Effects of Changing Vegetation Composition on Community Structure, Ecosystem Functioning, and Predator–Prey Interactions at the Saltmarsh-Mangrove Ecotone
Abstract
:1. Introduction
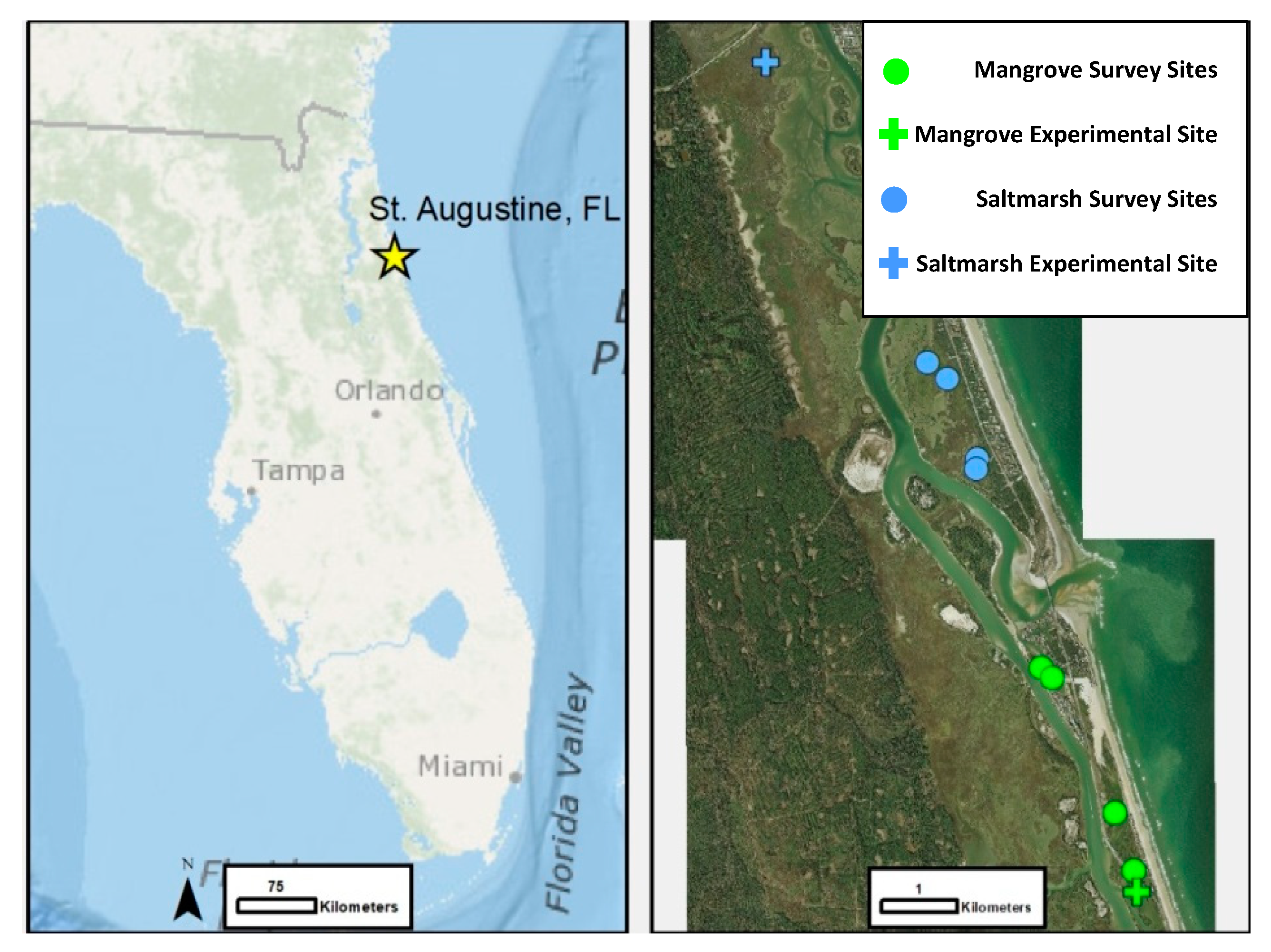
2. Materials and Methods
2.1. Predation Exclusion Experiment
2.2. Ecotone Survey
2.3. Data Analysis
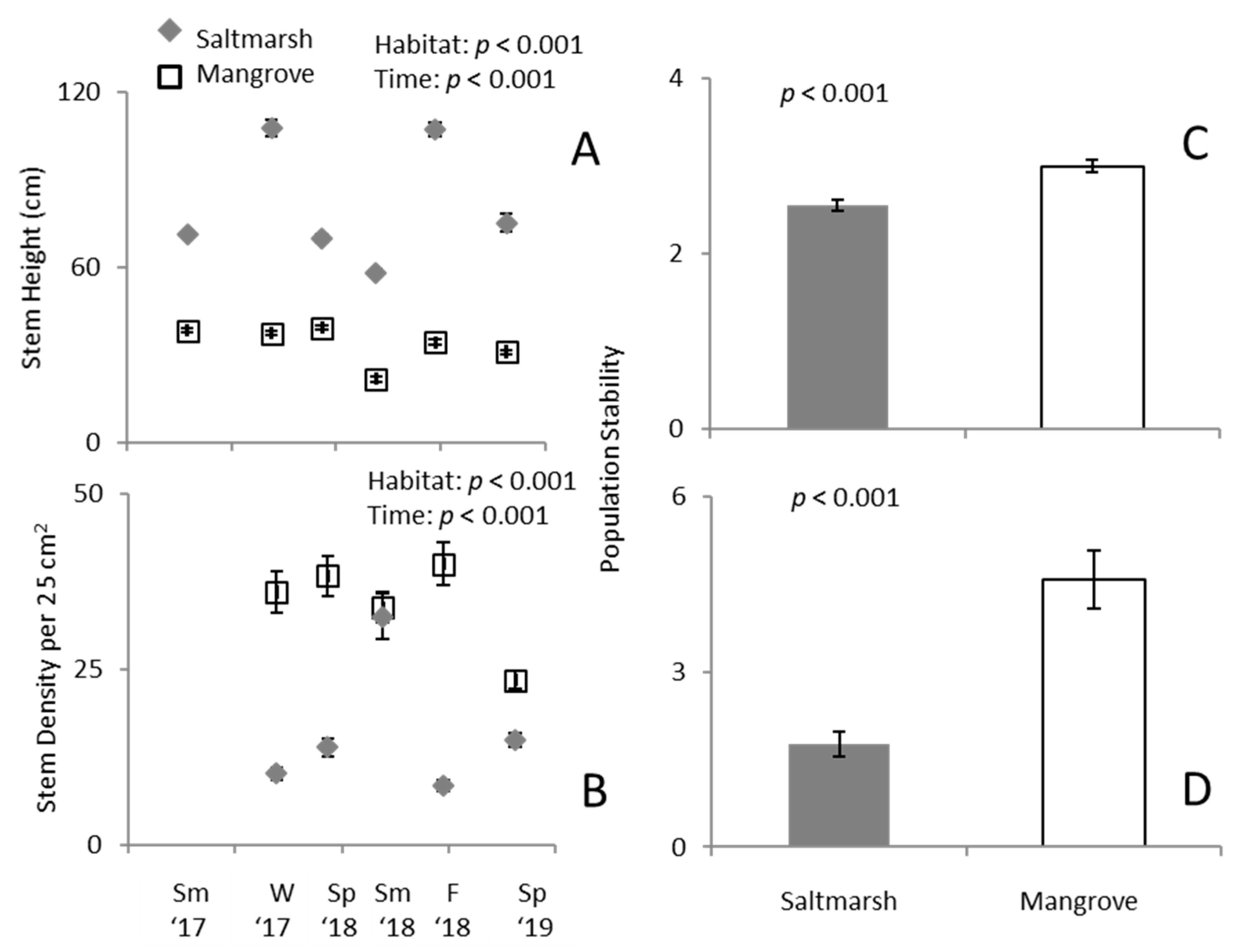
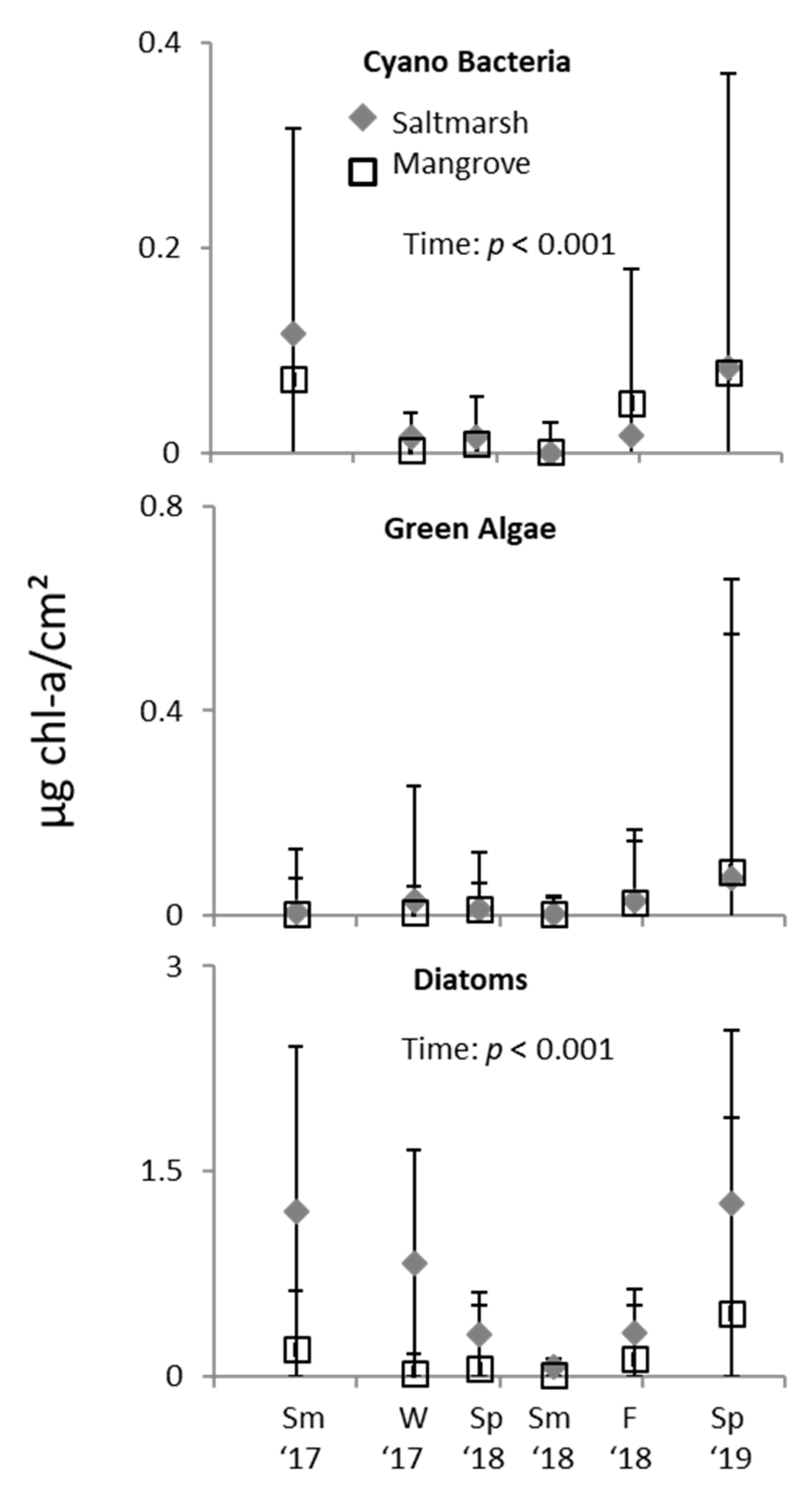
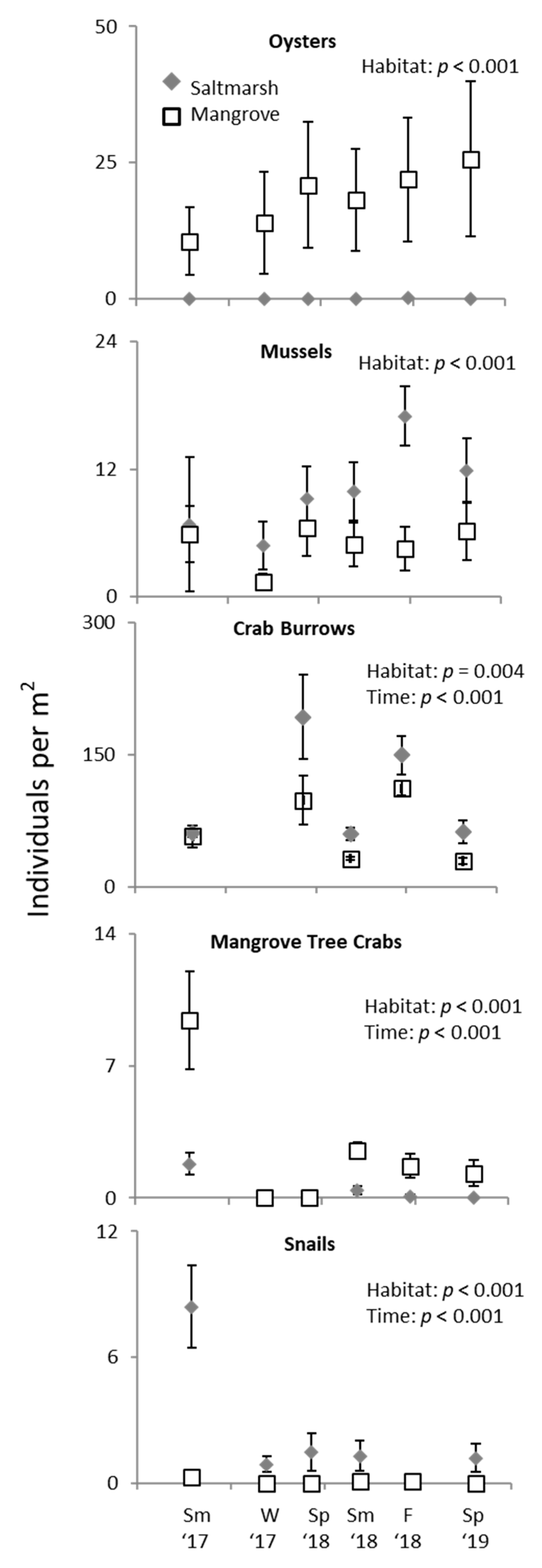
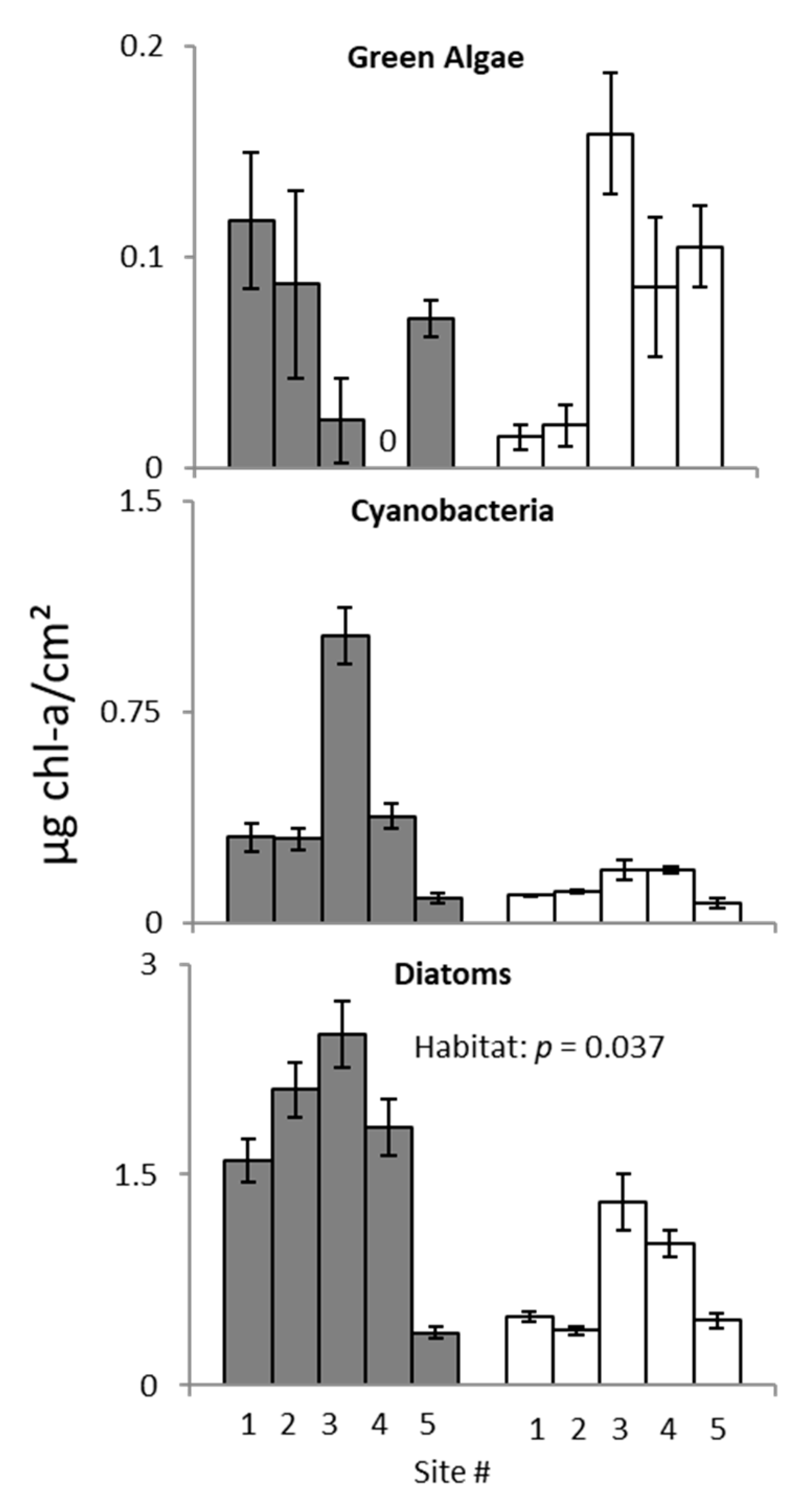
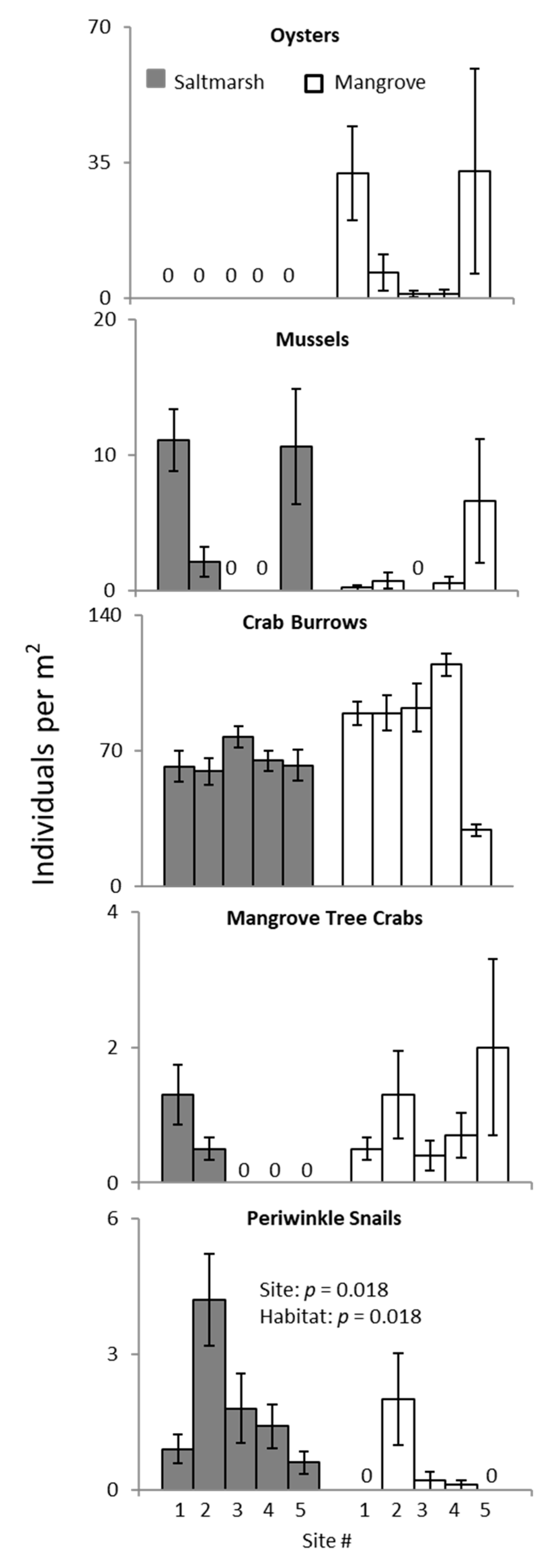
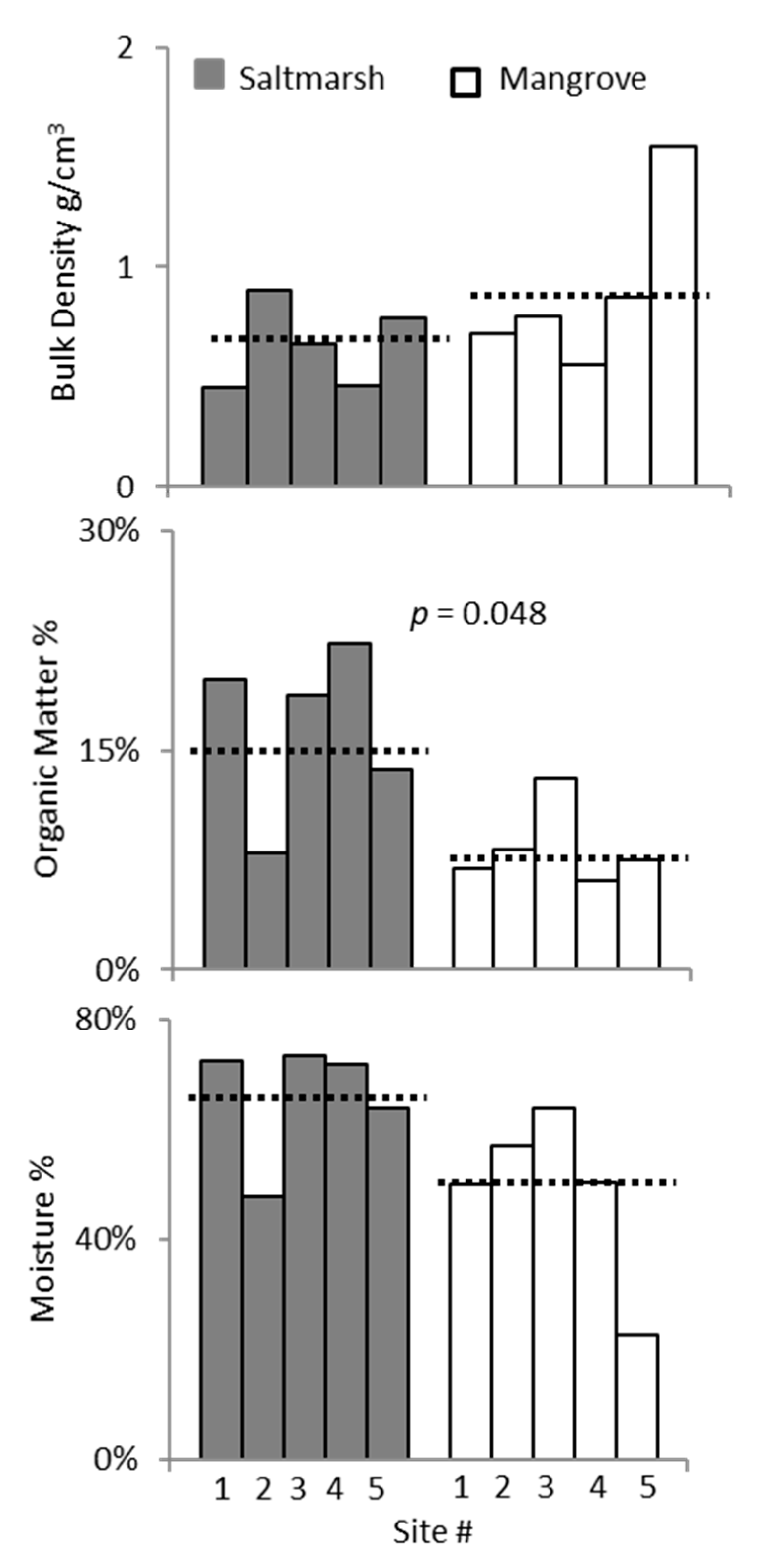
3. Results
3.1. Predation Exclusion Experiment
3.2. Ecotone Survey
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boesch, D.F.; Turner, R.E. Dependence of fishery species on salt marshes: The role of food and refuge. Estuaries 1984, 7, 460–468. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Costanza, R.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; Raskin, R.G.; Sutton, P. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Hatcher, B.G.; Johannes, R.E.; Robertson, A.I. Review of research relevant to the conservation of shallow tropical marine ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 1989, 27, 337–414. [Google Scholar]
- Mitsch, W.J.; Gosselink, J.G. The value of wetlands: Importance of scale and landscape setting. Ecol. Econ. 2000, 35, 25–33. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Allison, M.A.; Zhao, J.; Li, X.; Comeaux, R.S.; Feagin, R.A.; Kulawardhana, R.W. Historical reconstruction of mangrove expansion in the Gulf of Mexico: Linking climate change with carbon sequestration in coastal wetlands. Estuar. Coast. Shelf Sci. 2013, 119, 7–16. