Abstract
Seagrass meadows are globally important sinks of ‘Blue Carbon’, but warming water temperatures due to climate change may undermine their capacity to sequester and retain organic carbon (Corg). We tested the effects of warming on seagrass Corg stocks in situ by transplanting seagrass soil cores along a thermal plume generated by a coal-fired power plant in a seagrass-dominated estuary (Lake Macquarie, Australia). Transplanted cores were subjected to temperatures 2 and 4 °C above ambient temperatures and Corg content was measured after 7, 30, 90 and 180 days. We were unable to detect any significant effect of warming on Corg concentration, stocks, chemical composition (as measured by labile, recalcitrant, refractory ratios), or microbial abundance at any time point. In fact, Corg levels were temporally variable. These findings contrast those of previous studies (mostly laboratory-based) that have reported increases in microbial remineralisation (breakdown) of Corg in response to warming. To explain the lack of any detectable warming effect, we suggest that higher temperatures, longer durations of warming exposure, or additional stressors (e.g., oxygen exposure) may be needed to overcome microbial activation barriers and stimulate Corg remineralisation.
1. Introduction
Seagrass meadows are regarded as one the most efficient and long-term organic carbon (Corg) sinks on earth [1,2], but disturbances could threaten this capacity [3]; therefore, understanding the effects of disturbance on Corg stored within seagrass meadows—termed ‘Blue Carbon’—is important. Warming represents one form of disturbance that will affect all of the ocean’s seagrass meadows. Warming can affect the Corg-sink capacity of seagrasses in two main ways. The first is through its impact on the plants themselves—e.g., by diminishing their biomass and photosynthetic capacity [4,5], leading to the loss of seagrass meadows and concomitant loss of sequestration capacity. The second is by stimulating microbial activity within the sediments beneath seagrass meadows, which is where the majority of Blue Carbon reserves are held [1,2], and therefore where the greatest immediate impact of disturbance typically manifests. For example, Macreadie et al. [6] reported loss of ~1000 years’ worth of sediment Blue Carbon within 50 years of a disturbance to a large seagrass meadow on the east coast of Australia, and found high proportions of microbes (aerobic heterotrophs) typically associated with Corg break down or decomposition (remineralisation) in those disturbed areas. The Corg-sink capacity of seagrass Corg stocks with respect to warming should therefore depend on both the plant and soil microbe responses.
In the broader literature, disagreement exists in the effects of climate change on global soil Corg stocks. Microbial activity is typically assumed to follow first-order kinetics, with elevated temperature being associated with increased microbial activity [7], resulting in higher rates of soil Corg decomposition and accelerating climate change [8]. Conversely, warming can increase plant-derived Corg inputs to soils due to increased plant productivity, which can result in net sequestration if the inputs exceed the increases in microbial decomposition [9]. Difficulty in developing a consensus on the effects of warming on soil Corg sequestration and storage by ecosystems is partly due to the wide range of Corg sources and their susceptibility to microbial attack [10]. Soil Corg can be broadly classified into labile, recalcitrant or refractory Corg, based on their susceptibility to remineralisation. These classifications have been used to estimate vulnerability of seagrass Blue Carbon to microbial remineralisation [11], but not within the context of warming.
Studies testing the effects of warming on seagrass Corg stocks are rare. Pederson et al. [12] simulated warming (15, 20, 25, 30, and 35 °C) under anoxic conditions for seven months in the laboratory with Posidonia oceanica seagrass mats from the Mediterranean and found that a temperature rise from 15 °C to 25 °C increased remineralization rates 4.5-fold, indicating that microbial activity was enhanced by increasing temperatures. Beyond 25 °C, however, bacterial growth declined, suggesting a thermal optimum for enhanced remineralisation rate. Recently Arias-Ortiz et al. [13] estimated major losses of seagrass C stocks (2–9 Tg CO2 over 3 years) following a heat wave in Shark Bay (Western Australia). To our knowledge, no studies to date have taken an experimental approach to measuring the effects of warming on seagrass Blue Carbon in situ.
