Native and Invasive Small Mammals in Urban Habitats along the Commercial Axis Connecting Benin and Niger, West Africa
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Species-Specific Identifications
2.3. Ethics Statements
2.4. Data Analyses
2.4.1. Distribution of Native and Invasive Small Mammals
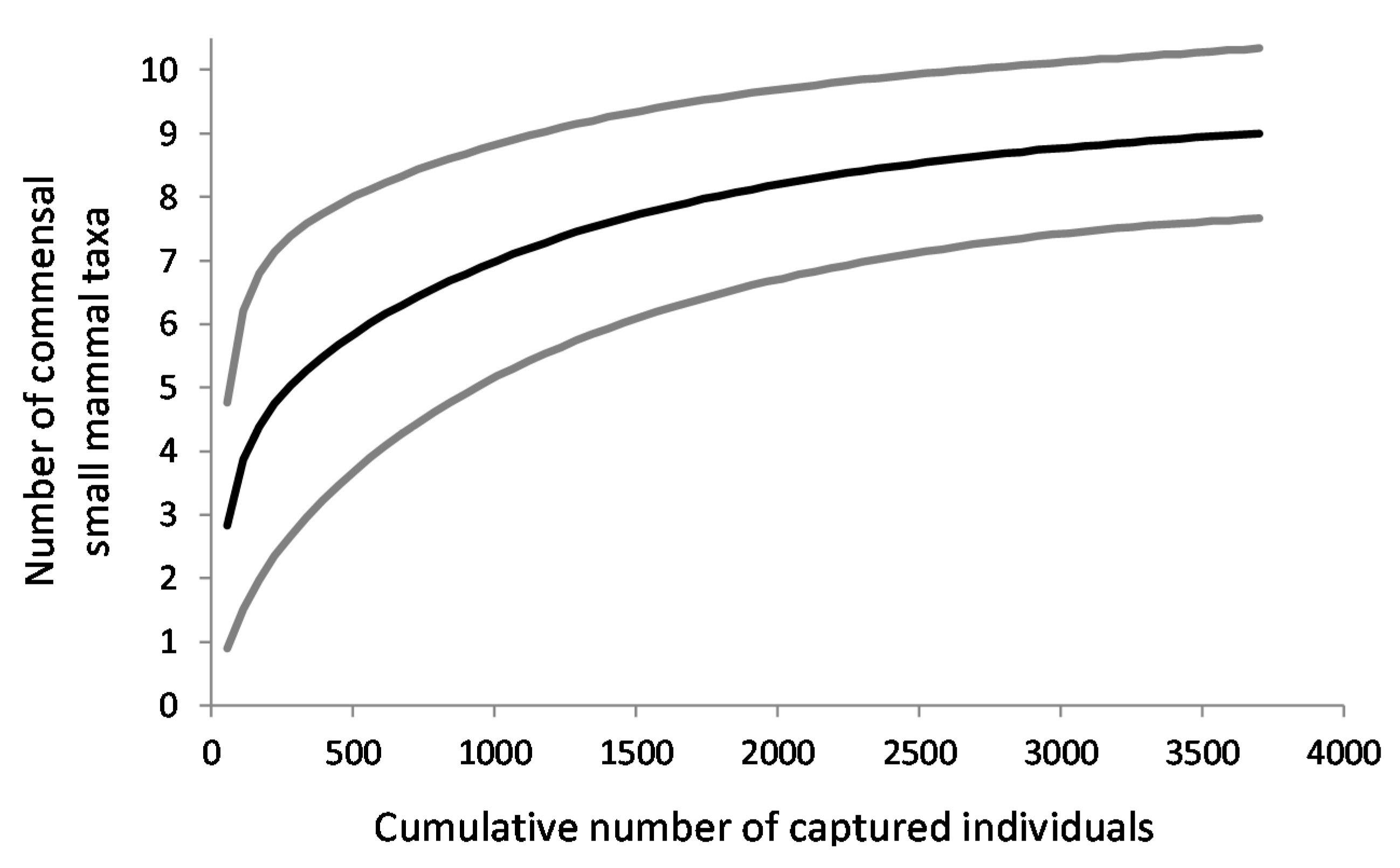
2.4.2. Species Diversity
2.4.3. Co-Occurrence Patterns
3. Results
3.1. Trapping Results
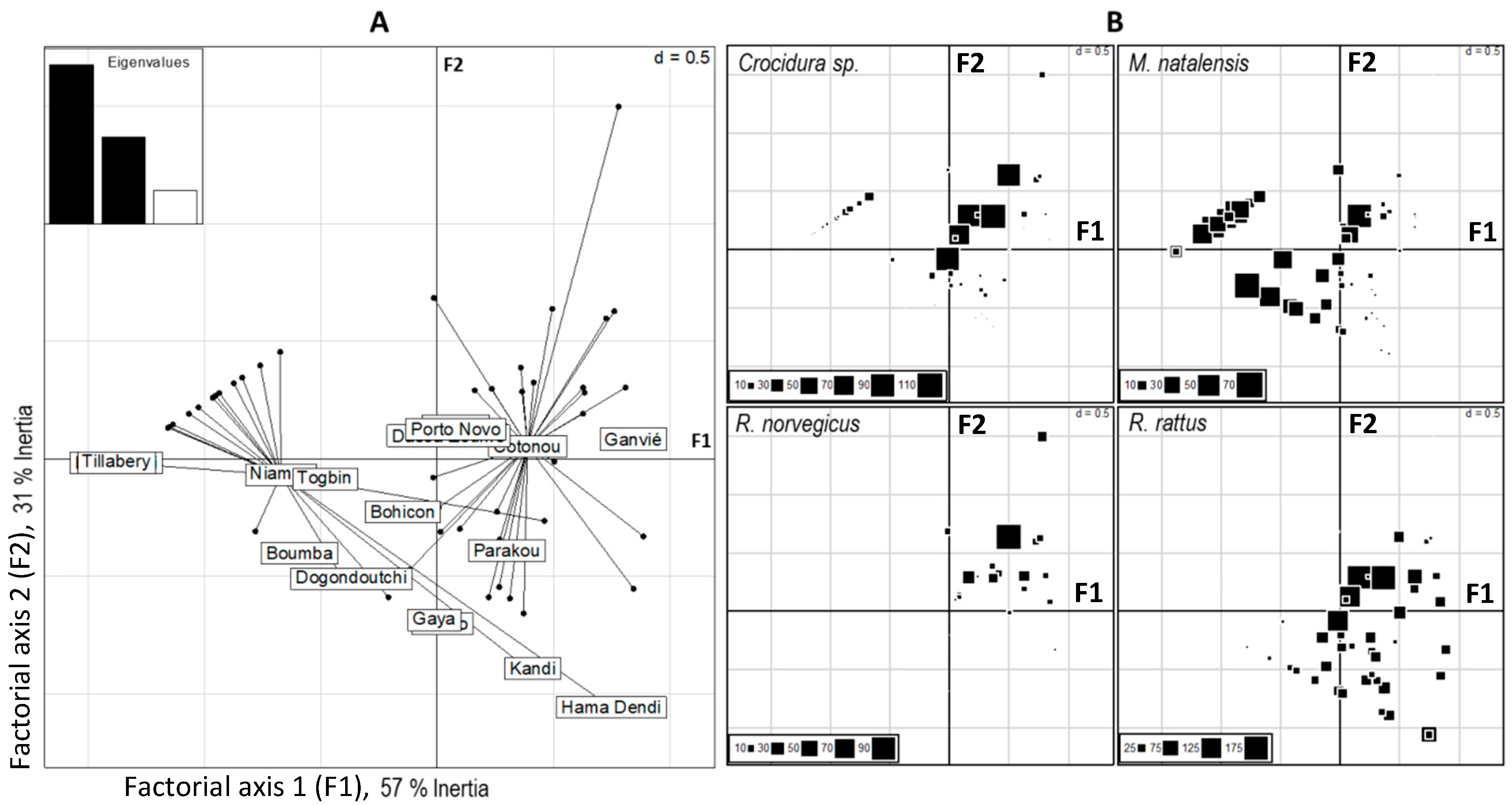
3.2. Distribution of Native and Invasive Small Mammals
3.3. Species Diversity
3.4. Co-Occurrence Patterns
4. Discussion
4.1. Distributions of Native and Invasive Small Mammals
4.2. Species Diversity and Co-Occurrence Patterns
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lebel, T.; Cappelaere, B.; Galle, S.; Hanan, N.; Kergoat, L.; Levis, S.; Vieux, B.; Descroix, L.; Gosset, M.; Mougin, E.; et al. AMMA-CATCH studies in the Sahelian region of West-Africa: An overview. J. Hydrol. 2009, 375, 3–13. [Google Scholar] [CrossRef]
- Sarr, B. Present and future climate change in the semi-arid region of West Africa: A crucial input for practical adaptation in agriculture. Atmos. Sci. Lett. 2012, 13, 108–112. [Google Scholar] [CrossRef]
- Ahmed, K.F.; Wang, G.; You, L.; Yu, M. Potential impact of climate and socioeconomic changes on future agricultural land use in West Africa. Earth Syst. Dyn. 2016, 7, 151–165. [Google Scholar] [CrossRef]
- Hitimana, L.; Heinrigs, P.; Tremolieres, M. West African urbanization trends. West Afr. Futures 2011, 1, 1–8. [Google Scholar]
- Denis, E.; Moriconi-Ebrard, F.; Harre-Roger, D.; Thiam, O.; Séjourné, M.; Chatel, C. Dynamique de l’urbanisation, 1950–2020: Approche géo-statistique Afrique de l’ouest. In Africapolis; HAL Id: Hal-00357271; Paris, 2008; 124p, Available online: https://hal.archives-ouvertes.fr/hal-00357271 (accessed on 13 March 2019).
- Bocquier, P.; Mukandila, A.K. African urbanization trends and prospects. Afr. Popul. Stud. 2011, 25, 337–361. [Google Scholar] [CrossRef]
- Guillaumont, P.; Simonet, C. To What Extent Are African Countries Vulnerable to Climate Change? Lessons from a New Indicator of Physical Vulnerability to Climate Change. Ferdi Working Paper I08. 2011. Available online: https://ferdi.fr/publications/to-what-extent-are-african-countries-vulnerable-to-climate-change-lessons-from-a-new-indicator-of-physical-vulnerability-to-climate-change (accessed on 7 October 2019).
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef]
- Pratt, C.F.; Contantine, K.L.; Murphy, S.T. Economic impacts of invasive alien species on African smallholder livelihoods. Glob. Food Secur. 2017, 14, 31–37. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Ascensão, F.; Capinha, C. Aliens on the Move: Transportation Networks and Non-native Species. In Railway Ecology; Ebook; de Água, B., Borda-de-Água, L., Barrientos, R., Beja, P., Pereira, H.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 65–80. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef]
- Kotliar, N.B.; Wiens, J.A. Multiple scales of patchiness and patch structure: A hierarchical framework for the study of heterogeneity. Oikos 1990, 59, 253–260. [Google Scholar] [CrossRef]
- Levin, S.A. The problem of pattern and scale in ecology. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Community diversity: Relative roles of local and regional processes. Science 1987, 235, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.A.; Stenseth, N.C.; Van Horne, B.; Ims, R.A. Ecological mechanisms and landscape ecology. Oikos 1993, 66, 369–380. [Google Scholar] [CrossRef]
- Alberti, M.; Marzluff, J.M. Ecological resilience in urban ecosystems: Linking urban patterns to human and ecological functions. Urban Ecosyst. 2004, 7, 241–265. [Google Scholar] [CrossRef]