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Kellner, J.R.; Forde, A.J.; Gruner, D.S.; Parker, J.D.; Rodriguez, W.; Feller, I.C. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc. Natl. Acad. Sci. USA 2014, 111, 723–727. [Google Scholar] [CrossRef]
- Schaeffer-Novelli, Y.; Soriano-Sierra, E.J.; do Vale, C.C.; Bernini, E.; Rovai, A.S.; Pinheiro, M.A.A.; Schmidt, A.J.; de Almeida, R.; Júnior, C.C.; Menghini, R.P.; et al. Climate changes in mangrove forests and salt marshes. Braz. J. Oceanogr. 2016, 64, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Simpson, L.T.; Osborne, T.Z.; Feller, I.C. Wetland soil CO2 efflux along a latitudinal gradient of spatial and temporal complexity. Estuaries Coasts 2019, 42, 45–54. [Google Scholar] [CrossRef]
- Perillo, G.; Wolanski, E.; Cahoon, D.R.; Hopkinson, C.S. Coastal Wetlands: An Integrated Ecosystem Approach; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-444-63894-6. [Google Scholar]
- Kelleway, J.J.; Cavanaugh, K.; Rogers, K.; Feller, I.C.; Ens, E.; Doughty, C.; Saintilan, N. Review of the ecosystem service implications of mangrove encroachment into salt marshes. Glob. Chang. Biol. 2017, 23, 3967–3983. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, M.L.; Megonigal, J.P. Tidal wetland stability in the face of human impacts and sea-level rise. Nature 2013, 504, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bretsch, K.; Allen, D.M. Tidal migrations of nekton in salt marsh intertidal creeks. Estuaries Coasts 2006, 29, 474–486. [Google Scholar] [CrossRef]
