Lichens and Bromeliads as Bioindicators of Heavy Metal Deposition in Ecuador
Abstract
1. Introduction
2. Materials and Methods
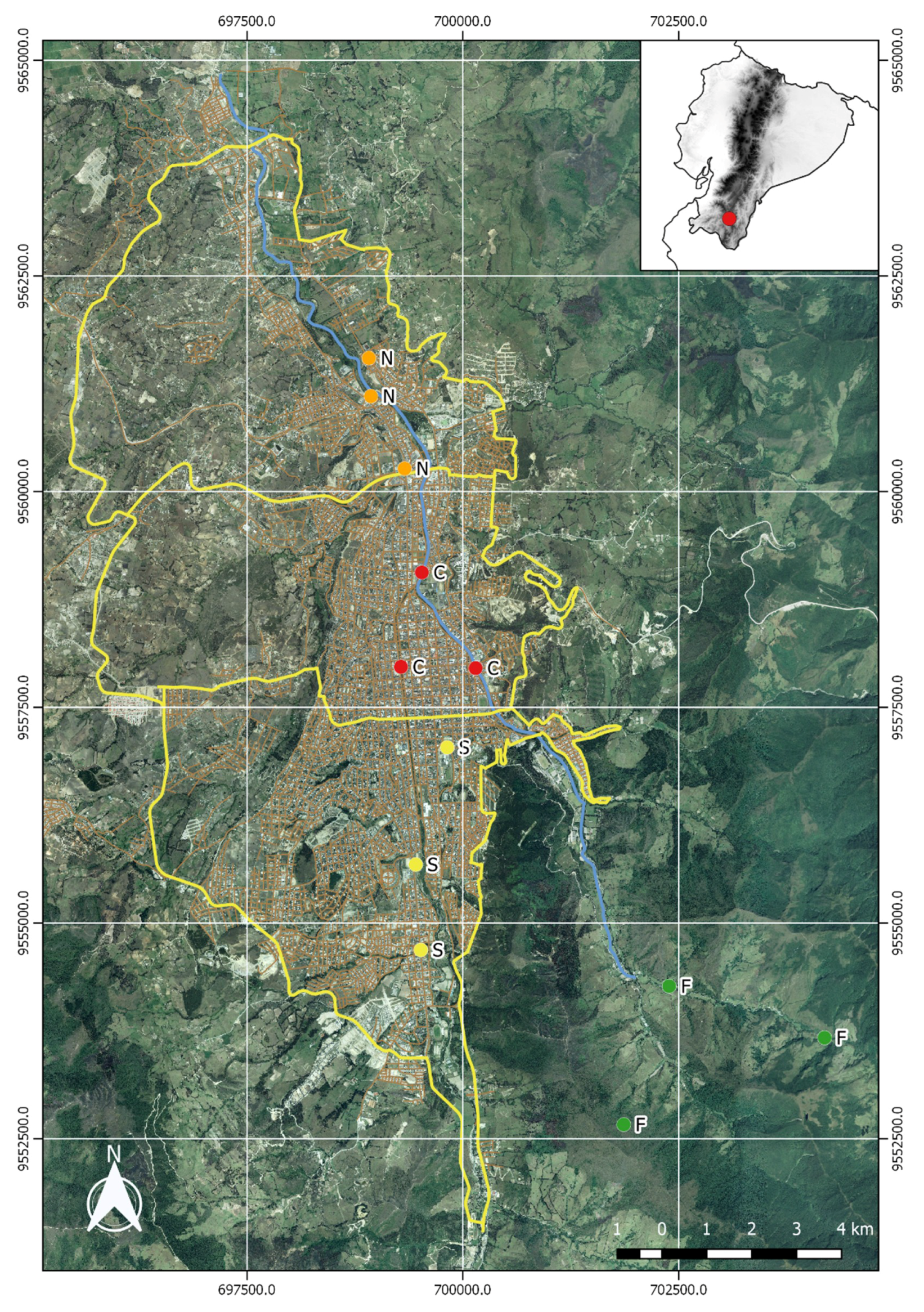
2.1. Study Area
- (1)
- Forested Zone (F): Our control zone is characterized by fragments of evergreen tropical forest close to the Podocarpus National Park. The area is generally densely vegetated with a low human population and very little rural traffic. This zone presumably acts as an air pollution buffer for the larger area surrounding the city of Loja.