- Shochat, E.; Stefanov, W.L.; Whitehouse, M.E.A.; Faeth, S.H. Urbanization and spider diversity: Influences of human modification of habitat structure and productivity. Ecol. Appl. 2004, 14, 268–280. [Google Scholar] [CrossRef]
- Cavia, R.; Cueto, G.R.; Suárez, O.V. Changes in rodent communities according to the landscape structure in an urban ecosystem. Landsc. Urban Plan. 2009, 90, 11–19. [Google Scholar] [CrossRef]
- Melles, S.; Glenn, S.M.; Martin, K. Urban bird diversity and landscape complexity: Species–environment associations along a multiscale habitat gradient. Conserv. Ecol. 2003, 7, art5. [Google Scholar] [CrossRef]
- Audoin-Rouzeau, F.; Vigne, J.D. La colonisation de l’Europe par le rat noir. Rev. Paléobiol. 1994, 13, 125–145. [Google Scholar]
- Aplin, K.P.; Suzuki, H.; Chinen, A.A.; Chesser, R.T.; ten Have, J.; Donnellan, S.C.; Austin, J.; Frost, A.; Gonzalez, J.P.; Herbreteau, V.; et al. Multiple Geographic Origins of Commensalism and Complex Dispersal History of Black Rats. PLoS ONE 2011, 6, e26357. [Google Scholar] [CrossRef]
- Dalecky, A.; Bâ, K.; Piry, S.; Lippens, C.; Diagne, C.A.; Kane, M.; Sow, A.; Diallo, M.; Niang, Y.; Konečný, A.; et al. Range expansion of the invasive house mouse Mus musculus domesticus in Senegal, West Africa: A synthesis of trapping data over three decades, 1983–2014. Mammal Rev. 2015, 45, 176–190. [Google Scholar] [CrossRef]
- Berthier, K.; Garba, M.; Leblois, R.; Navascues, M.; Tatard, C.; Gauthier, P.; Gagare, S.; Piry, S.; Brouat, C.; Dalecky, A.; et al. Black rat invasion of inland Sahel: Insights from interviews and population genetics in south-western Niger. Biol. J. Linn. Soc. 2016, 119, 748–765. [Google Scholar] [CrossRef]
- Drake, D.R.; Hunt, T.L. Invasive rodents on islands: Integrating historical and contemporary ecology. Biol. Invasions 2009, 11, 1483–1487. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [PubMed]
- Vogler, A.J.; Chan, F.; Nottingham, R.; Andersen, G.; Drees, K.; Beckstrom-Sternberg, S.M.; Wagner, D.M.; Chanteau, S.; Keim, P. A Decade of Plague in Mahajanga, Madagascar: Insights into the Global Maritime Spread of Pandemic Plague. mBio 2013, 4, e00623. [Google Scholar] [CrossRef]
- Wu, Y.W.; Hsu, E.L.; Lin, T.H.; Huang, J.H.; Chang, S.F.; Pai, H.H. Seaports as a source of Hantavirus: A study in isolated islands. Int. J. Environ. Health Res. 2007, 17, 25–32. [Google Scholar] [CrossRef]
- Lin, X.D.; Guo, W.P.; Wang, W.; Zou, Y.; Hao, Z.Y.; Zhou, D.J.; Dong, X.; Qu, Y.G.; Li, M.H.; Tian, H.F.; et al. Migration of Norway rats resulted in the worldwide distribution of Seoul Hantavirus today. J. Virol. 2012, 86, 972–981. [Google Scholar] [CrossRef]
- Kuo, C.C.; Wardrop, N.; Chang, C.T.; Wang, H.C.; Atkinson, P. Significance of major international seaports in the distribution of murine typhus in Taiwan. PLoS Trop. Negl. Dis. 2017, 11, e5430. [Google Scholar]
- Olson, L.J. The economics of terrestrial invasive species: A review of the literature. Agric. Res. Econ. Rev. 2006, 35, 178–194. [Google Scholar] [CrossRef]
- Suarez, V.A.; Tsutsui, N.D. The evolutionary consequences of biological invasions. Mol. Ecol. 2008, 17, 351–360. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Ann. Rev. Environ. Res. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Garba, M.; Dalecky, A.; Kadaoure, I.; Kane, M.; Hima, K.; Veran, S.; Gagare, S.; Gauthier, P.; Tatard, C.; Rossi, J.P.; et al. Spatial segregation between invasive and native commensal rodents in an urban environment: A case study in Niamey, Niger. PLoS ONE 2014, 9, e110666. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.A.; Bunbury, N. Invasive rats on tropical islands: Their population biology and impacts on native species. Glob. Ecol. Conserv. 2015, 3, 607–627. [Google Scholar] [CrossRef]