- Sheridan, P.; Hays, C. Are mangroves nursery habitat for transient fishes and decapods? Wetlands 2003, 23, 449–458. [Google Scholar] [CrossRef]
- Minello, T.J.; Able, K.W.; Weinstein, M.P.; Hays, C.G. Salt marshes as nurseries for nekton: Testing hypotheses on density, growth and survival through meta-analysis. Mar. Ecol. Prog. Ser. 2003, 246, 39–59. [Google Scholar] [CrossRef]
- Skilleter, G.A.; Warren, S. Effects of habitat modification in mangroves on the structure of mollusc and crab assemblages. J. Exp. Mar. Biol. Ecol. 2000, 244, 107–129. [Google Scholar] [CrossRef] [Green Version]
- McGarigal, K.; Marks, B.J. Fragstats: Spatial Pattern Analysis Program for Quantifying Landscape Structure; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; p. 122.
- Cuddington, K.; Yodzis, P. Predator-prey dynamics and movement in fractal environments. Am. Nat. 2002, 160, 119–134. [Google Scholar] [CrossRef]
- Vince, S.; Valiela, I.; Backus, N.; Teal, J.M. Predation by the salt marsh killifish Fundulus heteroclitus (L.) in relation to prey size and habitat structure: Consequences for prey distribution and abundance. J. Exp. Mar. Biol. Ecol. 1976, 23, 255–266. [Google Scholar] [CrossRef]
- Kenyon, R.A.; Loneragan, N.R.; Hughes, J.M. Habitat type and light affect sheltering behaviour of juvenile tiger prawns (Penaeus esculentus Haswell) and success rates of their fish predators. J. Exp. Mar. Biol. Ecol. 1995, 192, 87–105. [Google Scholar] [CrossRef]
