The Red Alien vs. the Blue Destructor: The Eradication of Cherax destructor by Procambarus clarkii in Latium (Central Italy)
Abstract
1. Introduction
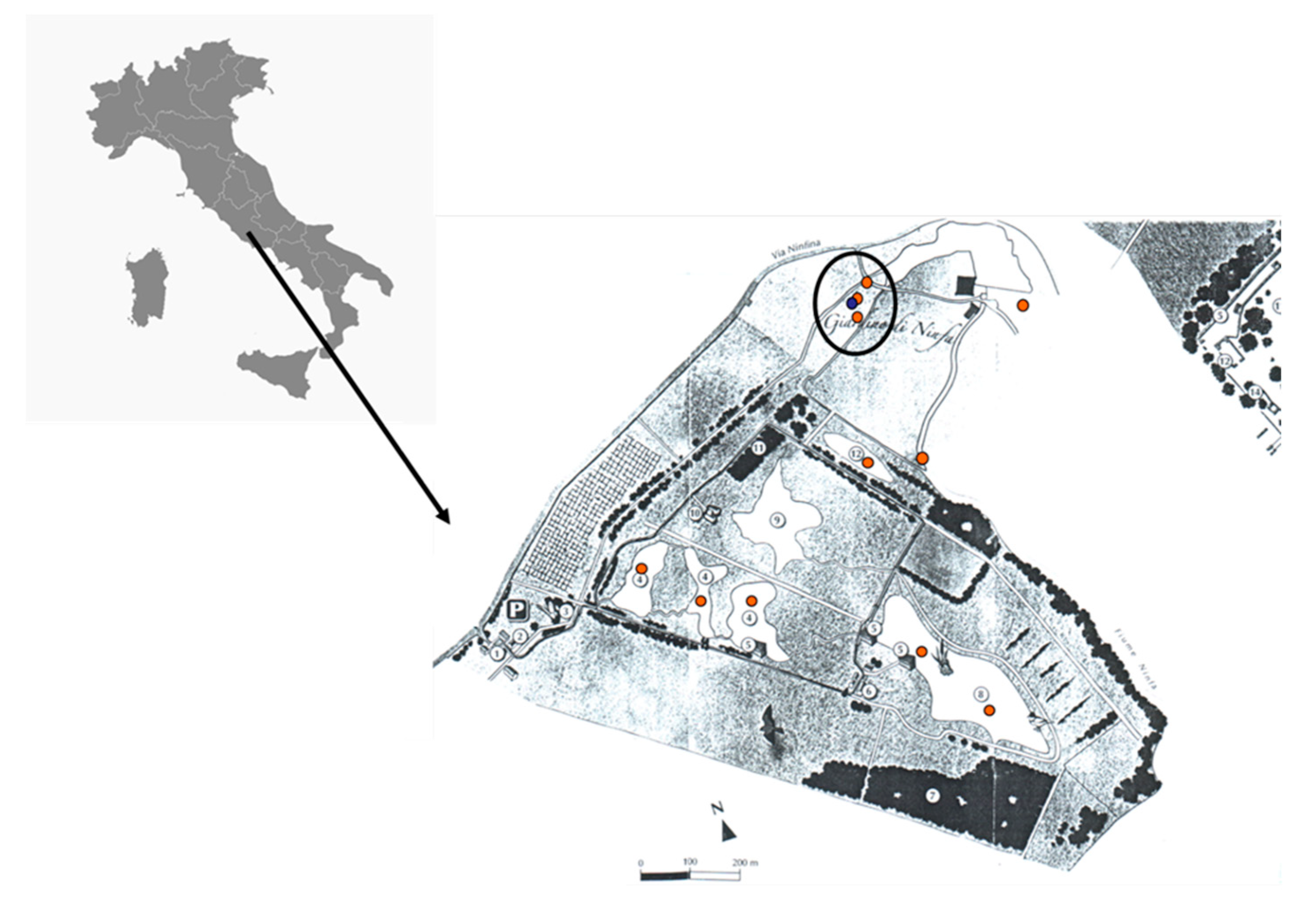
2. Materials and Methods
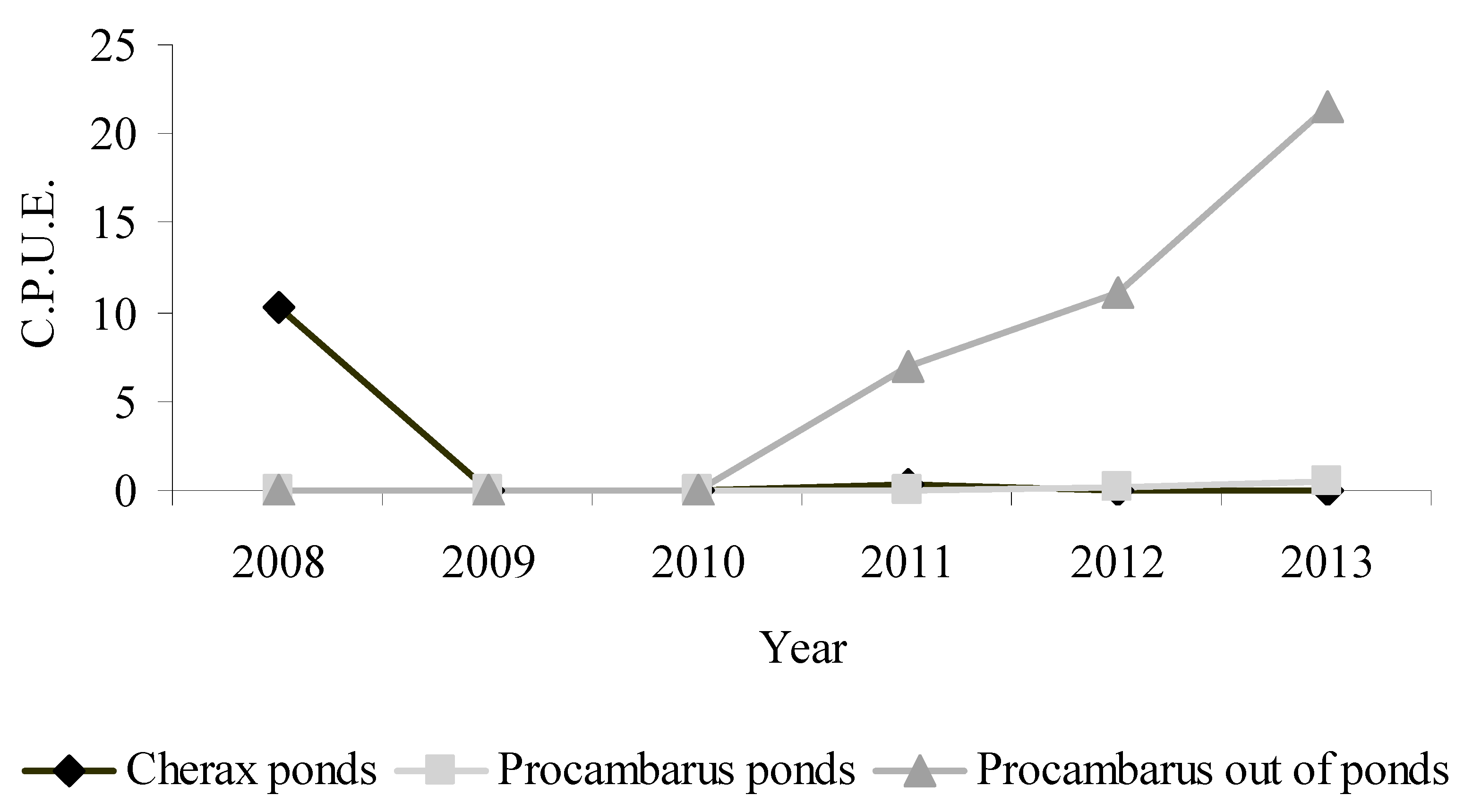
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gherardi, F.; Gollasch, S.; Minchin, D.; Olenin, S.; Panov, V.E. Alien invertebrates and fish in European inland waters. In Handbook of Alien Species in Europe; Springer: Dordrecht, The Netherlands, 2009; pp. 81–92. [Google Scholar]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef]
- Tricarico, E.; Junqueira, A.O.; Dudgeon, D. Alien species in aquatic environments: A selective comparison of coastal and inland waters in tropical and temperate latitudes. Aquat. Conserv. 2016, 26, 872–891. [Google Scholar] [CrossRef]
- Mazza, G.; Tricarico, E. Invasive Species and Human Health; CABI Invasives Series 10; CPI Group: Preston, UK, 2018; 186p, ISBN 13 978-1-78639-098-1. [Google Scholar]
- Lodge, D.M.; Deines, A.; Gherardi, F.; Yeo, D.C.; Arcella, T.; Baldridge, A.K.; Barnes, M.A.; Lindsay Chadderton, W.; Feder, J.L.; Gantz, C.A.; et al. Global introductions of crayfishes: Evaluating the impact of species invasions on ecosystem services. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 449–472. [Google Scholar] [CrossRef]
- Kouba, A.; Petrusek, A.; Kozák, P. Continental-wide distribution of crayfish species in Europe: Update and maps. Knowl. Manag. Aquat. Ecosyst. 2014, 413, 5. [Google Scholar] [CrossRef]
- Aquiloni, L.; Tricarico, E.; Gherardi, F. Crayfish in Italy: Distribution, threats and management. Int. Aquat. Res. 2010, 2, 1–14. [Google Scholar]
- Scalici, M.; Chiesa, S.; Gherardi, F.; Ruffini, M.; Gibertini, G.; Marzano, F.N. The new threat to Italian inland waters from the alien crayfish “gang”: The Australian Cherax destructor Clark, 1936. Hydrobiologia 2009, 632, 341–345. [Google Scholar] [CrossRef]