Our study capitalised on a thermal gradient in Lake Macquarie, NSW Australia, generated from the release of hot water into the lake from a nearby power plant, to explore the effect of warming (up to 4 °C above ambient) on seagrass sediments. Cores from a donor site were transplanted along the thermal plume, and the Corg content measured over a period of seven months (spring 2013 to summer 2014). We predicted that the higher temperature treatments would increase Corg remineralisation by microbes in the sediment, leading to lower Corg stocks.
2. Materials and Methods
We performed this study in Lake Macquarie, Australia, an open barrier estuary which is open to the Tasman Sea via a small channel on the eastern side. Transplant samples were sourced from Wyee Point (33°8′23.8″ S, 151°31′16.2″ E) which served as our experimental control site. The site was dominated by meadows of Zostera mulleri (syn Zostera capricornii), interspersed with small, scattered patches of Halophila ovalis, and surrounded by mangroves (Avicennia marina) at the intertidal zone.
We examined the effect of elevated temperature on Corg stocks in seagrass sediment, using sediment cores from a control site transplanted along a temperature gradient within Wyee Bay. This thermal plume is downstream of the Vales Point Power Station (Figure 1A), allowing us to measure the effect of warming in situ. We chose three treatment sites along this plume which, on average, exhibited a 0 °C, 2 °C and 4 °C temperature rise above the control treatment (which at the beginning of the experiment was ~19 °C).
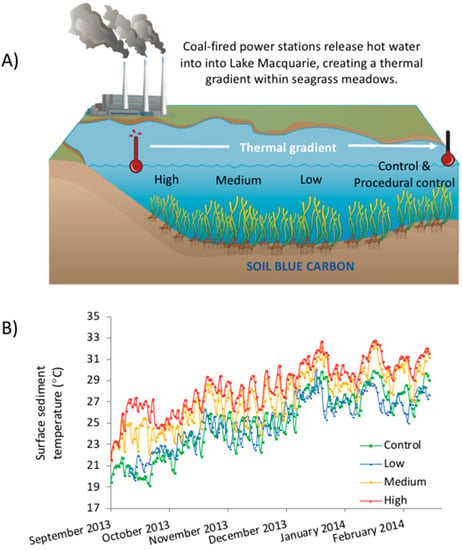
Figure 1.
(A) Experimental setup showing the position of the different warming treatments along the thermal gradient created by the power station. (B) Average daily sediment surface temperatures according to logger data; temperatures at the low treatment were similar to that of the control site, within 0.5 °C of each other, temperature at medium were 2 °C higher than control and temperature at high were 4 °C higher than control.
The ‘high’ temperature site was located approximately 300 m from the power station’s outlet (33°9′29.6″ S, 151° 31′ 54.3″ E), and contained mainly bare sediment with a small amount of H. ovalis that grew in the summer. The ‘low’ and ‘medium’ temperature sites were approximately 1 km (33°9′10.7″ S, 151°32′3.3″ E), and 2 km (33°8′37.2″ S, 151°32′10.2″ E) from the station’s outlet respectively. Both the ‘low’ and ‘medium’ treatment sites contained small patches of Z. mulleri in an otherwise sandy matrix.
Site temperatures were recorded using HOBOware temperature loggers (HOBO Pendant Temperature/Light Data Logger 64 K—UA-002-64), which were sunk at each site to depths of 0, 5, 10 and 20 cm and set to take hourly recordings from September 2013 to February 2014. Generally, the control and low sites had similar temperatures (within 0.5 °C of each other), the medium being 2 °C warmer than these and the high site being 4 °C above the control (Figure 1B). Due to seasonal changes, the temperature increased through the duration of the experiments. This induced a change of at least 10 °C for each treatment within four months.