- Primavera, J.H. Fish predation on mangrove-associated penaeids: The role of structures and substrate. J. Exp. Mar. Biol. Ecol. 1997, 215, 205–216. [Google Scholar] [CrossRef]
- Laegdsgaard, P.; Johnson, C. Why do juvenile fish utilise mangrove habitats? J. Exp. Mar. Biol. Ecol. 2001, 257, 229–253. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.H.; Tremain, D.M. Association of large juvenile red drum, Sciaenops ocellatus, with an Estuarine Creek on the Atlantic Coast of Florida. Environ. Biol. Fishes 2000, 58, 183–194. [Google Scholar] [CrossRef]
- Orth, R.J. The importance of sediment stability in seagrass communities. In Ecology of Marine Benthos; University of South Carolina Press: Columbia, SC, USA, 1977; pp. 281–300. [Google Scholar]
- Virnstein, R.W. The importance of predation by crabs and fishes on benthic infauna in Chesapeake Bay. Ecology 1977, 58, 1200–1217. [Google Scholar] [CrossRef]
- Wang, M.; Gao, X.; Wang, W. Differences in burrow morphology of crabs between Spartina alterniflora marsh and mangrove habitats. Ecol. Eng. 2014, 69, 213–219. [Google Scholar] [CrossRef]
- Angelini, C.; van der Heide, T.; Griffin, J.N.; Morton, J.P.; Derksen-Hooijberg, M.; Lamers, L.P.M.; Smolders, A.J.P.; Silliman, B.R. Foundation species’ overlap enhances biodiversity and multifunctionality from the patch to landscape scale in southeastern United States salt marshes. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150421. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Thomas, J.; Proffitt, C.E. Oysters Crassostrea virginica on red mangrove Rhizophora mangle prop roots: Facilitation of one foundation species by another. Mar. Ecol. Prog. Ser. 2014, 503, 177–194. [Google Scholar] [CrossRef]
