Long-Term Changes in the Composition, Ecology, and Structure of Pinus mugo Scrubs in the Apennines (Italy)
Abstract
1. Introduction
2. Materials and Methods
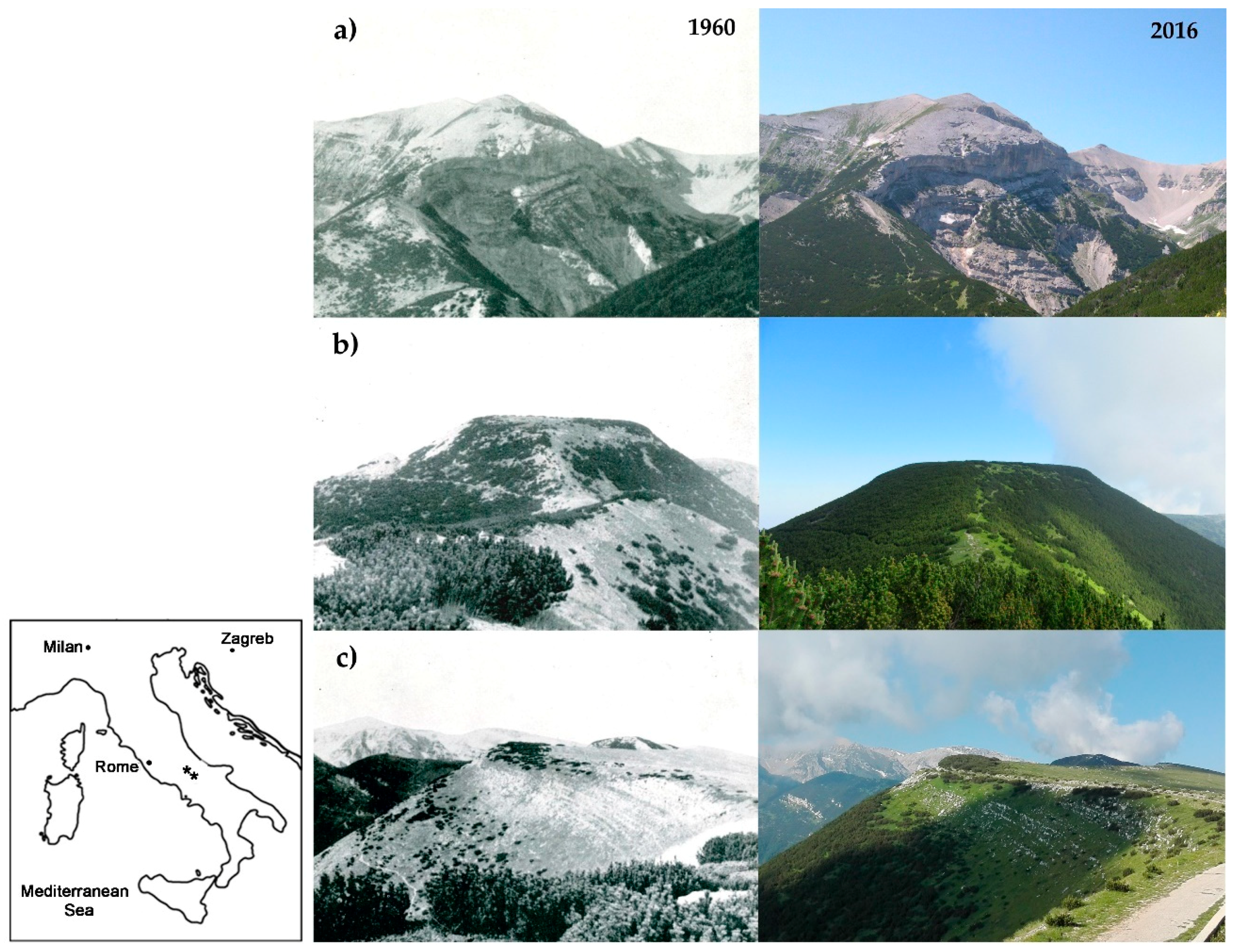
2.1. Study Area
2.2. Vegetation Data
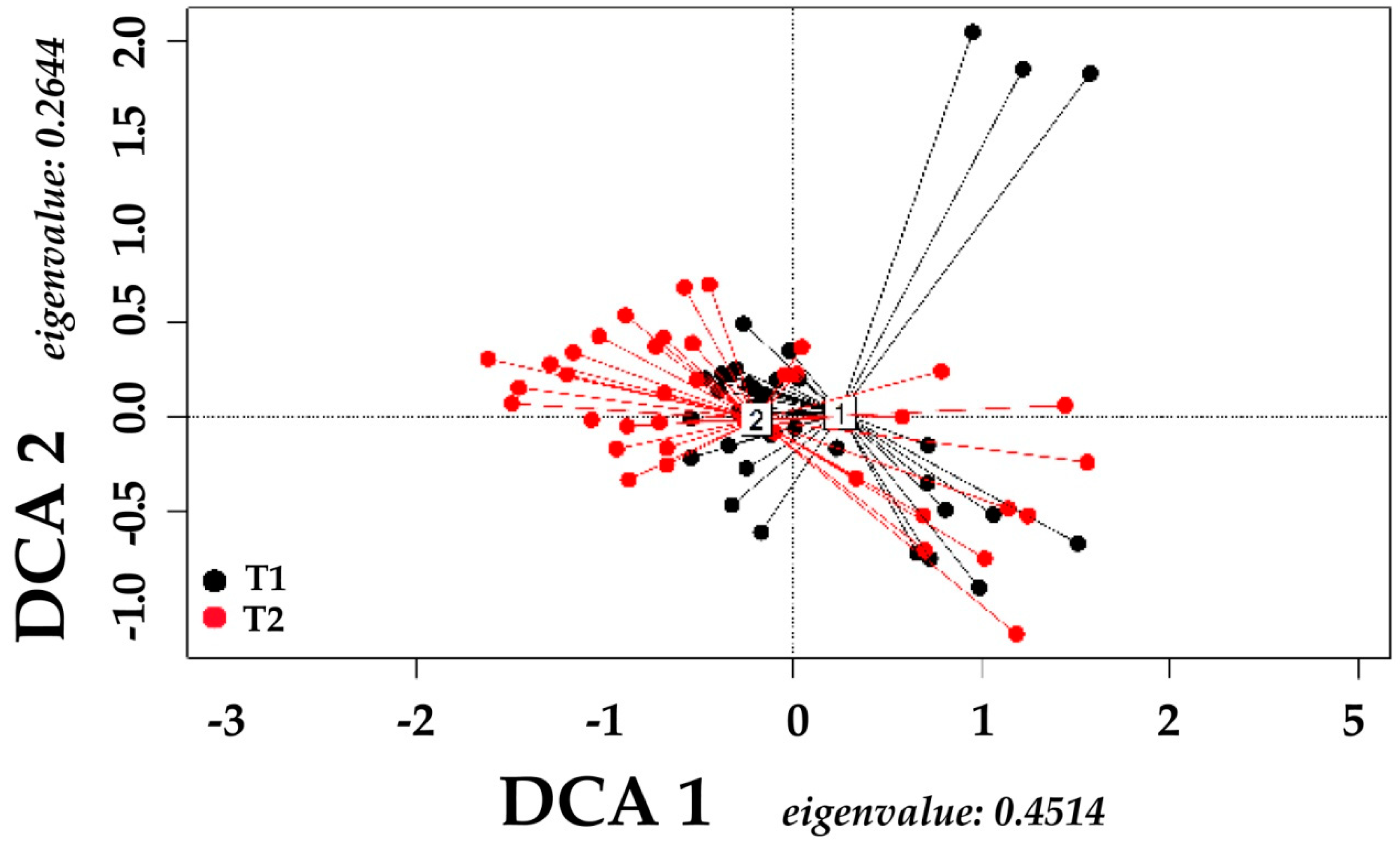
2.3. Data Analysis
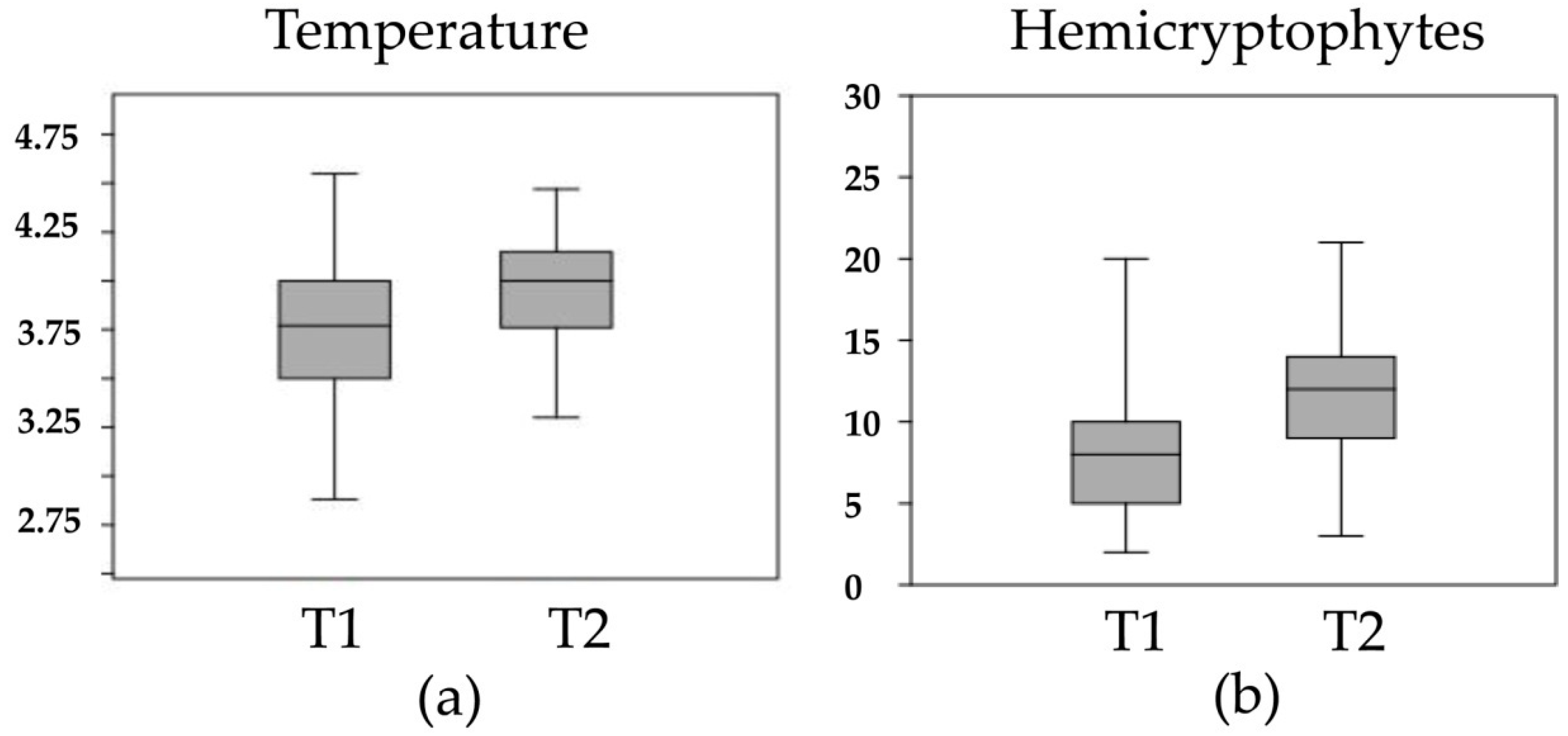
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Taxon | Ecological Indicator Value | Vegetation Belt | |||