Sediment cores were taken from the control site using 50 mm diameter PVC tubes using the methods of Ewers Lewis [14]. To ensure no live plants were present in the cores, cores were taken in natural gaps between individual plants within a large, continuous seagrass meadow. Cores extracted from the control site were transported to the treatment site (thermal gradient) on the same day of collection. The tubes were thrust 50 cm into the sediment and PVC caps were tightly attached to the bottom of the cores upon removal. These remained for the duration of the study. Foam caps were placed on top of each core during transportation to prevent sediment movement. Four replicate cores were sunk at each treatment site, until the sediment within the core was level with the sediment outside. Three of these replicate cores were removed at each sampling point, and one was left as a spare.
At each collection time point, (t = 7, 30, 90, 180 days) cores (n = 3) were removed at random from each treatment (high, medium, low and control), sealed, and transported to the University of Technology Sydney. These were stored in a 4 °C fridge until analysis. The cores were then extruded every 0.5 cm at 0–2 cm depth, every 1 cm at 2–6 cm and every 2 cm from 6–10 cm. The wet weight was recorded for each sectioned sediment sample, before being dried at 60 °C for 72 h and the dry sample weight recorded.
The %Corg was measured using a Leco Truspec Carbon Nitrogen Analyser at every depth interval with three replicates. A differential thermogravimetric analysis (TG-DTA (Q600, TA Instruments, New Castle, DE, USA)) was run in order to determine the structural complexity of the Corg within the sediment as per Trevathan-Tackett et al. [15]. Ball-ground samples from depth= 0–0.5 cm and 8–10 cm were chosen to illustrate the change of Corg composition with depth. Experimental conditions included: A heating rate of 15 °C/min from ambient temperature to 670 °C, 20% O2 in He (50 cm3/min), an aluminium crucible, and an approximate sample mass of 25 mg. Prior to Corg analyses, inorganic carbon (carbonate) was removed via acidification with 1 M HCl.
The composition of the Corg analysed was separated based on their ‘percentage weight loss’ into three categories as defined by Capel et al. [16]; labile being 200–400 °C, recalcitrant being 400–550 °C and refractory being 550–650 °C. In short, the terms ‘labile’, ‘recalcitrant’ and ‘refractory’ refer to a spectrum of susceptibility to oxidation or microbial attack, and in the case of this study the thermal stability is assumed to be a proxy for ‘susceptibility’. Due to the varying amount of Corg per core the carbon composition was standardised to reflect their percentage contribution to the total weight loss, as opposed to their weight loss to the total sediment weight.
To check if the process of transplanting cores had any effect on the sulfide and Corg concentrations (which could bias results), cores that were incubated at the control site were compared against fresh cores following a procedure by De Boer [17]. The sulfide concentration in samples at each treatment was analysed at t = 90 days. Fresh samples were sectioned (as described above) on the same day as collection, the porewater extracted via centrifugation (10 min, 1250× g), and passed through a 0.45 µm GF/F filter. Zinc acetate (30 µL, 2%) was added to 1 mL of the filtered porewater, and the sulphide concentrations determined spectrophotometrically using the Cline [18] method.
For determination of microbial cell abundance, 300 µL sub-samples were taken from the top 0–2 cm. The 300 µL sediment samples were immediately mixed with 1 mL of 0.2 μm pre-filtered seawater, collected from the overlaying seawater at the time of sampling. Samples were transferred to cryotubes, fixed with glutaraldehyde (1% final concentration), flash frozen in liquid nitrogen, then stored −80 °C prior to flow cytometry analysis. Immediately before flow cytometric analysis, samples were quickly thawed in warm water and diluted 1:10 in TE. Samples were vortexed for 1 min, Tween 80 (2.5% final concentration) added to the Eppendorf tubes, vortexed again for 2 min. Supernatant was filtered onto 20 µm mesh in order to remove larger particles or cells and approximately 0.5 mL was transferred into FCM tubes [19]. SYBR Green (at a final dilution of 1:10,000) (Invitrogen Molecular Probes USA, Carlsbad, CA, USA) was added to each vial. Fluorescent microspheres (1 µm, Invitrogen Molecular Probes USA) were added as an internal reference (final concentration 3.6 × 106 microspheres mL−1) before cell counting was performed using a Becton & Dickinson LSR II flow cytometer (BD Biosciences). Side-scatter and SYBR green fluorescence were used to discriminate bacterial populations [20]. Data was analysed using Cyflogic [version 1.2.1] flow cytometry analysis software (http://www.cyflogic.com; Perttu Terho & CyFlo Ltd., Turku, Finland).