- Young, H.S.; Parker, I.M.; Gilbert, G.S.; Guerra, A.S.; Nunn, C.L. Introduced Species, Disease Ecology, and Biodiversity–Disease Relationships. Trends Ecol. Evol. 2017, 32, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Galan, M.; Tamisier, L.; d’Ambrosio, J.; Dalecky, A.; Bâ, K.; Kane, M.; Niang, Y.; Diallo, M.; Sow, A.; et al. Ecological and sanitary impacts of bacterial communities associated to biological invasions in African commensal rodent communities. Nat. Sci. Rep. 2017, 7, 14995. [Google Scholar] [CrossRef] [PubMed]
- Duplantier, J.-M.; Granjon, L.; Bâ, K. Répartition biogéographique des petits rongeurs au Sénégal. J. Afr. Zool. 1997, 111, 17–26. [Google Scholar]
- Kaleme, P.K.; Bates, J.M.; Belesi, H.K.; Bowie, R.C.K.; Gambalemoke, M.; Kerbis-Peterhans, J.; Michaux, J.; Mwanga, J.M.; Ndara, B.R.; Taylor, P.J.; et al. Origin and putative colonization routes for invasive rodent taxa in the Democratic Republic of Congo. Afr. Zool. 2011, 46, 133–145. [Google Scholar] [CrossRef]
- Lippens, C.; Estoup, A.; Hima, M.K.; Loiseau, A.; Tatard, C.; Dalecky, A.; Bâ, K.; Kane, M.; Diallo, M.; Sow, A.; et al. Genetic structure and invasion history of the house mouse (Mus musculus domesticus) in Senegal, West Africa: A legacy of colonial and contemporary times. Heredity 2017, 119, 64–75. [Google Scholar] [CrossRef]
- Dobigny, G.; Poirier, P.; Hima, K.; Cabaret, O.; Gauthier, P.; Tatard, C.; Costa, J.M.; Bretagne, S. Molecular survey of rodent-borne Trypanosoma in Niger with special emphasis on T. lewisi imported by invasive black rats. Acta Trop. 2011, 117, 183–188. [Google Scholar]
- Tollenaere, C.; Brouat, C.; Duplantier, J.-M.; Rahalison, L.; Rahelinirina, S.; Pascal, M.; Moné, H.; Mouahid, G.; Leirs, H.; Cosson, J.-F. Phylogeography of the introduced species Rattus rattus in the western Indian Ocean, with special emphasis on the colonization history of Madagascar. J. Biogeogr. 2010, 37, 398–410. [Google Scholar] [CrossRef]
- Bonhomme, F.; Orth, A.; Cucchi, T.; Rajabi-Maham, H.; Catalan, J.; Boursot, P.; Auffray, J.C.; Britton-Davidian, J. Genetic differentiation of the house mouse around the Mediterranean basin: Matrilineal footprints of early and late colonization. Proc. R. Soc. Lond. B 2011, 278, 1034–1043. [Google Scholar] [CrossRef]
- Konečný, A.; Estoup, A.; Duplantier, J.-M.; Bryja, J.; Bâ, K.; Galan, M.; Tatard, C.; Cosson, J.-F. Invasion genetics of the introduced black rat (Rattus rattus) in Senegal, West Africa. Mol. Ecol. 2013, 22, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Stragier, C.; Piry, S.; Loiseau, A.; Kane, M.; Sow, A.; Niang, Y.; Diallo, M.; Ndiaye, A.; Gauthier, P.; Borderon, M.; et al. Interplay between historical and current features of the cityscape in shaping the genetic structure of the house mouse (Mus musculus domesticus) in Dakar (Senegal, West Africa). bioRxiv 2019. [Google Scholar] [CrossRef]
- Sounouvou, M.G.J. Contribution à L’amélioration du Transport International de Marchandises: Cas du Corridor Bénin-Niger. Bachelor’s Thesis, Université Internationale du Benin (UPIB), Benin, Benin, 2007; 57p. [Google Scholar]
- Chambre de Commerce d’Industrie et d’Artisanat du Niger. Organisation des trafics routiers et échanges commerciaux le long du corridor Bénin-Niger. In Rapport D’Etudes; Chambre de Commerce d’Industrie et d’Artisanat du Niger: Niamey, Niger, 2018; 34p. [Google Scholar]
- Boluvi, G.M. Malanville-Gaya: Comptoir commercial et couloir de spéculations (pays-frontière de l’informel). Club du Sahel et de l’Afrique de l’ouest. In West African Borders and Integration; Paris, 2004; 29p, Available online: http://www.hubrural.org/IMG/pdf/wabi_malanville_gaya.pdf (accessed on 7 October 2019).