- (2)
- Southern Zone (S): This district is characterized by extensive green areas and recreational parks (1,053,000 m2), and a low quantity of green area per inhabitant (15.38 m2/inhabitant), but is nevertheless subject to relatively high traffic due to the transit between this area and the city.
- (3)
- Central Zone (C): The downtown district is a mostly urban area, with a low quantity of green area per inhabitant (11.58 m2/inhabitant) and very little vegetation (green areas cover only 635,000 m2); and is subject to high volumes of traffic.
- (4)
- Northern Zone (N): With a relatively large quantity of green space (1,060,000 m2), this city district has a high amount of green space per inhabitant (38.95 m2/inhabitant); it is subject to moderate traffic only.
2.2. Heavy Metal Measures
2.3. Data Analysis
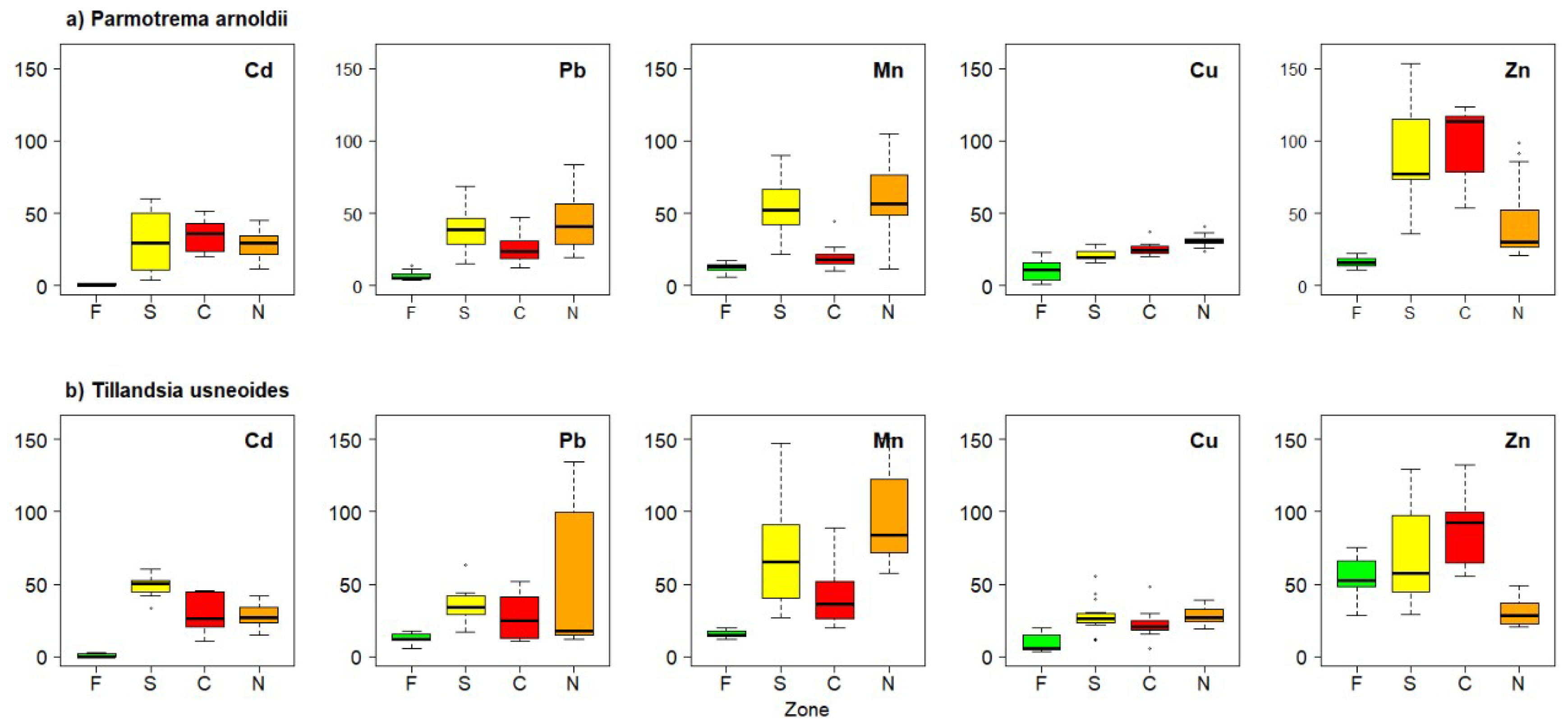
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Heavy Metal | Forest | South | Center | North |
|---|---|---|---|---|---|
| Parmotrema arnoldii | Cd | 0.60 ± 0.81 | 30.83 ± 19.12 | 34.66 ± 9.22 | 27.99 ± 9.06 |
| Cu | 10.41 ± 7.37 | 21.27 ± 3.93 | 25.41 ± 4.44 | 31.02 ± 4.13 | |
| Mn | 12.30 ± 3.43 | 53.49 ± 18.97 | 20.03 ± 8.42 | 56.81 ± 25.59 | |
| Pb | 7.14 ± 3.05 | 39.48 ± 14.66 | 25.29 ± 9.46 | 42.95 ± 18.03 | |
| Zn | 16.19 ± 3.69 | 91.37 ± 35.78 | 100.54 ± 23.92 | 44.46 ± 26.49 | |
| Tillandsia usneoides | Cd | 1.10 ± 1.27 | 49.16 ± 6.93 | 28.93 ± 12.16 | 28.11 ± 8.17 |
| Cu | 9.08 ± 5.98 | 27.57 ± 11.55 | 22.36 ± 9.69 | 28.44 ± 5.97 | |
| Mn | 15.60 ± 2.52 | 71.43 ± 34.94 | 43.27 ± 22.31 | 96.12 ± 29.60 | |
| Pb | 12.29 ± 3.68 | 35.53 ± 10.75 | 27.74 ± 14.53 | 49.93 ± 10.81 | |
| Zn | 54.65 ± 13.00 | 70.97 ± 33.70 | 89.54 ± 24.09 | 30.11 ± 8.49 |
References
- Mateos, A.C.; Amarillo, A.C.; Carreras, H.A.; González, C.M. Land use and air quality in urban environments: Human health risk assessment due to inhalation of airborne particles. Environ. Res. 2018, 161, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H. Air pollution in cities. Atmos. Environ. 1999, 33, 4029–4037. [Google Scholar] [CrossRef]
- Han, X.; Naeher, L.P. A review of traffic-related air pollution exposure assessment studies in the developing world. Environ. Int. 2006, 32, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, M.A.; El Shahawy, A.M. Environmental heavy metals and mental disorders of children in developing countries. In Environmental Heavy Metal Pollution and Effects on Child Mental Development: Risk Assessment and Prevention Strategies; Simeonov, L.I., Kochubovski, M.V., Simeonova, B.G., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–25. [Google Scholar]