- Bertness, M.D. Fiddler crab regulation of Spartina alterniflora production on a New England salt marsh. Ecology 1985, 66, 1042–1055. [Google Scholar] [CrossRef]
- Levin, L.A.; Neira, C.; Grosholz, E.D. Invasive cordgrass modifies wetland trophic function. Ecology 2006, 87, 419–432. [Google Scholar] [CrossRef]
- Neira, C.; Grosholz, E.D.; Levin, L.A.; Blake, R. Mechanisms generating modification of benthos following tidal flat invasion by a Spartina hybrid. Ecol. Appl. 2006, 16, 1391–1404. [Google Scholar] [CrossRef]
- Igulu, M.M.; Nagelkerken, I.; van der Velde, G.; Mgaya, Y.D. Mangrove fish production is largely fueled by external food sources: A stable isotope analysis of fishes at the individual, species, and community levels from across the globe. Ecosystems 2013, 16, 1336–1352. [Google Scholar] [CrossRef]
- Perry, C.L.; Mendelssohn, I.A. Ecosystem effects of expanding populations of Avicennia germinans in a Louisiana salt marsh. Wetlands 2009, 29, 396–406. [Google Scholar] [CrossRef]
- Bouillon, S.; Koedam, N.; Raman, A.; Dehairs, F. Primary producers sustaining macro-invertebrate communities in intertidal mangrove forests. Oecologia 2002, 130, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, B.J.; Howarth, R.W. Sulfur, carbon, and nitrogen isotopes used to trace organic matter flow in the salt-marsh estuaries of Sapelo Island, Georgia 1. Limnol. Oceanogr. 1987, 32, 1195–1213. [Google Scholar] [CrossRef]