- Coughran, J.; Daly, G. Potential threats posed by a translocated crayfish: The case of Cherax destructor in coastal drainages of New South Wales, Australia. Crustac. Res. 2012, 7, 5–13. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Holdich, D.M.; Noe¨l, P.Y.; Reynolds, J.D.; Haffner, P. Atlas of Crayfish in Europe; Patrimoines Naturels 64; Muséum National d’ Histoire Naturelle: Paris, France, 2006. [Google Scholar]
- Holdich, D.M.; Reynolds, J.D.; Souty-Grosset, C.; Sibley, P.J. A review of the ever increasing threat to European crayfish from nonindigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2009, 11, 394–395. [Google Scholar]
- Deidun, A.; Sciberras, A.; Formosa, J.; Zava, B.; Insacco, G.; Corsini-Foka, M.; Crandall, K.A. Invasion by non-indigenous freshwater decapods of Malta and Sicily, central Mediterranean Sea. J. Crustac. Biol. 2018. [Google Scholar] [CrossRef]
- Tricarico, E.; Vilizzi, L.; Gherardi, F.; Copp, G.H. Calibration of FI-ISK, an invasiveness screening tool for non-indigenous freshwater invertebrates. Risk Anal. 2010, 30, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Veselý, L.; Buřič, M.; Kouba, A. Hardy exotics species in temperate zone: Can “warm water” crayfish invaders establish regardless of low temperatures? Sci. Rep. 2015, 5, 16340. [Google Scholar] [CrossRef] [PubMed]
- Scalici, M.; Pitzalis, M.; Gibertini, G. Crayfish distribution updating in central Italy. Knowl. Manag. Aquat. Ecosyst. 2009, 6, 394–395. [Google Scholar] [CrossRef]
- Cerenius, L.; Bangyeekhun, E.; Keyser, P.; Söderhäll, I.; Söderhäll, K. Host prophenoloxidase expression in freshwater crayfish is linked to increased resistance to the crayfish plague fungus, Aphanomyces astaci. Cell. Microbiol. 2003, 5, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, F.; Pretto, T.; Dundon, W.; Zambon, M.; Giustinelli, A.; Manfrin, A. First occurrence of Aphanomyces astaci epidemic infection in cultured yabby Cherax destructor (Clark, 1936) in Northern Italy. In Proceedings of the 19 Symposium International Association of Astacology, Innsbruck, Austria, 26–31 August 2012. [Google Scholar]
- Mrugała, A.; Veselý, L.; Petrusek, A.; Viljamaa-Dirks, S.; Kouba, A. May Cherax destructor contribute to Aphanomyces astaci spread in Central Europe? Aquat. Invasions 2016, 11, 53–64. [Google Scholar] [CrossRef]