- Adamiec, E. Chemical fractionation and mobility of traffic-related elements in road environments. Environ. Geochem. Health 2017, 39, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Ares, Á.; Itouga, M.; Kato, Y.; Sakakibara, H. Differential Metal Tolerance and Accumulation Patterns of Cd, Cu, Pb and Zn in the Liverwort Marchantia polymorpha L. Bull. Environ. Contam. Toxicol. 2017, 100, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.K. Understanding Environmental Pollution; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Pescott, O.L.; Simkin, J.M.; August, T.A.; Randle, Z.; Dore, A.J.; Botham, M.S. Air pollution and its effects on lichens, bryophytes, and lichen-feeding Lepidoptera: Review and evidence from biological records. Biol. J. Linn. Soc. 2015, 115, 611–635. [Google Scholar] [CrossRef]
- Sánchez-Chardi, A. Biomonitoring potential of five sympatric Tillandsia species for evaluating urban metal pollution (Cd, Hg and Pb). Atmos. Environ. 2016, 131, 352–359. [Google Scholar] [CrossRef]
- Jurado, J.; Southgate, D. Dealing with air pollution in Latin America: The case of Quito, Ecuador. Environ. Dev. Econ. 1999, 4, 375–388. [Google Scholar] [CrossRef]
- Ministerio del Ambiente. Plan Nacional de Calidad del Aire; Agencia Suiza para el Desarrollo y la Cooperación, COSUDE y del Ministerio del Ambiente: Quito, Ecuador, 2010. [Google Scholar]
- Programa de las. Naciones Unidas para el Medio Ambiente, Municipalidad de Loja & Naturaleza y Cultura Internacional; Geo-Loja: Loja, Ecuador, 2007. [Google Scholar]
- Espinoza, E.P.; Molina, C.E. Contaminación del aire exterior Cuenca-Ecuador, 2009–2013. Posibles efectos en la salud. Revista de la Facultad de Ciencias Médicas 2014, 32, 6–17. [Google Scholar]
- Cevallos, V.M.; Díaz, V.; Sirois, C.M. Particulate matter air pollution from the city of Quito, Ecuador, activates inflammatory signaling pathways in vitro. Innate Immun. 2017, 23, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Raysoni, A.U.; Armijos, R.X.; Weigel, M.M.; Echanique, P.; Racines, M.; Pingitore, N.E.; Li, W.W. Evaluation of sources and patterns of elemental composition of PM2.5 at three low-income neighborhood schools and residences in Quito, Ecuador. Int. J. Environ. Res. Public Health 2017, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, G.M.A.; Rodriguez, J.H.; Pignata, M.L. Comparison of the air pollution biomonitoring ability of three Tillandsia species and the lichen Ramalina celastri in Argentina. Environ. Res. 2009, 109, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Demiray, A.D.; Yolcubal, I.; Akyol, N.H.; Çobanoğlu, G. Biomonitoring of airborne metals using the Lichen Xanthoria parietina in Kocaeli Province, Turkey. Ecol. Ind. 2012, 18, 632–643. [Google Scholar] [CrossRef]
- Garty, J. Biomonitoring atmospheric heavy metals with lichens: Theory and application. Crit. Rev. Plant Sci. 2001, 20, 309–371. [Google Scholar] [CrossRef]
- Adamo, P.; Giordano, S.; Vingiani, S.; Cobianchi, R.C.; Violante, P. Trace element accumulation by moss and lichen exposed in bags in the city of Naples (Italy). Environ. Pollut. 2003, 122, 91–103. [Google Scholar] [CrossRef]