The effect of coring on sulphide concentrations (µM) and Corg (%Corg) was determined from paired t-tests. Corg stock (g C/cm3) was calculated from %Corg × bulk density. Where possible, data was transformed to fit normality (Kolmogorov-Smirnov test) and variance homogeneity (Levene’s test). The effect of temperature treatments and time on both %Corg and g C/cm3 were tested using a two-way Analysis of Variance (ANOVA) and a three-way ANOVA was used to test time, treatment and depth effects on %Corg. The Corg compositions were tested as independent categories (i.e., labile, recalcitrant and refractory) using a two-way ANOVA with time and depth as fixed factors. Significance was established when p < 0.05.
3. Results and Discussion
Contrary to our predictions, warming (4 °C above ambient) had no effect on the percentage of Corg or the Corg stock in the top 10 cm of seagrass sediment over the six-month testing period (Table 1, Figure 2). Furthermore, there was no change in the Corg ‘quality’ (as inferred by the proportions of labile, refractory, and recalcitrant Corg; Figure 2) or the bacterial cell count (Figure 3) in response to warming for any of the treatments. The only change was a decline in %Corg and Corg stock with time, which occurred across all treatments (Table 1, Figure 4). Given the (surprisingly) high temporal variability in Corg levels during experiment, we are left to ask: Was there no treatment effect, or were we simply unable to detect an effect? In the following sections we will discuss both of these possibilities.

Table 1.
Results of ANOVA test on the effects of time (7, 30, 90, 180 days), warming treatment (high, medium, low, control, procedural control), and their interactions on the % organic carbon (Corg) and the organic carbon stock within seagrass cores. Significant p-values (<0.05) down in bold.
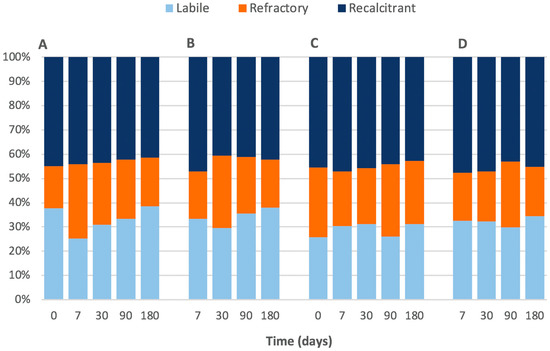
Figure 2.
Thermogravimetric analysis (TGA) showing the labile, refractory, and recalcitrant percentages of organic carbon in the 0–0.5 cm portion of (A) procedural control and (B) high treatment, and the 8–10 cm portion of (C) procedural control and (D) high treatment.
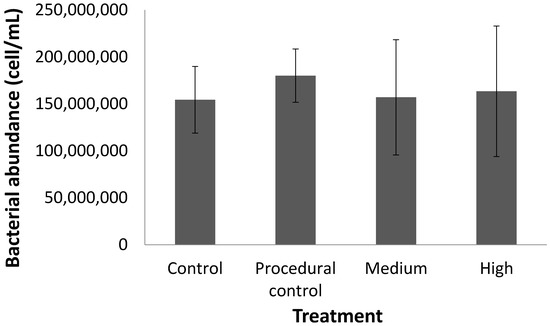
Figure 3.