- Newell, R.I.E.; Marshall, N.; Sasekumar, A.; Chong, V.C. Relative importance of benthic microalgae, phytoplankton, and mangroves as sources of nutrition for penaeid prawns and other coastal invertebrates from Malaysia. Mar. Biol. 1995, 123, 595–606. [Google Scholar] [CrossRef]
- Sullivan, M.; Moncreiff, C. Edaphic algae are an important component of salt marsh food-webs: Evidence from multiple stable isotope analyses. Mar. Ecol. Prog. Ser. 1990, 62, 149–159. [Google Scholar] [CrossRef]
- Williams, A.A.; Eastman, S.F.; Eash-Loucks, W.E.; Kimball, M.E.; Lehmann, M.L.; Parker, J.D. Record northernmost endemic mangroves on the United States Atlantic coast with a note on latitudinal migration. Southeast. Nat. 2014, 13, 56–63. [Google Scholar] [CrossRef]
- Rodriguez, W.; Feller, I.C.; Cavanaugh, K.C. Spatio-temporal changes of a mangrove–saltmarsh ecotone in the northeastern coast of Florida, USA. Glob. Ecol. Conserv. 2016, 7, 245–261. [Google Scholar] [CrossRef]
- Silliman, B.R.; Bertness, M.D. A trophic cascade regulates salt marsh primary production. Proc. Natl. Acad. Sci. USA 2002, 99, 10500–10505. [Google Scholar] [CrossRef] [Green Version]
- Crotty, S.M.; Sharp, S.J.; Bersoza, A.C.; Prince, K.D.; Cronk, K.; Johnson, E.E.; Angelini, C. Foundation species patch configuration mediates salt marsh biodiversity, stability and multifunctionality. Ecol. Lett. 2018, 21, 1681–1692. [Google Scholar] [CrossRef] [Green Version]
- Lehman, C.L.; Tilman, D. Biodiversity, stability, and productivity in competitive communities. Am. Nat. 2000, 156, 534–552. [Google Scholar] [CrossRef]