- Sougue, E. Malanville-Gaya, une dynamique de territorialisation à la frontière Benin-Niger. Territ. En Mouv. Rev. Géogr. Aménage. 2016, 29. [Google Scholar] [CrossRef]
- Mills, J.N.; Yates, T.L.; Childs, J.E.; Parmenter, R.R.; Ksiazek, T.E. Guidelines for Working with Rodents Potentially Infected with Hantavirus. J. Mammal. 1995, 76, 716–722. [Google Scholar] [CrossRef]
- Granjon, L.; Duplantier, J.-M. Les Rongeurs de L’Afrique Sahélo-Soudanienne; IRD, MNHN: Marseille, France, 2009; 215p. [Google Scholar]
- Dobigny, G.; Lecompte, E.; Tatard, C.; Gauthier, P.; Bâ, K.; Duplantier, J.-M.; Granjon, L.; Denys, C. An update on the taxonomy and geographic distribution of the cryptic species Mastomys kollmannspergeri (Muridae, Murinae) using combined cytogenetic and molecular data. J. Zool. 2008, 276, 368–374. [Google Scholar] [CrossRef]
- Tatard, C.; Garba, M.; Gauthier, P.; Hima, K.; Artige, E.; Dossou, H.J.; Gagaré, S.; Genson, G.; Truc, P.; Dobigny, G. Rodent-borne Trypanosoma from cities and villages of Niger and Nigeria: A special role for the invasive genus Rattus? Acta Trop. 2017, 17, 151–158. [Google Scholar] [CrossRef]
- Sikes, R.S.; Gannon, W.L. and the Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Colwell, R.K. Estimate S Version 9.1: Statistical Estimation of Species Richness and Shared Species from Samples (Software and User’s Guide). Freeware for Windows and Mac OS. 2013. Available online: http://viceroy.eeb.uconn.edu/Colwell/#Software (accessed on 20 February 2019).
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Chessel, D.; Dufour, A.B.; Thioulouse, J. The ade4 package-I-One-table methods. R News 2004, 4, 5–10. [Google Scholar]
- Dray, S.; Dufour, A.B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22. [Google Scholar] [CrossRef]
- Dray, S.; Siberchicot, A. Adegraphics: An S4 Lattice-Based Package for the Representation of Multivariate Data. 2017. Available online: https://CRAN.R-project.org/package=adegraphics (accessed on 20 February 2019).
- Hardy, O.J. BiodivR 1.2. A Program to Compute indices of Species Diversity within Sample and Species Similarity between Samples Using Rarefaction Principles to Reduce Sampling Bias. 2010. Available online: http://ebe.ulb.ac.be/ebe/Software.html (accessed on 22 February 2019).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; 256p. [Google Scholar]
- Chao, A.; Chazdon, R.L.; Cowell, R.K.; Shen, T.J. Abundance-Based Similarity Indices and Their Estimation When There Are Unseen Species in Samples. Biometrics 2006, 62, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Borcard, D.; Legendre, P.; Dapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Cottenie, K.; Michels, E.; Nuytten, N.; De Meester, L. Zooplankton metacommunity structure: Regional vs. local processes in highly interconnected ponds. Ecology 2003, 84, 991–1000. [Google Scholar] [CrossRef]
- Cottenie, K. Integrating environment and spatial processes in ecological community dynamics. Ecol. Lett. 2005, 8, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT, Version 2.9.3, a Program to Estimate and Test Gene Diversities and Fixation Indices; Lausanne University: Lausanne, Switzerland, 2001; Available online: https://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 9 December 2019).
- Gotelli, N.J. Null model analysis of species co-occurrence patterns. Ecology 2000, 81, 2606–2621. [Google Scholar] [CrossRef]
- Stone, L.; Roberts, A. The checkerboard score and species distributions. Oecologia 1990, 85, 74–79. [Google Scholar] [CrossRef]
- Ulrich, W. Pairs—A FORTRAN Program for Studying Pair-Wise Species Associations in Ecological Matrices. 2008. Available online: http://www.keib.umk.pl/pairs/ (accessed on 31 January 2019).
- Gotelli, N.J.; Ulrich, W. The empirical Bayes approach as a tool to identify non-random species associations. Oecologia 2010, 162, 463–477. [Google Scholar] [CrossRef]
- Dobigny, G.; Tatard, C.; Gauthier, P.; Bâ, K.; Duplantier, J.-M.; Granjon, L.; Kergoat, G.J. Mitochondrial and Nuclear Genes-Based Phylogeography of Arvicanthis niloticus (Murinae) and Sub-Saharan Open Habitats Pleistocene History. PLoS ONE 2013, 8, e77815. [Google Scholar] [CrossRef]
- Bryja, J.; Colangelo, P.; Lavrenchenko, L.A.; Meheretu, Y.; Šumbera, R.; Bryjová, A.; Verheyen, E.; Leirs, H.; Castiglia, R. Diversity and evolution of African Grass Rats (Muridae: Arvicanthis)—From radiation in East Africa to repeated colonization of northwestern and southeastern savannas. J. Zool. Syst. Evol. Res. 2019, 57, 970–988. [Google Scholar] [CrossRef]
