-and-energy/provincial-stream-water-quality-monitoring-network (accessed on 22 April 2017).
- National Water Data Archive: HYDAT. Available online: http://www.ec.gc.ca/rhc-wsc/default.asp?lang=En&n=9018B5EC-1 (accessed on 22 April 2017).
- Monk, W.A.; Peters, D.L.; Baird, D.J. Assessment of ecologically relevant hydrological variables influencing a cold-region river and its delta: the Athabasca River and the Peace–Athabasca Delta, northwestern Canada. Hydrol. Process. 2012, 26, 1827–1839. [Google Scholar] [CrossRef]
- Peters, D.L.; Monk, W.A.; Baird, D.J. Cold-regions Hydrological Indicators of Change (CHIC) for ecological flow needs assessment. Hydrol. Sci. J. 2014, 59, 502–516. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R package Version 1.17-4. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 12 January 2016).
- R Development Core Team. R Foundation for Statistical Computing; Version 2.12.0. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 12 January 2016).
- Kendall, M.G. Rank Correlation Measures; Charles Griffin: London, UK, 1975. [Google Scholar]
- Mann, H.B. Non-parametric tests against trend. Economtrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Marchetto, A. Mann-Kendall test, Seasonal and Regional Kendall Tests; The Comprehensive R Archive Network: Vienna, Austria, 2015. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amersterdam, The Netherlands, 1998. [Google Scholar]