- Long, Z.T.; Bruno, J.F.; Duffy, J.E. Food chain length and omnivory determine the stability of a marine subtidal food web. J. Anim. Ecol. 2011, 80, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, J.E.; Ziegler, S.L.; Campbell, J.E.; Bippus, P.M.; Lefcheck, J.S. Squidpops: A simple tool to crowdsource a global map of marine predation intensity. PLoS ONE 2015, 10, e0142994. [Google Scholar] [CrossRef] [PubMed]
- Silliman, B.R.; Bortolus, A. Underestimation of Spartina productivity in western atlantic marshes: Marsh invertebrates eat more than just detritus. Oikos 2003, 101, 549–554. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Currin, C.A. Community structure and functional dynamics of benthic microalgae in salt marshes. In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 81–106. [Google Scholar]
- Atkins, R.L.; Griffin, J.N.; Angelini, C.; O’Connor, M.I.; Silliman, B.R. Consumer–plant interaction strength: Importance of body size, density and metabolic biomass. Oikos 2015, 124, 1274–1281. [Google Scholar] [CrossRef]
- Altieri, A.H.; Bertness, M.D.; Coverdale, T.C.; Herrmann, N.C.; Angelini, C. A trophic cascade triggers collapse of a salt-marsh ecosystem with intensive recreational fishing. Ecology 2012, 93, 1402–1410. [Google Scholar] [CrossRef]
- Holdredge, C.; Bertness, M.D.; Altieri, A.H. Role of crab herbivory in die-off of New England salt marshes. Conserv. Biol. 2009, 23, 672–679. [Google Scholar] [CrossRef]
- National Marine Fisheries Service, Fisheries Statistics Division; Silver Spring, MD, USA. Personal Communication, 2019.