- Gherardi, F.; Aquiloni, L.; Bertocchi, S.; Brusconi, S.; Inghilesi, A.F.; Mazza, G.; Scalici, M.; Tricarico, E. Un contributo multidisciplinare alla conoscenza dei gamberi alloctoni del Lazio. In Alieni: La Minaccia Delle Specie Alloctone per la Biodiversità del Lazio; Monaco, A., Ed.; Palombi Editori: Roma, Italy, 2014. [Google Scholar]
- Oidtmann, B.; Geiger, S.; Steinbauer, P.; Culas, A.; Hoffmann, R.W. Detection of Aphanomyces astaci in North American crayfish by polymerase chain reaction. Dis. Aquat. Organ. 2006, 72, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Scalici, M.; Lucentini, L.; Marzano, F.N. Molecular identification of an alien temnocephalan crayfish parasite in Italian freshwaters. Aquat. Invasions 2015, 10, 209–216. [Google Scholar] [CrossRef]
- Marino, F.; Pretto, T.; Tosi, F.; Monaco, S.; De Stefano, C.; Manfrin, A.; Quaglio, F. Mass mortality of Cherax quadricarinatus (von Martens, 1868) reared in Sicily (Italy): Crayfish plague introduced in an intensive farming. Freshw. Crayfish 2014, 20, 93–96. [Google Scholar] [CrossRef]
- Schrimpf, A.; Schimdt, T.; Schulz, R. Invasive Chinese mitten crab (Eriocheir sinensis) transmits crayfish plague pathogen (Aphanomyces astaci). Aquat. Invasions 2014, 9, 203–209. [Google Scholar] [CrossRef]
- Svoboda, J.; Strand, D.A.; Vrålstad, T.; Grandjean, F.; Edsman, L.; Kozák, P.; Kouba, A.; Fristad, R.F.; Koca, S.B.; Petrusek, A. The crayfish plague pathogen can infect freshwater-inhabiting crabs. Freshw. Biol. 2014, 59, 918–929. [Google Scholar] [CrossRef]
- Svoboda, J.; Mrugała, A.; Kozubíková-Balcarová, E.; Kouba, A.; Diéguez-Uribeondo, J.; Petrusek, A. Resistance to the crayfish plague pathogen, Aphanomyces astaci, in two freshwater shrimps. J. Invertebr. Pathol. 2014, 121, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, L.; Pyšek, P. Manipulating alien plant species propagule pressure as a prevention strategy for protected areas. In Plant Invasions in Protected Areas: Patterns, Problems and Challenges; Foxcroft, L.C., Pyšek, P., Richardson, D.M., Genovesi, P., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 473–486. [Google Scholar]