Bacterial cell abundance (as measured by flow cytometry) in seagrass cores under different treatments.
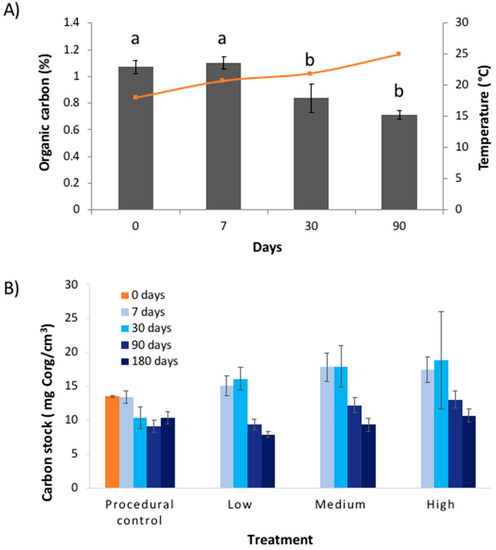
Figure 4.
Results (mean ± SE) of experimental warming study showing: (A) change in the percentage organic carbon (all treatments pooled) within seagrass soil cores against time (‘days’), overlayed with temperature (orange line); and (B) change in carbon stocks for the different treatments and times. Letters above bars in (A) indicate differences according to Tukey HSD test.
This study opportunistically tested the effects of warming from an industrial thermal plume; consequently, there were aspects of the experimental design that were beyond our control. For example, we did not have control over water temperatures, and there is a risk that artefacts were introduced by our experimental transplants. We tested for experimental artefacts of the coring procedure on sulphide and Corg availability within the cores, but found no difference in levels of sulphide and Corg between fresh cores (‘controls’) and sediments confined within PVC and transplanted (‘procedural controls’) (Figure 4). We were also concerned that retaining sediments in PVC cores during the experiment might bias results by limiting sulphide and Corg availability to microbes, but thermogravimetric data showed that quantities of labile Corg and sulphate levels were high (Figure 2 and Figure 4), implying that substrate availability was not an issue. Since we were unable to find any evidence of experimental artefacts, we assumed that our experimental approach was robust and the results are reliable.
The lack of treatment effect could be because the level of warming (4 °C above ambient) was too low to elicit a response from microbes. Microbial activity is typically assumed to follow first-order kinetics, with elevated temperature being associated with increased activity. At higher temperatures (up to a thermal optimum), microbes have more energy, allowing them to metabolise Corg more effectively [21]. In most biological systems, the Q10 efficiency of microbes generally doubles to triples with an increase of 10 °C [10]. In addition, many microbes lie dormant in soils, awakening only once they have enough energy to grow and respire [22], akin to a ‘on-off’ switch as opposed to a ‘dimmer’. The laboratory experiment by Pedersen et al. [12] that tested microbial remineralisation of seagrass Corg in response to warming used a much higher temperature range (15–35 °C). Our data suggests that when the warming gradient from the industrial plume is combined with a seasonal increase in water temperature (which ranged from 12–32 °C over the entire year), there was an increase in microbial remineralisation. This is shown in Figure 4B where the seasonal increase in water temperatures is mirrored by an opposing decline in %Corg in the warming treatments. Microbes at the site are therefore potentially adapted to variable temperatures.
Another possibility is that warming alone (even at high temperatures) is not enough to trigger microbial remineralisation of seagrass C. Recent research suggests that oxygen concentration could have an important influence on the effects of warming on seagrass C. Indeed, Trevathan-Tackett et al. [23] showed that the effects of warming on C remineralisation rates within seagrass meadows depends on oxygen availability. Using 16S-rDNA sequencing, solid-state NMR and microsensor profiling, Trevathan-Tackett et al. [23] demonstrated that the anoxic conditions ubiquitous to seagrass sediments can provide a degree of Corg protection under warming seawater temperatures. We therefore suggest that the effects of warming tested in this study may need to be coupled with an increase in oxygen exposure to trigger a significant increase in C remineralisation.