- Kalela-Brundin, M. Climatic information from tree-rings of Pinus sylvestris L. and a reconstruction of summer temperatures back to AD 1500 in Femundsmarka, eastern Norway, using partial least squares regression (PLS) analysis. Holocene 1999, 9, 59–77. [Google Scholar] [CrossRef]
- Smoliak, B.V.; Wallace, J.M.; Stoelinga, M.T.; Mitchell, T.P. Application of partial least squares regression to the diagnosis of year-to-year variations in Pacific Northwest snowpack and Atlantic hurricanes. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Kinnard, C.; Zdanowicz, C.M.; Fisher, D.A.; Isaksson, E.; de Vernal, A.; Thompson, L.G. Reconstructed changes in Arctic sea ice over the past 1,450 years. Nature 2011, 479, 509. [Google Scholar] [CrossRef]
- Bougeard, S.; Qannari, E.M.; Lupo, C.; Hanafi, M. From Multiblock Partial Least Squares to Multiblock Redundancy Analysis. A Continuum Approach. Informatica 2011, 22, 11–26. [Google Scholar]
- Eriksson, L.; Hermens, J.L.M.; Johansson, E.; Verhaar, H.J.M.; Wold, S. Multivariate analysis of aquatic toxicity data with PLS. Aquat Sci 1995, 57, 217–241. [Google Scholar] [CrossRef]
- Trap, J.; Hättenschwiler, S.; Gattin, I.; Aubert, M. Forest ageing: An unexpected driver of beech leaf litter quality variability in European forests with strong consequences on soil processes. For. Ecol. Manag. 2013, 302, 338–345. [Google Scholar] [CrossRef]
- Mevik, B.-H.; Wehrens, R. The pls Package: Principal Component and Partial Least Squares Regression in R. J. Stat. Softw. 2007, 18, 1–23. [Google Scholar] [CrossRef]
- Woodward, G.; Jones, J.I.; Hildrew, A.G. Community persistence in Broadstone Stream (U.K.) over three decades. Freshw. Biol. 2002, 47, 1419–1435. [Google Scholar] [CrossRef]