- Bryja, J.; Granjon, L.; Dobigny, G.; Patzenhauerova, H.; Konečný, A.; Duplantier, J.-M.; Gauthier, P.; Colyn, M.; Durnez, L.; Lalis, A.; et al. Plio-Pleistocene history of West African Sudanian savanna and the phylogeography of the Praomys daltoni complex (Rodentia): The environment/geography/genetic interplay. Mol. Ecol. 2010, 19, 4783–4799. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, F.; Denys, C.; Verheyen, E.; Bryja, J.; Hutterer, R.; Kerbis Peterhans, J.C.; Stanley, W.T.; Goodman, S.M.; Couloux, A.; Colyn, M.; et al. Phylogeography and evolutionary history of the Crocidura olivieri complex (Mammalia, Soricomorpha): From a forest origin to broad ecological expansion across Africa. BMC Evol. Biol. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Olayemi, A.; Obadare, A.; Oyeyiola, A.; Fasogbon, S.; Igbokwe, J.; Igbahenah, F.; Ortsega, D.; Günther, S.; Verheyen, E.; Fichet-Calvet, E. Small mammal diversity and dynamics within Nigeria, with emphasis on reservoirs of the Lassa virus. Syst. Biodivers. 2018, 16, 118–127. [Google Scholar] [CrossRef]
- Lecompte, E.; Fichet-Calvet, E.; Daffis, S.; Koulémou, K.; Sylla, O.; Kourouma, F.; Doré, A.; Soropogui, B.; Aniskin, V.; Allali, B.; et al. Mastomys natalensis and Lassa Fever, West Africa. Emerg. Infect. Dis. 2006, 12, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly-N’Golo, D.; Allali, B.; Kouassi, S.K.; Fichet-Calvet, E.; Becker-Ziaja, B.; Rieger, T.; Gunther, S. Novel arenavirus sequences in Hylomyscus sp. And Mus (Nannomys) setulosus from Côte d’Ivoire: Implications for evolution of arenaviruses in Africa. PLoS ONE 2011, 6, e20893. [Google Scholar]
- Kronmann, K.C.; Nimo-Paintsil, S.; Guirguis, F.; Kronmann, L.C.; Bonney, K.; Obiri-Danso, K.; Fichet-Calvet, E. Two novel arenaviruses detected in pygmy mice, Ghana. Emerg. Infect. Dis. 2013, 19, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Fichet-Calvet, E.; Becker-Ziaja, B.; Koivogui, L.; Gunther, S. Lassa serology in natural populations of rodents and horizontal transmission. Vector Borne Zoonotic Dis. 2014, 14, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Traweger, D.; Travnitzky, R.; Moser, C.; Walzer, C.; Bernatzky, G. Habitat preferences and distribution of the brown rat (Rattus norvegicus Berk.) in the city of Salzburg (Austria): Implications for an urban rat management. J. Pest Sci. 2006, 79, 113–125. [Google Scholar] [CrossRef]
- Langton, S.; Cowan, D.; Meyer, A. The occurrence of commensal rodents in dwellings as revealed by the 1996 English House Condition Survey. J. Appl. Ecol. 2001, 38, 699–709. [Google Scholar] [CrossRef]
- Feng, A.Y.T.; Himsworth, C.G. The secret life of the city rat: A review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosyst. 2014, 17, 149–162. [Google Scholar] [CrossRef]
- Kajdacsi, B.; Costa, F.; Hyseni, C.; Porter, F.; Brown, J.; Rodrigues, G.; Farias, H.; Reis, M.G.; Childs, J.E.; Ko, A.I.; et al. Urban population genetics of slum-dwelling rats (Rattus norvegicus) in Salvador, Brazil. Mol. Ecol. 2013, 22, 5056–5070. [Google Scholar] [CrossRef] [PubMed]
- Rosevear, D.R. The Rodents of West Africa; British Museum (Natural History): London, UK, 1969; 604p. [Google Scholar]
- Meinig, H. Notes on the mammal fauna of the southern part of the Republic of Mali, West Africa. Bonn. Zool. Beitr. 2000, 49, 101–114. [Google Scholar]
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef] [PubMed]
- Kosoy, M.; Khlyap, L.; Cosson, J.-F.; Morand, S. Aboriginal and Invasive Rats of Genus Rattus as Hosts of Infectious Agents. Vector Borne Zoonotic Dis. 2015, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dobigny, G.; Nomao, A.; Gautun, J.C. A cytotaxonomic survey of rodents from Niger: Implications for systematics, biodiversity and biogeography. Mammalia 2002, 66, 495–523. [Google Scholar] [CrossRef]
- Klimant, P.; Klimantová, A.; Baláž, I.; Jakab, I.; Tulis, F.; Rybanský, L.; Vadel, L.; Krumpálová, Z. Small mammals in an urban area: Habitat preferences and urban rural gradient in Nitra City, Slovakia. Pol. J. Ecol. 2017, 65, 144–157. [Google Scholar] [CrossRef]
- McDevitt, A.D.; Montgomery, W.I.; Tosh, D.G.; Lusby, J.; Reid, N.; White, T.A.; McDevitt, C.D.; O’Halloran, J.; Searle, J.B.; Yearsley, J.M. Invading and Expanding: Range Dynamics and Ecological Consequences of the Greater White-Toothed Shrew (Crocidura russula) Invasion in Ireland. PLoS ONE 2014, 9, e100403. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Lecompte, E.; Veyrunes, F.; Barriere, P.; Nicolas, V.; Koulemou, K. Diversity and dynamics in a community of small mammals in coastal Guinea, West Africa. Belg. J. Zool. 2009, 139, 93–102. [Google Scholar]