- Figueiredo, A.M.G.; Alcalá, A.L.; Ticianelli, R.B.; Domingos, M.; Saiki, M. The use of Tillandsia usneoides L. as bioindicator of air pollution in São Paulo, Brazil. J. Radioanal. Nucl. Chem. 2004, 259, 59–63. [Google Scholar] [CrossRef]
- Käffer, M.I.; Lemos, A.T.; Apel, M.A.; Rocha, J.V.; de Azevedo Martins, S.M.; Vargas, V.M.F. Use of bioindicators to evaluate air quality and genotoxic compounds in an urban environment in Southern Brazil. Environ. Pollut. 2012, 163, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Llop, E.; Pinho, P.; Matos, P.; Pereira, M.J.; Branquinho, C. The use of lichen functional groups as indicators of air quality in a Mediterranean urban environment. Ecol. Ind. 2012, 13, 215–221. [Google Scholar] [CrossRef]
- Conti, M.E.; Tudino, M.; Stripeikis, J.; Cecchetti, G. Heavy metal accumulation in the lichen Evernia prunastri transplanted at urban, rural and industrial sites in Central Italy. J. Atmos. Chem. 2004, 49, 83–94. [Google Scholar] [CrossRef]
- Balabanova, B.; Stafilov, T.; Sajn, R.; Baèeva, K. Characterization of heavy metals in lichen species Hypogymnia physodes and Evernia prunastri due to biomonitoring of air pollution in the vicinity of copper mine. Int. J. Environ. Res. Public Health 2012, 6, 779–792. [Google Scholar]
- Lackovičová, A.; Guttova, A.; Bačkor, M.; Pišút, P.; Pišút, I. Response of Evernia prunastri to urban environmental conditions in Central Europe after the decrease of air pollution. Lichenologist 2013, 45, 89–100. [Google Scholar] [CrossRef]
- Rhzaoui, G.; Divakar, P.K.; Crespo, A.; Tahiri, H. Biomonitoring of air pollutants by using lichens (Evernia prunastri) in areas between Kenitra and Mohammedia cities in Morocco. Lazaroa 2015, 36, 21–30. [Google Scholar] [CrossRef]
- Loppi, S.; Frati, L.; Paoli, L.; Bigagli, V.; Rossetti, C.; Bruscoli, C.; Corsini, A. Biodiversity of epiphytic lichens and heavy metal contents of Flavoparmelia caperata thalli as indicators of temporal variations of air pollution in the town of Montecatini Terme (central Italy). Sci. Total Environ. 2004, 326, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Godinho, R.M.; Wolterbeek, H.T.; Verburg, T.; Freitas, M.C. Bioaccumulation behaviour of transplants of the lichen Flavoparmelia caperata in relation to total deposition at a polluted location in Portugal. Environ. Pollut. 2008, 151, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Jeran, Z.; Jaćimović, R.; Batič, F.; Mavsar, R. Lichens as integrating air pollution monitors. Environ. Pollut. 2002, 120, 107–113. [Google Scholar] [CrossRef]
- Cañas, M.S.; Orellana, L.; Pignata, M.L. Chemical response of the lichens Parmotrema austrosinense and P. conferendum transplanted to urban and non-polluted environments. Ann. Bot. Fenn. 1997, 34, 27–34. [Google Scholar]