- Fore, L.S.; Karr, J.R.; Wisseman, R.W. Assessing Invertebrate Responses to Human Activities: Evaluating Alternative Approaches. J. N. Am. Benthol. Soc. 1996, 15, 212–231. [Google Scholar] [CrossRef]
- Vinson, M.R.; Hawkins, C.P. Effects of sampling area and subsampling procedure on comparisons of taxa richness among streams. J. N. Am. Benthol. Soc. 1996, 15, 392–399. [Google Scholar] [CrossRef]
- Chen, K.; Hughes, R.M.; Wang, B. Effects of fixed-count size on macroinvertebrate richness, site separation, and bioassessment of Chinese monsoonal streams. Ecol. Indic. 2015, 53, 162–170. [Google Scholar] [CrossRef]
- Maloney, K.O.; Munguia, P.; Mitchell, R.M. Anthropogenic disturbance and landscape patterns affect diversity patterns of aquatic benthic macroinvertebrates. J. N. Am. Benthol. Soc. 2011, 30, 284–295. [Google Scholar] [CrossRef]
- Krynak, E.M.; Yates, A.G. Benthic invertebrate taxonomic and trait associations with land use in an intensively managed watershed: Implications for indicator identification. Ecol. Indic. 2018, 93, 1050–1059. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L. Ecological processes driving biotic homogenization: Testung a mechanistic model using fish faunas. Ecology 2004, 85, 1867–1875. [Google Scholar] [CrossRef]
- Culp, J.M.; Armanini, D.G.; Dunbar, M.J.; Orlofske, J.M.; Poff, N.L.; Pollard, A.I.; Yates, A.G.; Hose, G.C. Incorporating traits in aquatic biomonitoring to enhance causal diagnosis and prediction. Integr. Environ. Assess. Manag. 2011, 7, 187–197. [Google Scholar] [CrossRef]