- Fernández, T.V.; D’Anna, G.; Badalamenti, F.; Pérez-Ruzafa, A. Habitat connectivity as a factor affecting fish assemblages in temperate reefs. Aquat. Biol. 2008, 1, 239–248. [Google Scholar] [CrossRef] [Green Version]
- McCraith, B.J.; Gardner, L.R.; Wethey, D.S.; Moore, W.S. The effect of fiddler crab burrowing on sediment mixing and radionuclide profiles along a topographic gradient in a southeastern salt marsh. J. Mar. Res. 2003, 61, 359–390. [Google Scholar] [CrossRef]
- Reis-Filho, J.A.; Giarrizzo, T.; Barros, F. Tidal migration and cross-habitat movements of fish assemblage within a mangrove ecotone. Mar. Biol. 2016, 163, 111. [Google Scholar] [CrossRef]
- Grabowski, J.H.; Powers, S.P. Habitat complexity mitigates trophic transfer on oyster reefs. Mar. Ecol. Prog. Ser. 2004, 277, 291–295. [Google Scholar] [CrossRef]
- O’Connor, N.E.; Grabowski, J.H.; Ladwig, L.M.; Bruno, J.F. Simulated predator extinctions: Predator identity affects survival and recruitment of oysters. Ecology 2008, 89, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.D.; Smee, D.L. Predators influence the tidal distribution of oysters (Crassostrea virginica). Mar. Biol. 2014, 161, 1557–1564. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, M.; Lee, S.Y. Food availability and predation risk drive the distributional patterns of two pulmonate gastropods in a mangrove-saltmarsh transitional habitat. Mar. Environ. Res. 2017, 130, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, K.L.; Brown, B.L.; Lennon, J.T. Biodiversity may regulate the temporal variability of ecological systems. Ecol. Lett. 2001, 4, 72–85. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Methuen: London, UK, 1958. [Google Scholar]
- MacArthur, R. Fluctuations of animal populations and a measure of community stability. Ecology 1955, 36, 533–536. [Google Scholar] [CrossRef]
- Riley, M.E.; Johnston, C.A.; Feller, I.C.; Griffen, B.D. Range expansion of Aratus pisonii (Mangrove Tree Crab) into novel vegetative habitats. Southeast. Nat. 2014, 13. [Google Scholar] [CrossRef]
- Schuler, M.S.; Chase, J.M.; Knight, T.M. Habitat size modulates the influence of heterogeneity on species richness patterns in a model zooplankton community. Ecology 2017, 98, 1651–1659. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Kon, K.; Kurokura, H.; Tongnunui, P. Effects of the physical structure of mangrove vegetation on a benthic faunal community. J. Exp. Mar. Biol. Ecol. 2010, 383, 171–180. [Google Scholar] [CrossRef]
- Henry, K.M.; Twilley, R.R. Soil development in a coastal Louisiana wetland during a climate-induced vegetation shift from salt marsh to mangrove. J. Coast. Res. 2013, 29, 1273–1283. [Google Scholar]
- Saintilan, N.; Wilson, N.C.; Rogers, K.; Rajkaran, A.; Krauss, K.W. Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob. Chang. Biol. 2014, 20, 147–157. [Google Scholar] [CrossRef] [PubMed]
| Habitat | S. alterniflora | A. germinans | df | f | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Max | Min | Mean | Max | Min | ||||
| Height (cm) | 79.00 | 86.5 | 73.66 | 25.96 | 31.56 | 73.66 | 1 | 412.39 | <0.001 |
| Stem Density (per 0.25 m2) | 14.60 | 17.15 | 11.30 | 27.86 | 34.40 | 11.30 | 1 | 75.68 | 0.001 |
| Inter-stem Distance (cm) | 8.20 | 9.29 | 7.54 | 4.778 | 5.86 | 7.54 | 1 | 42.97 | 0.003 |
| Sightlines (cm) | 284.69 | 374.4 | 223.11 | 260.36 | 330.71 | 223.11 | 1 | 23.89 | 0.008 |
| Flexure (N) | 1.65 | 2.09 | 1.42 | 1.10 | 1.22 | 0.88 | 1 | 3.70 | 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, J.E.; Angelini, C.; Safak, I.; Altieri, A.H.; Osborne, T.Z. Effects of Changing Vegetation Composition on Community Structure, Ecosystem Functioning, and Predator–Prey Interactions at the Saltmarsh-Mangrove Ecotone. Diversity 2019, 11, 208. https://doi.org/10.3390/d11110208
Walker JE, Angelini C, Safak I, Altieri AH, Osborne TZ. Effects of Changing Vegetation Composition on Community Structure, Ecosystem Functioning, and Predator–Prey Interactions at the Saltmarsh-Mangrove Ecotone. Diversity. 2019; 11(11):208. https://doi.org/10.3390/d11110208
Chicago/Turabian StyleWalker, Julie E., Christine Angelini, Ilgar Safak, Andrew H. Altieri, and Todd Z. Osborne. 2019. "Effects of Changing Vegetation Composition on Community Structure, Ecosystem Functioning, and Predator–Prey Interactions at the Saltmarsh-Mangrove Ecotone" Diversity 11, no. 11: 208. https://doi.org/10.3390/d11110208
APA StyleWalker, J. E., Angelini, C., Safak, I., Altieri, A. H., & Osborne, T. Z. (2019). Effects of Changing Vegetation Composition on Community Structure, Ecosystem Functioning, and Predator–Prey Interactions at the Saltmarsh-Mangrove Ecotone. Diversity, 11(11), 208. https://doi.org/10.3390/d11110208