We must also consider that of the Corg that is remineralised by bacteria, some of the decomposed Corg is assimilated into the bacteria to allow them to grow, meaning that some of the Corg in the seagrass sediments may simply have been converted into bacterial biomass. López-Urrutia [24] modelled bacterial growth efficiency (BGE) and found that while bacterial respiration is temperature dependent, there can be a maximum remineralisation efficiency, above which a higher proportion of biomass is produced compared to CO2. Pederson et al. [12] in their warming experiment with P. oceanica, found that while Q10 was 4.5 times faster from 15–25 °C, above 25 °C microbial efficiency dropped off. A similar effect may be occurring here, where the microbes are less efficient at producing CO2, and instead create more biomass. This could explain why the experimental warming did not result in any (detectable) net Blue Carbon loss.
4. Conclusions
Overall, our study worked within the ecologically relevant climate change scenarios forecasted by the IPCC, with the medium treatment (+2 °C) reflecting the 2100 best-case climate warming scenario, and the high treatment (+4 °C) the worst-case scenario. No significant losses of Corg were observed through time, which could be interpreted to mean that sedimentary Corg stocks in seagrass meadows are unlikely to be affected directly by global sea temperature rise. However, indirect release of Corg may occur through reduced seagrass primary productivity, and habitat loss at higher temperatures. Previous studies at this site (Lake Macquarie) have found prolonged bouts of temperatures above 30 °C would cause 100% seagrass mortality after 42 days [4], potentially causing extensive seagrass loss, and therefore reduced Corg sequestration and storage. Moreover, the effects of temperature might manifest when other stressors (e.g., oxygen exposure) occur, or if the duration of exposure was longer (this study was only 180 days in duration). For future research, we therefore recommend multi-stressor studies over longer time periods, incorporating more sophisticated analyses of microbes (e.g., species composition, function, Corg uptake).
Author Contributions
P.I.M. conceived the study. S.S.S.H. carried out the fieldwork and laboratory analyses. Both authors analysed the data and wrote the paper.
Funding
This research was funded by a UTS School of the Environment Honours Scholarship (to S.S.S.H.) and an Australian Research Council (ARC) Discovery Early Career Researcher Award DE130101084 (to P.I.M.).
Acknowledgments
The authors thank the support of the University of Technology Sydney Climate Change Cluster. We thank Daniel Nielsen, Katherina Petrou, and Peter Ralph for advice and feedback, and Paul Carnell and Tessa Evans for assistance with analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Macreadie, P.I.; Baird, M.E.; Trevathan-Tackett, S.M.; Larkum, A.W.D.; Ralph, P.J. Quantifying and modelling the carbon sequestration capacity of seagrass meadows-a critical assessment. Mar. Pollut. Bull. 2014, 83, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marba, N.; Holmer, M.; Angel Mateo, M.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.; Fourqurean, J.W.; Kauffman, J.B.; Marba, N.; et al. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef] [PubMed]