- Poff, N.L. Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J. N. Am. Benthol. Soc. 1997, 16, 391–409. [Google Scholar] [CrossRef]
- Reynoldson, T.B.; Logan, C.; Pascoe, T.; Thompson, S.P. Canadian Aquatic Biomonitoring Network Invertebrate Biomonitoring Field and Laboratory Manual; Environment Canada: Burlington, ON, Canada, 2007.
- Davies, P.E. Development of A National River Bioassessment System (AUSRIVAS) in Australia; Freshwater Biological Association (FBA): Ambleside, UK, 2000; pp. 113–124. [Google Scholar]
- Vieira, N.K.M.; Poff, N.L.; Carlisle, D.M.; Moulton Ii, S.R.; Koski, M.L.; Kondratieff, B.C. A Database of Lotic Invertebrate Traits for North America; U.S. Geological Survey: Reston, VA, USA, 2006.
- Schäfer, R.B.; Kefford, B.J.; Metzeling, L.; Liess, M.; Burgert, S.; Marchant, R.; Pettigrove, V.; Goonan, P.; Nugegoda, D. A trait database of stream invertebrates for the ecological risk assessment of single and combined effects of salinity and pesticides in South-East Australia. Sci. Total Environ. 2011, 409, 2055–2063. [Google Scholar] [CrossRef]
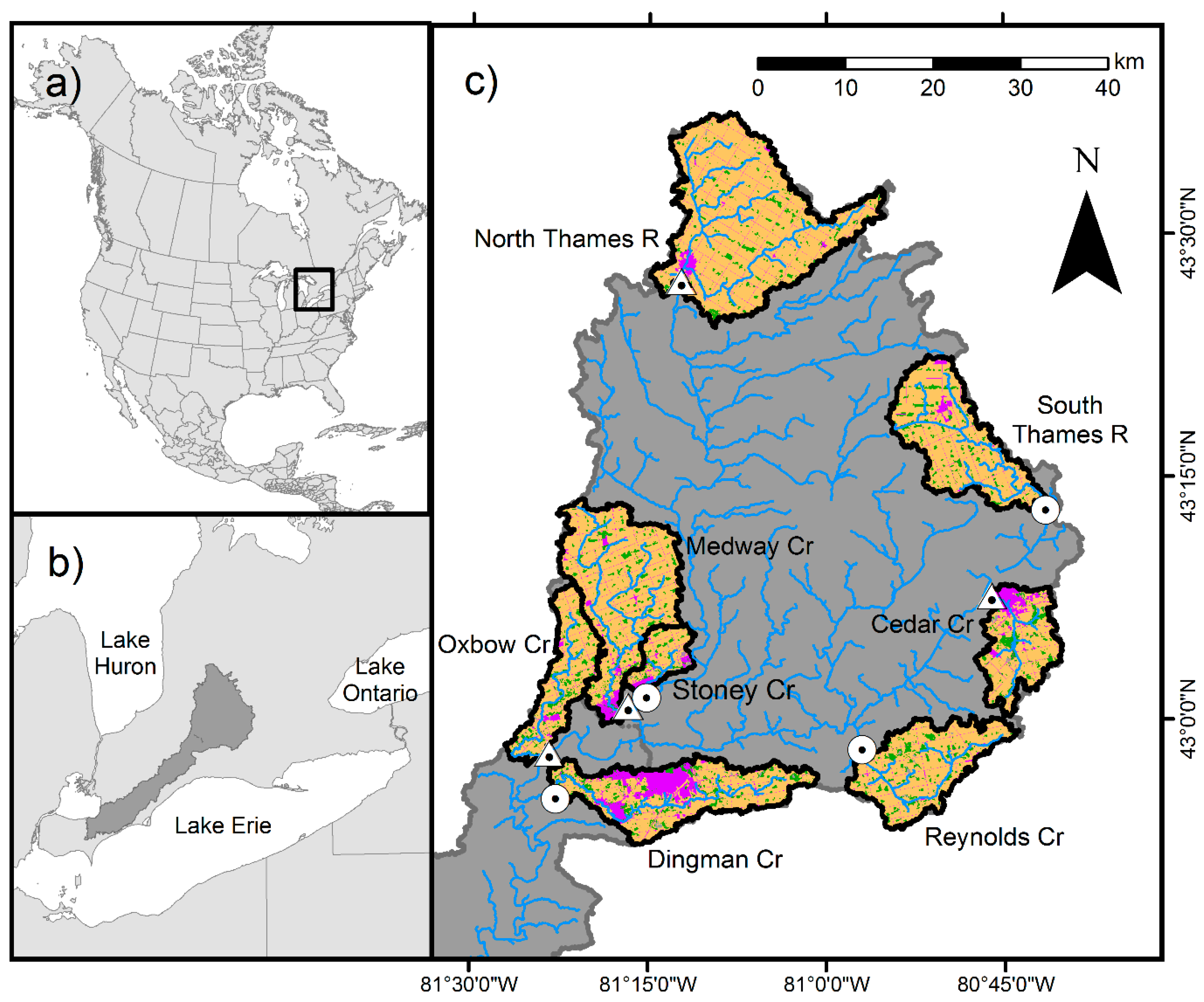
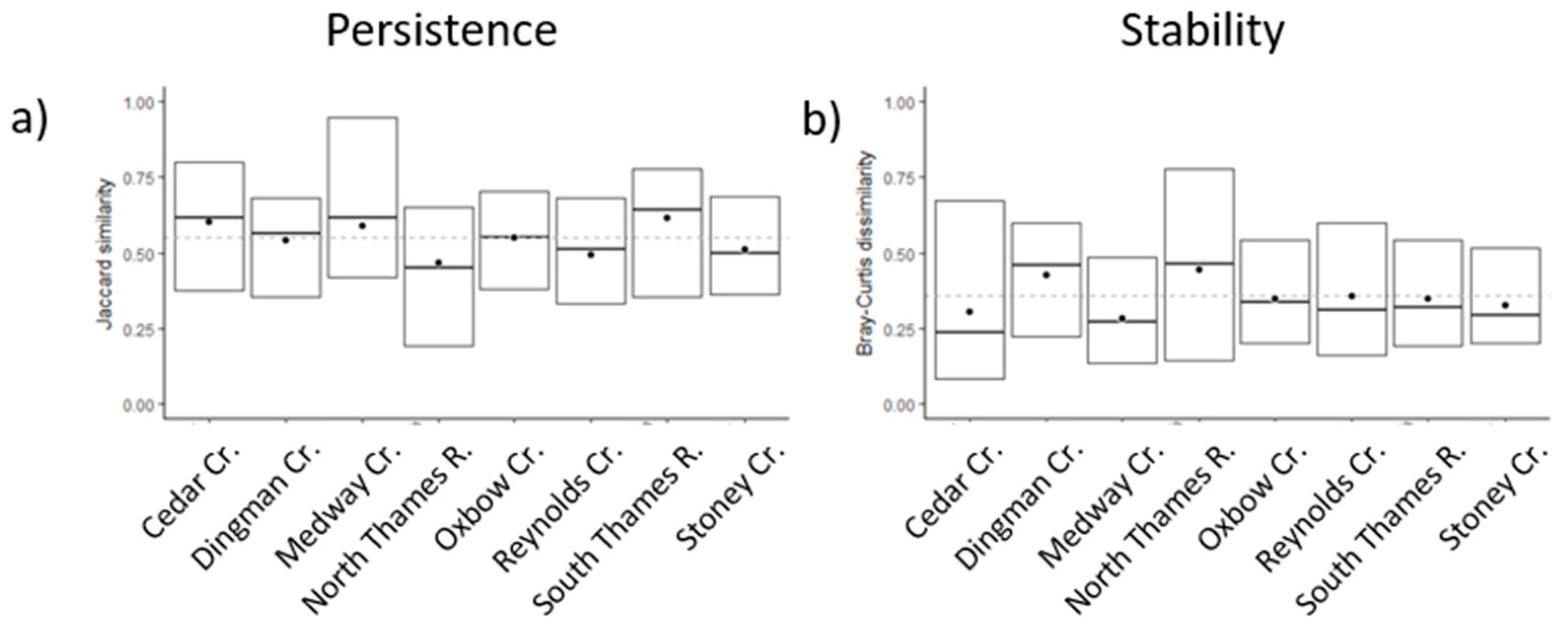
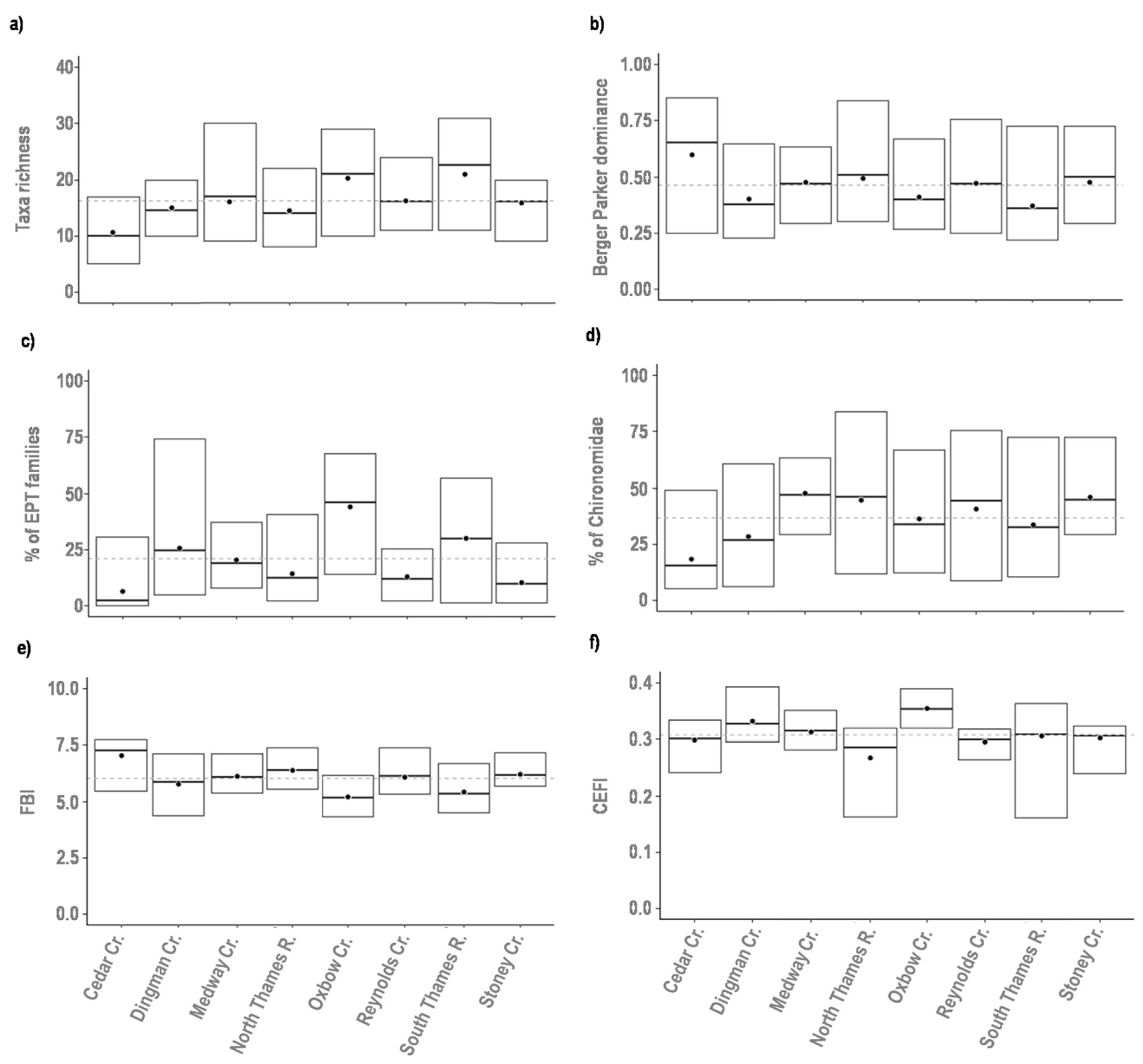
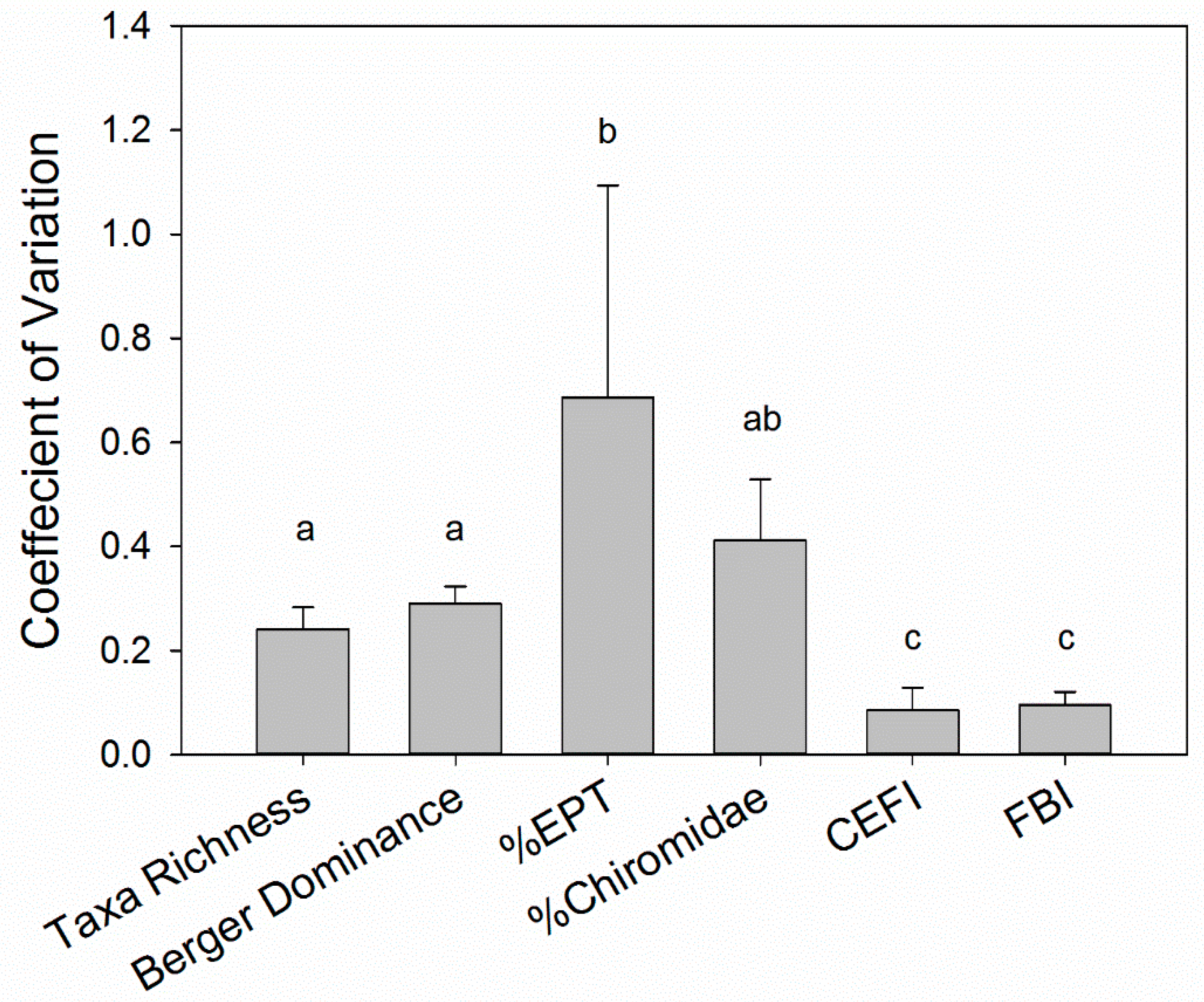
| Stream | Latitude | Longitude | Drainage Area (km2) | Urban (%) | Agriculture (%) | Forest (%) | Dam Present |
|---|---|---|---|---|---|---|---|
| Cedar Creek | 43.122 | −80.7515 | 87.75 | 10.0 | 70.6 | 3.8 | yes |
| Dingman Creek | 42.934 | −81.3513 | 148.59 | 15.5 | 64.0 | 8.7 | no |