- Khanam, S.; Mushtaq, M.; Kayani, A.R.; Nadeem, M.S.; Beg, M.A. Small mammal community composition and abundance in rural human habitations of Pothwar, Pakistan. Trop. Ecol. 2017, 58, 515–524. [Google Scholar]
- Wells, K.; Lakim, M.B.; O’Hara, R.B. Shifts from native to invasive small mammals across gradients from tropical forest to urban habitat in Borneo. Biodivers. Conserv. 2014, 23, 2289–2303. [Google Scholar] [CrossRef]
- Cully, J.F.; Collinge, S.K.; Van Nimwegen, R.E.; Ray, C.; Johnson, W.C.; Thiagarajan, B.; Conlin, D.B.; Holmes, B.E. Spatial variation in keystone effects: Small mammal diversity associated with black-tailed prairie dog colonies. Ecography 2010, 33, 667–677. [Google Scholar] [CrossRef]
- Jing-yuan, L.; Hong, D.; Geng-bai, T.; Pin-hong, Y.; Shen-wen, W.; Hong, P. Community Structure and Diversity Distributions of Small Mammals in Different Sample Plots in the Eastern Part of Wuling Mountains. Zool. Res. 2008, 29, 637–645. [Google Scholar]
- Butet, A.; Paillat, G.; Delettre, Y. Factors driving small rodents assemblages from field boundaries in agricultural landscapes of western France. Landsc. Ecol. 2006, 21, 449–461. [Google Scholar]
- Andrade, A.; Monjeau, A. Patterns in community assemblage and species richness of small mammals across an altitudinal gradient in semi-arid Patagonia. J. Arid Environ. 2014, 106, 18–26. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Shenbrot, G.I.; Mouillot, D.; Khokhlova, I.S.; Poulin, R. Spatial Variation in Species Diversity and Composition of Flea Assemblages in Small Mammalian Hosts: Geographical Distance or Faunal Similarity? J. Biogeogr. 2015, 32, 633–644. [Google Scholar] [CrossRef]
- Monath, T.P. A short history of Lassa fever: The first 10–15 years after discovery. Curr. Opin. Virol. 2019, 37, 77–83. [Google Scholar] [CrossRef]
- Attinsounon, C.A.; Ossibi, I.B.R.; Alassani, A.; Adé, S.; Saké, K.; Glèlè-Kakaï, C.; Dovonou, A. Report of a fatal case of Lassa fever in Parakou in 2018: Clinical, therapeutic and diagnostic aspects. BMC Infect. Dis. 2018, 18, 667. [Google Scholar] [CrossRef]
- Güneralp, B.; Lwasa, S.; Masundire, H.; Parnell, S.; Seto, K.C. Urbanization in Africa: Challenges and opportunities for conservation. Environ. Res. Lett. 2018, 13, 015002. [Google Scholar] [CrossRef]
- Neiderud, C.J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 2015, 5, 27060. [Google Scholar] [CrossRef]
- Eskew, E.A.; Olival, K.J. De-urbanization and Zoonotic Disease Risk. EcoHealth 2018, 15, 707–712. [Google Scholar] [CrossRef]
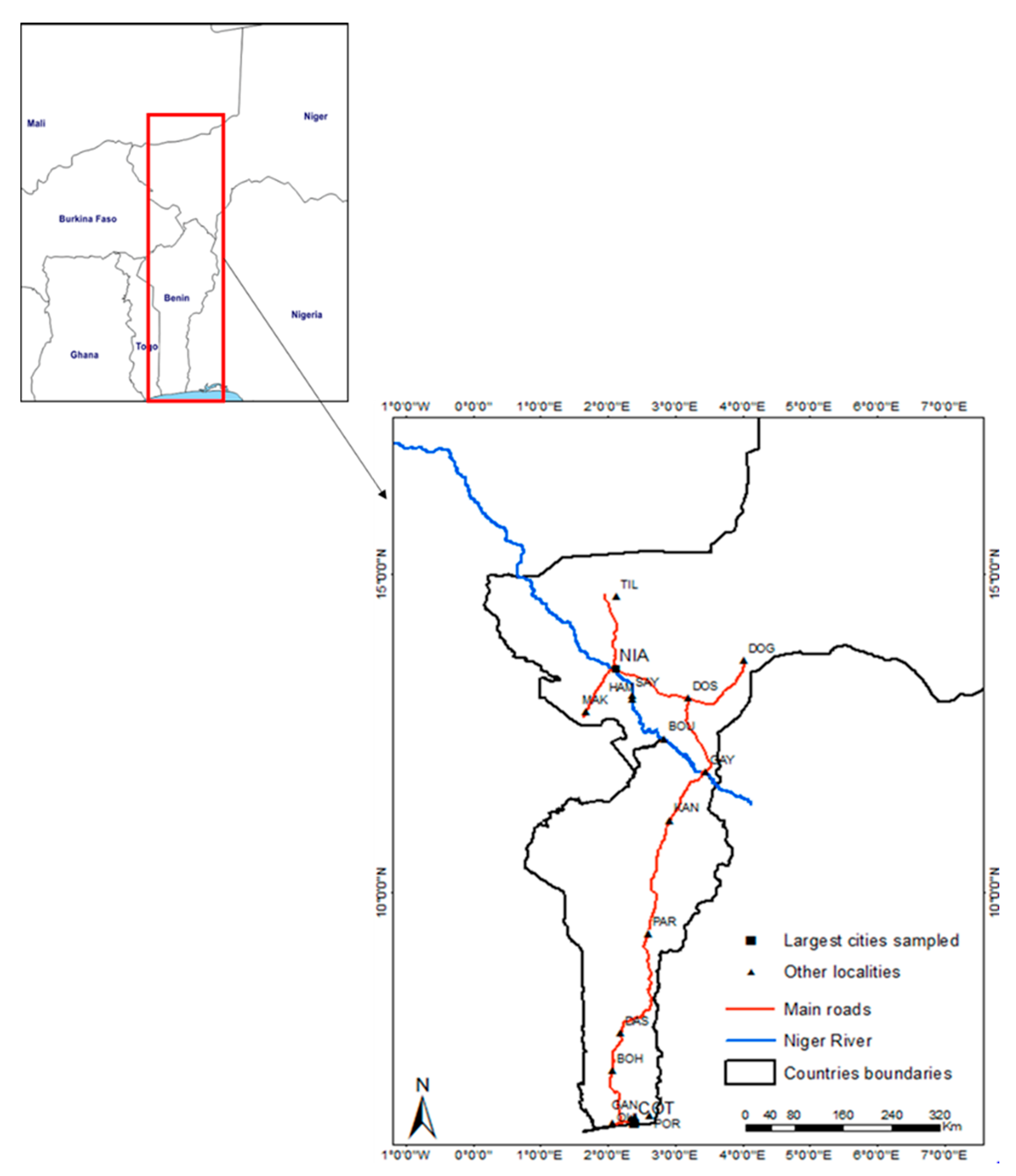
| Localities | Sites | Latitude (°N) | Longitude (°E) | Sampling Date(s) | Trapping Effort (Trap-Nights) | Invasive Species | Native Species | Total Captures | Diversity Indices | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R. ra. | R. no. | M. mu. | M. nat. | Cro. | Arvi. | Prao. | Cri. | M. ery. | S(10) | 1-D | H | |||||||
| Cotonou | PAC | 6.348 | 2.431 | 2006, 2014, 2015 | 840 | 38 | 99 | 12 | 3 | 87 | 0 | 0 | 0 | 0 | 239 | 3.35 | 0.67 | 1.23 |
| Cotonou | Wlacodji | 6.351 | 2.442 | 2006 | 270 | 9 | 7 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 22 | 3.71 | 0.69 | 1.27 |
| Cotonou | Marché Ganhi | 6.355 | 2.437 | 2005 | 60 | 0 | 18 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 25 | 1.99 | 0.40 | 0.59 |
| Togbin | Togbin | 6.355 | 2.305 | 2017 | 262 | 6 | 0 | 0 | 35 | 4 | 0 | 0 | 0 | 0 | 45 | 2.45 | 0.37 | 0.68 |
| Cotonou | Jacques | 6.358 | 2.457 | 2006 | 45 | 13 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 18 | 2.75 | 0.44 | 0.78 |
| Cotonou | Enagnon | 6.362 | 2.453 | 2006 | 180 | 17 | 9 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 32 | 3.36 | 0.62 | 1.14 |
| Cotonou | St-Jean | 6.363 | 2.418 | 2010, 2016, 2017 | 1791 | 128 | 0 | 0 | 17 | 93 | 0 | 14 | 12 | 0 | 264 | 3.28 | 0.63 | 1.19 |
| Cotonou | Abokicodji | 6.363 | 2.442 | 2009, 2010 | 240 | 37 | 1 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 43 | 2.21 | 0.25 | 0.55 |
| Cotonou | Bokossi | 6.365 | 2.438 | 2009, 2010 | 150 | 9 | 10 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 26 | 2.98 | 0.66 | 1.09 |
| Cotonou | Tokpa | 6.365 | 2.434 | 2006 | 150 | 18 | 7 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 27 | 2.59 | 0.48 | 0.81 |
| Cotonou | Dédokpo | 6.369 | 2.439 | 2006 | 210 | 16 | 8 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 26 | 2.76 | 0.52 | 0.91 |
| Ouidah | Ouidah | 6.372 | 2.076 | 2015 | 720 | 115 | 6 | 0 | 39 | 36 | 4 | 0 | 0 | 0 | 200 | 3.22 | 0.60 | 1.13 |
| Cotonou | Kpankpan | 6.373 | 2.439 | 2006 | 270 | 1 | 8 | 0 | 12 | 3 | 0 | 0 | 0 | 0 | 24 | 3.23 | 0.62 | 1.11 |