- Pyatt, F.B.; Grattan, J.P.; Lacy, D.; Pyatt, A.J.; Seaward, M.R.D. Comparative effectiveness of Tillandsia usneoides L. and Parmotrema praesorediosum (Nyl.) Hale as bio-indicators of atmospheric pollution in Louisiana (USA). Water Air Soil Pollut. 1999, 111, 317–326. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Chai, Z.F.; Mao, X.Y.; Chen, J.B. Biomonitoring trace element atmospheric deposition using lichens in China. Environ. Pollut. 2002, 120, 157–161. [Google Scholar] [CrossRef]
- Aprile, G.G.; Di Salvatore, M.; Carratù, G.; Mingo, A.; Carafa, A.M. Comparison of the suitability of two lichen species and one higher plant for monitoring airborne heavy metals. Environ. Monit. Assess. 2010, 162, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.A.; Lavornia, J.M.; Chaparro, M.A.; Sinito, A.M. Biomonitors of urban air pollution: Magnetic studies and SEM observations of corticolous foliose and microfoliose lichens and their suitability for magnetic monitoring. Environ. Pollut. 2013, 172, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, K.I.A.; De Freitas, C.R. Epiphytic lichens as biomonitors of airborne heavy metal pollution. Environ. Exp. Bot. 2013, 88, 24–32. [Google Scholar] [CrossRef]
- Calasans, C.F.; Malm, O. Elemental mercury contamination survey in a chlor-alkali plant by the use of transplanted Spanish moss, Tillandsia usneoides (L.). Sci. Total Environ. 1997, 208, 165–177. [Google Scholar] [CrossRef]
- Pignata, M.L.; Plá, R.R.; Jasan, R.C.; Martinez, M.S.; Rodriguez, J.H.; Wannaz, E.D.; Gudino, G.L.; Carreras, H.A.; Gonzalez, C.M. Distribution of atmospheric trace elements and assesment of air quality in Argentina employing the lichen, Ramalina celastri, as a passive biomonitor: Detection of air pollution emission sources. Int. J. Environ. Health 2007, 1, 29–46. [Google Scholar] [CrossRef]
- Rodriguez, J.H.; Weller, S.B.; Wannaz, E.D.; Klumpp, A.; Pignata, M.L. Air quality biomonitoring in agricultural areas nearby to urban and industrial emission sources in Córdoba province, Argentina, employing the bioindicator Tillandsia capillaris. Ecol. Indic. 2011, 6, 1673–1680. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Andrade, L.R.; Farina, M.; Malm, O. Hg localization in Tillandsia usneoides L. (Bromeliaceae), an atmospheric biomonitor. Atmos. Environ. 2002, 5, 881–887. [Google Scholar] [CrossRef]
- Alves, E.S.; Moura, B.B.; Domingos, M. Structural analysis of Tillandsia usneoides L. exposed to air pollutants in São Paulo City–Brazil. Water Air Soil Pollut. 2008, 189, 61–68. [Google Scholar] [CrossRef]
- Vianna, N.A.; Gonçalves, D.; Brandão, F.; de Barros, R.P.; Amado Filho, G.M.; Meire, R.O.; Andrade, L.R. Assessment of heavy metals in the particulate matter of two Brazilian metropolitan areas by using Tillandsia usneoides as atmospheric biomonitor. Environ. Sci. Pollut. Res. 2011, 18, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.T.; Cohen, R.A.; Vives, S.P. Evaluating relationships between mercury concentrations in air and in Spanish moss (Tillandsia usneoides L.). Ecol. Indic. 2014, 36, 392–399. [Google Scholar] [CrossRef]