| Medway Creek | 42.966 | −81.4180 | 85.66 | 3.8 | 82.4 | 6.6 | yes |
| North Thames River | 43.450 | −81.2068 | 315.4 | 1.3 | 90.4 | 3.1 | yes |
| Oxbow Creek | 43.014 | −81.2804 | 203.19 | 1.8 | 85.3 | 5.0 | yes |
| Reynolds Creek | 42.982 | −80.9546 | 145.14 | 2.4 | 79.6 | 9.6 | no |
| South Thames River | 43.215 | −80.6919 | 148.85 | 0.6 | 84.8 | 5.7 | no |
| Stoney Creek | 43.022 | −81.2534 | 37.33 | 14.4 | 62.8 | 7.2 | no |
| Stream | Benthic | Water Chemistry | Stream Flow |
|---|---|---|---|
| Cedar Creek | 1997 to 2016 (20) | 2004 to 2014 (11) | 1997 to 2016 (20) |
| Dingman Creek | 1998 to 2016 (19) | 2004 to 2014 (11) | 1997 to 2015 (19) |
| Medway Creek | 1997 to 2016 (20) | 1997 to 2014 (11) | 1997 to 2015 (19) |
| North Thames River | 1997 to 2016 (20) | 2004 to 2014 (11) | 1997 to 2015 (19) |
| Oxbow Creek | 1998 to 2016 (19) | 2004 to 2014 (11) | 2003 to 2015 (14) |
| Reynolds Creek | 1998 to 2016 (19) | 2004 to 2014 (11) | 2003 to 2016 (14) |
| South Thames River | 1997 to 2016 (20) | 2004 to 2014 (11) | 1997 to 2015 (19) |
| Stoney Creek | 2003 to 2016 (14) | 2004 to 2014 (11) | 2003 to 2015 (14) |
| Parameter | Description | Reference | |
|---|---|---|---|
| Bioassessment Metrics | Berger–Parker dominance index | Measure of taxa dominance based on the proportional abundance of the most abundant taxon | [22] |
| Canadian Ecological Flow Index (CEFI) | Measure to assess ecological responses to hydrological alterations | [23] | |
| Hilsenhoff Family Biotic Index (FBI) | Index based on the tolerance of family taxa to organic pollution | [24] | |