- Foxcroft, L.C.; Jarošík, V.; Pyšek, P.; Richardson, D.M.; Rouget, M. Protected-area boundaries as filters of plant invasions. Conserv. Biol. 2011, 25, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Mačić, V.; Albano, P.G.; Almpanidou, V.; Claudet, J.; Corrales, X.; Essl, F.; Evagelopoulos, A.; Giovos, I.; Jimenez, C.; Kark, S.; et al. Biological invasions in conservation planning: A global systematic review. Front. Mar. Sci. 2018, 5, 178. [Google Scholar] [CrossRef]
- Milt, A.W.; Diebel, M.W.; Doran, P.J.; Ferris, M.C.; Herbert, M.; Khoury, M.L.; Moody, A.T.; Neeson, T.M.; Ross, J.; Treska, T.; et al. Minimizing opportunity costs to aquatic connectivity restoration while controlling an invasive species. Conserv. Biol. 2018, 32, 894–904. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazza, G.; Scalici, M.; Inghilesi, A.F.; Aquiloni, L.; Pretto, T.; Monaco, A.; Tricarico, E. The Red Alien vs. the Blue Destructor: The Eradication of Cherax destructor by Procambarus clarkii in Latium (Central Italy). Diversity 2018, 10, 126. https://doi.org/10.3390/d10040126
Mazza G, Scalici M, Inghilesi AF, Aquiloni L, Pretto T, Monaco A, Tricarico E. The Red Alien vs. the Blue Destructor: The Eradication of Cherax destructor by Procambarus clarkii in Latium (Central Italy). Diversity. 2018; 10(4):126. https://doi.org/10.3390/d10040126
Chicago/Turabian StyleMazza, Giuseppe, Massimiliano Scalici, Alberto Francesco Inghilesi, Laura Aquiloni, Tobia Pretto, Andrea Monaco, and Elena Tricarico. 2018. "The Red Alien vs. the Blue Destructor: The Eradication of Cherax destructor by Procambarus clarkii in Latium (Central Italy)" Diversity 10, no. 4: 126. https://doi.org/10.3390/d10040126
APA StyleMazza, G., Scalici, M., Inghilesi, A. F., Aquiloni, L., Pretto, T., Monaco, A., & Tricarico, E. (2018). The Red Alien vs. the Blue Destructor: The Eradication of Cherax destructor by Procambarus clarkii in Latium (Central Italy). Diversity, 10(4), 126. https://doi.org/10.3390/d10040126