- Giampaoli, P.; Wannaz, E.D.; Tavares, A.R.; Domingos, M. Suitability of Tillandsia usneoides and Aechmea fasciata for biomonitoring toxic elements under tropical seasonal climate. Chemosphere 2016, 149, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Jimenez, D.A.; Cueva-Agila, A.; Prieto, M.; Aragon, G.; Benitez, A. Changes in the epiphytic lichen composition related with air quality in the city of Loja (Ecuador). Caldasia 2015, 37, 333–343. [Google Scholar]
- Wolterbeek, B. Biomonitoring of trace element air pollution: Principles, possibilities and perspectives. Environ. Pollut. 2002, 120, 11–21. [Google Scholar] [CrossRef]
- Hérnandez, O.H.Á.; Montaño, T.; Maldonado, J.; Caraballo, M.A.; Ojeda, C.G.S.; Granda, J.C.; Castillo, B.S. Método de selección para la ubicación de puntos de monitoreo de gases de combustión provenientes de fuentes fijas puntuales en la ciudad de Loja, Ecuador. Rev. Tecnol. ESPOL 2016, 29, 38–52. [Google Scholar]
- Smith, L.B.; Downs, R.J. Tillandsioideae (Bromeliaceae). Flora Neotrop. Monogr. 1997, 14, 663–1492. [Google Scholar]
- Sipman, H.J.M. Mason Hale’s Key to Parmotrema, Revised Edition: Key to Wide-Lobed Parmelioid Species Occurring in Tropical America (Genera Canomaculina, Parmotrema, Rimelia, Rimeliella). 2005. Available online: http://www.bgbm.org/sipman/keys/Neoparmo.htm (accessed on 2 February 2019).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models; R Package: Vienna, Austria, 2018. [Google Scholar]
- R Team Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Fuga, A.; Saiki, M.; Marcelli, M.P.; Saldiva, P.H. Atmospheric pollutants monitoring by analysis of epiphytic lichens. Environ. Pollut. 2008, 151, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Monna, F.; Marques, A.N.; Guillon, R.; Losno, R.; Couette, S.; Navarro, N.; Dongarra, G.; Tamburo, E.; Varrica, D.; Chateau, C.; et al. Perturbation vectors to evaluate air quality using lichens and bromeliads: A Brazilian case study. Environ. Monit. Assess. 2017, 189, 566. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Mielke, H.W.; Laidlaw, M.A.; Gonzales, C. Lead (Pb) legacy from vehicle traffic in eight California urbanized areas: Continuing influence of lead dust on children’s health. Sci. Total Environ. 2010, 408, 3965–3975. [Google Scholar] [CrossRef] [PubMed]
- Pulles, T.; Van der Gon, H.D.; Appelman, W.; Verheul, M. Emission factors for heavy metals from diesel and petrol used in European vehicles. Atmos. Environ. 2012, 61, 641–651. [Google Scholar] [CrossRef]
- Sysalová, J.; Sýkorová, I.; Havelcová, M.; Száková, J.; Trejtnarová, H.; Kotlík, B. Toxicologically important trace elements and organic compounds investigated in size-fractionated urban particulate matter collected near the Prague highway. Sci. Total Environ. 2012, 437, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Abril, G.A.; Wannaz, E.D.; Mateos, A.C.; Invernizzi, R.; Plá, R.R.; Pignata, M.L. Characterization of atmospheric emission sources of heavy metals and trace elements through a local-scale monitoring network using T. capillaris. Ecol. Indic. 2014, 40, 153–161. [Google Scholar] [CrossRef]