| Taxa richness | Total number of taxa in a sample | [25] | |
| % of Chironomidae | Percentage of Chironomidae taxa in the sample | [25] | |
| % of EPT families | Percentage of Ephemeroptera, Plecoptera, and Trichoptera taxa in the sample | [25] | |
| Water Quality | Conductivity | Mean annual conductivity of stream water (µS cm−1) | |
| Total Phosphorus (TP) | Mean annual concentration of Total Phosphorus (mg P/l) | ||
| Nitrate | Mean annual concentration of Nitrate (mg N/l) | ||
| Hydrology | Magnitude of spring freshet | Maximum flow rate (m3 s−1) measured during the spring freshet | [26] |
| Date of spring freshet | Hydrologic date the maximum flow rate during the spring freshet was measured | [26] | |
| Magnitude of peak flow in ice-influenced period | Maximum flow rate (m3 s−1) measured during the ice-influenced period | [26] | |
| Date of peak flow in ice-influenced period | Hydrologic date the maximum flow rate during the ice-influenced period was measured | [26] | |
| Magnitude of peak flow in open-water period | Maximum flow rate (m3 s−1) measured during the open-water period | [26] | |
| Date of peak flow in open-water period | Hydrologic date the maximum flow rate during the open-water period was measured | [26] |
| Biological Parameter | Cedar Cr | Dingman Cr | Medway Cr | North Thames R | Oxbow Cr | Reynolds Cr | South Thames R | Stoney Cr |
|---|---|---|---|---|---|---|---|---|
| Community Persistence | −0.009 (−56) | 0.014 (80) | 0.023 (47) | |||||
| Community Stability | 0.012 (50) | −0.008 (−53) | −0.014 (−67) | −0.013 (−59) | −0.023 (−38) | |||