| Cotonou | Marché Tokpa | 6.374 | 2.430 | 2006 | 60 | 22 | 7 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 31 | 2.60 | 0.44 | 0.80 |
| Cotonou | Agla | 6.375 | 2.363 | 2010, 2016, 2017 | 1728 | 168 | 27 | 0 | 57 | 82 | 0 | 6 | 1 | 0 | 341 | 3.54 | 0.66 | 1.28 |
| Cotonou | Chankpamè | 6.378 | 2.486 | 2006 | 270 | 18 | 0 | 0 | 4 | 6 | 0 | 0 | 0 | 0 | 28 | 2.80 | 0.52 | 0.89 |
| Cotonou | Zogbohouè | 6.379 | 2.389 | 2006 | 430 | 46 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 48 | 1.42 | 0.08 | 0.20 |
| Cotonou | Adogléta | 6.381 | 2.438 | 2005, 2006 | 60 | 5 | 2 | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 13 | 3.92 | 0.71 | 1.31 |
| Cotonou | Suru-Léré | 6.382 | 2.462 | 2006 | 240 | 35 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 38 | 1.73 | 0.15 | 0.33 |
| Cotonou | Djidjè | 6.384 | 2.434 | 2006 | 210 | 6 | 8 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 18 | 2.98 | 0.64 | 1.06 |
| Cotonou | Kowégbo | 6.387 | 2.469 | 2006 | 210 | 28 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 1.34 | 0.07 | 0.15 |
| Cotonou | Ahouansori | 6.388 | 2.423 | 2005 | 210 | 22 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 24 | 1.83 | 0.16 | 0.34 |
| Cotonou | Ladji | 6.389 | 2.433 | 2010, 2016, 2017 | 1744 | 182 | 16 | 0 | 5 | 101 | 0 | 1 | 0 | 0 | 305 | 2.59 | 0.53 | 0.91 |
| Cotonou | Avotrou | 6.389 | 2.476 | 2005 | 360 | 40 | 0 | 0 | 13 | 1 | 0 | 0 | 0 | 0 | 54 | 2.14 | 0.39 | 0.64 |
| Cotonou | Minonchou | 6.391 | 2.457 | 2006 | 150 | 23 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 28 | 2.21 | 0.30 | 0.56 |
| Cotonou | Ayimlonfidé | 6.392 | 2.567 | 2017 | 210 | 28 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 1.56 | 0.12 | 0.24 |
| Cotonou | Gankpodo | 6.393 | 2.456 | 2006 | 150 | 28 | 0 | 0 | 5 | 4 | 0 | 0 | 0 | 0 | 37 | 2.55 | 0.40 | 0.72 |
| Cotonou | Fifadji | 6.395 | 2.398 | 2006, 2016 | 210 | 69 | 19 | 0 | 1 | 6 | 0 | 0 | 0 | 0 | 95 | 2.51 | 0.43 | 0.78 |
| Cotonou | Vossa Kpodji | 6.397 | 2.400 | 2006 | 250 | 48 | 5 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 56 | 2.15 | 0.26 | 0.54 |
| Cotonou | Godomey | 6.413 | 2.312 | 2006 | 480 | 14 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 16 | 2.25 | 0.23 | 0.46 |
| Ganvié | Ganvié | 6.469 | 2.397 | 2017 | 230 | 36 | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 43 | 2.05 | 0.28 | 0.51 |
| Porto Novo | Porto Novo | 6.497 | 2.629 | 2015 | 580 | 129 | 4 | 0 | 30 | 67 | 0 | 0 | 0 | 0 | 230 | 2.89 | 0.58 | 1.02 |
| Bohicon | Bohicon | 7.192 | 2.076 | 2017 | 276 | 44 | 0 | 0 | 21 | 8 | 0 | 0 | 0 | 0 | 73 | 2.69 | 0.54 | 0.91 |
| Dassa-Zoumé | Dassa-Zoumé | 7.785 | 2.199 | 2017 | 169 | 15 | 2 | 0 | 9 | 5 | 0 | 0 | 0 | 0 | 31 | 3.41 | 0.65 | 1.18 |
| Parakou | Parakou | 9.376 | 2.630 | 2017 | 190 | 34 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 39 | 1.97 | 0.23 | 0.45 |
| Kandi | Kandi | 11.135 | 2.936 | 2017 | 280 | 38 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 39 | 1.26 | 0.05 | 0.12 |
| Gaya | Gaya | 11.877 | 3.451 | 2011 | 304 | 32 | 0 | 0 | 6 | 0 | 2 | 0 | 0 | 0 | 40 | 2.29 | 0.34 | 0.61 |
| Boumba | Boumba | 12.409 | 2.840 | 2011 | 201 | 8 | 0 | 0 | 41 | 0 | 3 | 0 | 0 | 0 | 52 | 2.32 | 0.35 | 0.64 |
| Makalondi | Makalondi | 12.836 | 1.687 | 2012 | 334 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 15 | 1.00 | 0.00 | 0.00 |
| Hamma Dendi | Hamma Dendi | 13.0327 | 2.3785 | 2011 | 326 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 1.00 | 0.00 | 0.00 |
| Dosso | Dosso | 13.042 | 3.198 | 2011 | 543 | 31 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 36 | 1.83 | 0.24 | 0.40 |
| Say | Say | 13.096 | 2.360 | 2011 | 338 | 0 | 0 | 0 | 18 | 0 | 7 | 0 | 0 | 0 | 25 | 1.99 | 0.40 | 0.59 |
| Niamey | Gnalga | 13.479 | 2.114 | 2010 | 400 | 0 | 0 | 0 | 27 | 3 | 0 | 0 | 0 | 0 | 30 | 1.72 | 0.18 | 0.33 |
| Niamey | Pont Kennedy | 13.485 | 2.102 | 2010 | 448 | 0 | 0 | 0 | 40 | 4 | 0 | 0 | 0 | 0 | 44 | 1.66 | 0.17 | 0.30 |
| Niamey | Abattoirs | 13.490 | 2.123 | 2010 | 478 | 77 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 77 | 1.0 | 0.00 | 0.00 |
| Niamey | Karadje 1–2 | 13.494 | 2.097 | 2009, 2011 | 1290 | 0 | 0 | 0 | 50 | 8 | 0 | 0 | 0 | 0 | 58 | 1.80 | 0.24 | 0.40 |
| Niamey | Gamkalé Q | 13.494 | 2.125 | 2010 | 452 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | 0 | 0 | 23 | 1.00 | 0.00 | 0.00 |
| Niamey | Kirkissoye | 13.495 | 2.110 | 2010 | 725 | 24 | 0 | 0 | 13 | 0 | 4 | 0 | 2 | 0 | 43 | 3.07 | 0.59 | 1.05 |
| Niamey | CGA | 13.502 | 2.112 | 2010 | 326 | 19 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 20 | 1.50 | 0.10 | 0.20 |
| Niamey | Lamorde | 13.507 | 2.077 | 2010 | 418 | 0 | 0 | 0 | 36 | 1 | 0 | 0 | 0 | 0 | 37 | 1.27 | 0.05 | 0.12 |