- Giordano, S.; Adamo, P.; Sorbo, S.; Vingiani, S. Atmospheric trace metal pollution in the Naples urban area based on results from moss and lichen bags. Environ. Pollut. 2005, 136, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Gope, M.; Masto, R.E.; George, J.; Hoque, R.R.; Balachandran, S. Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb and Zn) in street dust of Asansol, India. Ecotoxicol. Environ. Saf. 2017, 138, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Minganti, V.; Capelli, R.; Drava, G.; De Pellegrini, R.; Brunialti, G.; Giordani, P.; Modenes, I.P. Biomonitoring of trace metals by different species of lichens (Parmelia) in North-West Italy. J. Atmos. Chem. 2003, 45, 219–229. [Google Scholar] [CrossRef]
| LMM | Cd | Pb | Mn | Cu | Zinc | |||||
| Factor | F | p-Value | F | p-Value | F | p-Value | F | p-Value | F | p-Value |
| Zone | 51.84 | <0.001 | 26.37 | <0.001 | 33.48 | <0.001 | 28.12 | <0.001 | 53.35 | <0.001 |
| Specie | 3.80 | 0.054 | 0.005 | 0.944 | 22.17 | <0.001 | 0.03 | 0.863 | 0.051 | 0.822 |
| Zone x Specie | 6.27 | <0.001 | 1.061 | 0.37 | 2.32 | 0.08 | 2.457 | 0.067 | 15.67 | <0.001 |
| Tukey’s HSD Test | Cd | Pb | Mn | Cu | Zinc | |||||
| Zone | Est | p-Value | Est | p-Value | Est | p-Value | Est | p-Value | Est | p-Value |
| F - S | −38.79 | <0.001 | −0.865 | <0.001 | 16.58 | 0.419 | −14.68 | <0.001 | −0.73 | <0.001 |
| F - C | 30.54 | <0.001 | −0.583 | <0.001 | −61.38 | 0.001 | −14.14 | <0.001 | −0.92 | <0.001 |
| F - N | −26.99 | <0.001 | −0.891 | <0.001 | −47.00 | 0.004 | −19.98 | <0.001 | −0.08 | 0.823 |
| S - C | −8.25 | 0.185 | 0.282 | 0.039 | −30.41 | 0.042 | 0.53 | 0.993 | −0.19 | 0.132 |
| S - N | −11.79 | 0.043 | −0.026 | 0.994 | 14.38 | 0.457 | −5.30 | 0.045 | 0.652 | <0.001 |
| C - N | 3.54 | 0.798 | −0.309 | 0.028 | −44.79 | 0.006 | −5.84 | 0.033 | 0.841 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, Á.; Medina, J.; Vásquez, C.; Loaiza, T.; Luzuriaga, Y.; Calva, J. Lichens and Bromeliads as Bioindicators of Heavy Metal Deposition in Ecuador. Diversity 2019, 11, 28. https://doi.org/10.3390/d11020028
Benítez Á, Medina J, Vásquez C, Loaiza T, Luzuriaga Y, Calva J. Lichens and Bromeliads as Bioindicators of Heavy Metal Deposition in Ecuador. Diversity. 2019; 11(2):28. https://doi.org/10.3390/d11020028
Chicago/Turabian StyleBenítez, Ángel, Jefferson Medina, Cristina Vásquez, Talía Loaiza, Yesenia Luzuriaga, and James Calva. 2019. "Lichens and Bromeliads as Bioindicators of Heavy Metal Deposition in Ecuador" Diversity 11, no. 2: 28. https://doi.org/10.3390/d11020028
APA StyleBenítez, Á., Medina, J., Vásquez, C., Loaiza, T., Luzuriaga, Y., & Calva, J. (2019). Lichens and Bromeliads as Bioindicators of Heavy Metal Deposition in Ecuador. Diversity, 11(2), 28. https://doi.org/10.3390/d11020028