| Taxa richness | 0.273 (83) | 0.444 (102) | 0.500 (50) | 0.333 (54) | 0.631 (74) | |||
| % of EPT families | ||||||||
| % of Chironomidae | −0.005 (−56) | |||||||
| Berger–Parker dominance index | ||||||||
| Hilsenhoff Family Biotic Index | −0.004 (−56) | |||||||
| Canadian Ecological Flow Index |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idígoras Chaumel, A.L.; Armanini, D.G.; Schwindt, J.A.; Yates, A.G. Interannual Variation of Benthic Macroinvertebrate Communities at Long-Term Monitoring Sites Impacted by Human Activities: Implications for Bioassessment. Diversity 2019, 11, 167. https://doi.org/10.3390/d11090167
Idígoras Chaumel AL, Armanini DG, Schwindt JA, Yates AG. Interannual Variation of Benthic Macroinvertebrate Communities at Long-Term Monitoring Sites Impacted by Human Activities: Implications for Bioassessment. Diversity. 2019; 11(9):167. https://doi.org/10.3390/d11090167
Chicago/Turabian StyleIdígoras Chaumel, Almudena L., David G. Armanini, John A. Schwindt, and Adam G. Yates. 2019. "Interannual Variation of Benthic Macroinvertebrate Communities at Long-Term Monitoring Sites Impacted by Human Activities: Implications for Bioassessment" Diversity 11, no. 9: 167. https://doi.org/10.3390/d11090167
APA StyleIdígoras Chaumel, A. L., Armanini, D. G., Schwindt, J. A., & Yates, A. G. (2019). Interannual Variation of Benthic Macroinvertebrate Communities at Long-Term Monitoring Sites Impacted by Human Activities: Implications for Bioassessment. Diversity, 11(9), 167. https://doi.org/10.3390/d11090167