| Niamey | CYA | 13.512 | 2.099 | 2010 | 500 | 4 | 0 | 0 | 61 | 0 | 0 | 0 | 0 | 0 | 65 | 1.50 | 0.12 | 0.23 |
| Niamey | Petit Marché | 13.514 | 2.110 | 2011 | 374 | 13 | 0 | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 37 | 1.99 | 0.46 | 0.65 |
| Niamey | Wadata | 13.518 | 2.144 | 2010 | 497 | 0 | 0 | 0 | 11 | 6 | 0 | 0 | 0 | 0 | 17 | 2.00 | 0.46 | 0.65 |
| Niamey | Grand Marché | 13.519 | 2.115 | 2011 | 305 | 7 | 0 | 61 | 0 | 3 | 0 | 0 | 0 | 0 | 71 | 2.04 | 0.25 | 0.49 |
| Niamey | Entrepôt CYA | 13.520 | 2.081 | 2009 | 661 | 19 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 22 | 1.86 | 0.24 | 0.40 |
| Niamey | Route Filingué | 13.521 | 2.152 | 2011 | 370 | 0 | 0 | 0 | 15 | 15 | 0 | 0 | 0 | 0 | 30 | 2.00 | 0.50 | 0.69 |
| Niamey | Yantala Bas | 13.527 | 2.082 | 2010 | 449 | 0 | 0 | 0 | 27 | 1 | 0 | 0 | 0 | 0 | 28 | 1.36 | 0.07 | 0.15 |
| Niamey | Yantala Haut | 13.534 | 2.082 | 2010 | 484 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | 0 | 0 | 23 | 1.00 | 0.00 | 0.00 |
| Niamey | Boukoki | 13.537 | 2.113 | 2010 | 449 | 0 | 0 | 0 | 47 | 2 | 0 | 0 | 0 | 0 | 49 | 1.37 | 0.08 | 0.17 |
| Niamey | Banifandou | 13.544 | 2.136 | 2010 | 370 | 0 | 0 | 0 | 33 | 8 | 0 | 0 | 0 | 0 | 41 | 1.92 | 0.31 | 0.49 |
| Niamey | Daressalam | 13.546 | 2.096 | 2010 | 531 | 0 | 0 | 0 | 40 | 1 | 0 | 0 | 0 | 0 | 41 | 1.24 | 0.05 | 0.11 |
| Niamey | Koubia | 13.552 | 2.054 | 2010 | 378 | 0 | 0 | 0 | 26 | 2 | 0 | 0 | 0 | 0 | 28 | 1.60 | 0.13 | 0.26 |
| Niamey | Koirategui | 13.589 | 2.109 | 2010 | 266 | 0 | 0 | 0 | 10 | 2 | 0 | 0 | 0 | 0 | 12 | 1.98 | 0.28 | 0.45 |
| Niamey | Tchangare | 13.589 | 2.101 | 2010 | 228 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 16 | 1.00 | 0.00 | 0.00 |
| Dogondoutchi | Dogondoutchi | 13.644 | 4.034 | 2012 | 193 | 16 | 0 | 0 | 22 | 0 | 0 | 1 | 0 | 0 | 39 | 2.25 | 0.51 | 0.78 |
| Tillabery | Tillabery | 14.218 | 1.455 | 2011 | 619 | 0 | 0 | 0 | 6 | 0 | 1 | 0 | 0 | 8 | 15 | 2.67 | 0.55 | 0.88 |
| Total | 27,142 | 1765 | 272 | 73 | 912 | 610 | 24 | 22 | 15 | 8 | 3701 | |||||||
| Variation | Morisita-Horn Similarity Index | Jaccard Similarity Index | ||
|---|---|---|---|---|
| Variance (%) | P | Variance (%) | P | |
| [I + S] | 44.6 | <0.0001 | 24.4 | <0.0001 |
| [I] | 41.1 | <0.0001 | 19.2 | <0.0001 |
| [S] | 18.9 | <0.0001 | 15.0 | <0.0001 |
| [I|S] | 25.7 | <0.0001 | 9.4 | <0.0001 |
| [S|I] | 3.5 | <0.0001 | 5.2 | <0.0001 |
| [I∩S] | 15.4 | - | 9.9 | - |
| 1 − [I + S] | 55.4 | - | 75.6 | - |
| Species 1 | Number of Occurrences | Species 2 | Number of Occurrences | Number of Joint Occurrences | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|---|---|
| SCS | P | SCS | P | |||||
| M. natalensis | 55 | R. rattus | 47 | 37 | 1.49 | 0.14 | 1.70 | 0.088 |
| M. natalensis | 55 | Crocidura spp. | 46 | 40 | −1.42 | 0.16 | −1.18 | 0.24 |
| M. natalensis | 55 | R. norvegicus | 22 | 15 | 2.91 | 0.003 | 2.46 | 0.014 |
| R. rattus | 47 | Crocidura spp. | 46 | 33 | −0.33 | 0.74 | −0.21 | 0.83 |
| R. rattus | 47 | R. norvegicus | 22 | 21 | −2.62 | 0.009 | −2.65 | 0.008 |
| Crocidura spp. | 46 | R. norvegicus | 22 | 20 | −2.20 | 0.027 | −2.35 | 0.018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hima, K.; Houémenou, G.; Badou, S.; Garba, M.; Dossou, H.-J.; Etougbétché, J.; Gauthier, P.; Artige, E.; Fossati-Gaschignard, O.; Gagaré, S.; et al. Native and Invasive Small Mammals in Urban Habitats along the Commercial Axis Connecting Benin and Niger, West Africa. Diversity 2019, 11, 238. https://doi.org/10.3390/d11120238
Hima K, Houémenou G, Badou S, Garba M, Dossou H-J, Etougbétché J, Gauthier P, Artige E, Fossati-Gaschignard O, Gagaré S, et al. Native and Invasive Small Mammals in Urban Habitats along the Commercial Axis Connecting Benin and Niger, West Africa. Diversity. 2019; 11(12):238. https://doi.org/10.3390/d11120238
Chicago/Turabian StyleHima, Karmadine, Gualbert Houémenou, Sylvestre Badou, Madougou Garba, Henri-Joel Dossou, Jonas Etougbétché, Philippe Gauthier, Emma Artige, Odile Fossati-Gaschignard, Sama Gagaré, and et al. 2019. "Native and Invasive Small Mammals in Urban Habitats along the Commercial Axis Connecting Benin and Niger, West Africa" Diversity 11, no. 12: 238. https://doi.org/10.3390/d11120238
APA StyleHima, K., Houémenou, G., Badou, S., Garba, M., Dossou, H.-J., Etougbétché, J., Gauthier, P., Artige, E., Fossati-Gaschignard, O., Gagaré, S., Dobigny, G., & Dalecky, A. (2019). Native and Invasive Small Mammals in Urban Habitats along the Commercial Axis Connecting Benin and Niger, West Africa. Diversity, 11(12), 238. https://doi.org/10.3390/d11120238