- York, P.H.; Gruber, R.K.; Hill, R.; Ralph, P.J.; Booth, D.J.; Macreadie, P.I. Physiological and Morphological Responses of the Temperate Seagrass Zostera muelleri to Multiple Stressors: Investigating the Interactive Effects of Light and Temperature. PLoS ONE 2013, 8, e76377. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.J.; Uthicke, S.; Waycott, M. Thermal tolerance of two seagrass species at contrasting light levels: Implications for future distribution in the Great Barrier Reef. Limnol. Oceanogr. 2011, 56, 2200–2210. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Trevathan-Tackett, S.M.; Skilbeck, C.G.; Sanderman, J.; Curlevski, N.; Jacobsen, G.; Seymour, J.R. Losses and recovery of organic carbon from a seagrass ecosystem following disturbance. Proc. R. Soc. B 2015, 282, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, T.; King, G.M.; Blackburn, T.H. Bacterial Biogeochemistry: The Ecophysiology of Mineral Cycling; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Janssens, I.A.; Luo, Y.Q. On the variability of respiration in terrestrial ecosystems: Moving beyond Q(10). Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Trevathan-Tackett, S.M.; Kelleway, J.; Macreadie, P.I.; Beardall, J.; Ralph, P.; Bellgrove, A. Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology 2015, 96, 3043–3057. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.O.; Serrano, O.; Mateo, M.A.; Holmer, M. Temperature effects on decomposition of a Posidonia oceanica mat. Aquat. Microb. Ecol. 2011, 65, 169–182. [Google Scholar] [CrossRef]
- Arias-Ortiz, A.; Serrano, O.; Masque, P.; Lavery, P.S.; Mueller, U.; Kendrick, G.A.; Rozaimi, M.; Esteban, A.; Fourqurean, J.W.; Marba, N.; et al. A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat. Clim. Chang. 2018, 8, 338–344. [Google Scholar] [CrossRef]
- Lewis, C.J.E.; Carnell, P.E.; Sanderman, J.; Baldock, J.A.; Macreadie, P.I. Variability and vulnerability of coastal ‘blue carbon’ stocks: A case study from Southeast Australia. Ecosystems 2017, 21, 263–279. [Google Scholar] [CrossRef]
- Trevathan-Tackett, S.M.; Macreadie, P.I.; Sanderman, J.; Baldock, J.; Howes, J.M.; Ralph, P.J. A global assessment of the chemical recalcitrance of seagrass tissues: Implications for long-term carbon sequestration. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Capel, E.L.; Arranz, J.; Gonzalez-Vila, F.J.; Gonzalez-Perez, J.A.; Manning, D.A.C. Elucidation of different forms of organic carbon in marine sediments from the Atlantic coast of Spain using thermal analysis coupled to isotope ratio and quadrupole mass spectrometry. Org. Geochem. 2006, 37, 1983–1994. [Google Scholar] [CrossRef]
- de Boer, W.F. Seagrass-sediment interactions, positive feedbacks and critical thresholds for occurrence: A review. Hydrobiologia 2007, 591, 5–24. [Google Scholar] [CrossRef]
- Cline, J.D. Spectrophotometric determination of hydorgen sulfide in natural waters. Limnol. Oceanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Duhamel, S.; Jacquet, S. Flow cytometric analysis of bacteria- and virus-like particles in lake sediments. J. Microbiol. Methods 2006, 64, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Marie, D.; Simon, N.; Guillou, L.; Partensky, F.; Vaulot, D. Flow Cytometry Analysis of Marine Picoplankton. In Living Color: Protocols in Flow Cytometry and Cell Sorting; Diamond, R.A., DeMaggio, S., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2000. [Google Scholar]
- Chen, S.; Huang, Y.; Zou, J.; Shi, Y. Mean residence time of global topsoil organic carbon depends on temperature, precipitation and soil nitrogen. Glob. Planet. Chang. 2013, 100, 99–108. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Rodler, A.; Kuffner, M.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S. Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biol. Biochem. 2011, 43, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Trevathan-Tackett, S.M.; Seymour, J.R.; Nielsen, D.A.; Macreadie, P.I.; Jeffries, T.C.; Sanderman, J.; Baldock, J.; Howes, J.M.; Steven, A.D.L.; Ralph, P.J. Sediment anoxia limits microbial-driven seagrass carbon remineralization under warming conditions. FEMS Microbiol. Ecol. 2017, 93, fix033. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Urrutia, A.; Moran, X.A.G. Resource limitation of bacterial production distorts the temperature dependence of oceanic carbon cycling. Ecology 2007, 88, 817–822. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