|---|---|---|---|---|---|
| L | T | U | N | ||
| Achillea barrelieri s.l. | 11 | 3 | 2 | 1 | 1,2 |
| Adenostyles australis | 6 | 2 | 7 | 4 | 1,2 |
| Anthemis cretica subsp. petraea | 10 | 3 | 2 | 1 | 2 |
| Anthoxanthum nipponicum | 8 | 2 | 5 | 4 | 1,2 |
| Anthyllis montana subsp. jacquinii | 10 | 6 | 3 | 3 | 1,2 |
| Anthyllis vulneraria subsp. pulchella | 8 | 5 | 3 | 3 | 1 |
| Arctostaphylos uva-ursi | 6 | 3 | 3 | 2 | 1,2 |
| Arenaria bertolonii | 7 | 4 | 3 | 1 | 1,2 |
| Armeria gracilis subsp. majellensis | 11 | 3 | 3 | 2 | 1,2 |
| Asperula cynanchica s.l. | 7 | 7 | 3 | 3 | 1 |
| Bellidiastrum michelii | 7 | 3 | 7 | 2 | 1,2 |
| Bellis perennis | 9 | 5 | 7 | 5 | 1,2 |
| Betonica alopecuros subsp. divulsa | 7 | 3 | 4 | 2 | 1,2 |
| Biscutella laevigata subsp. laevigata | 8 | 2 | 3 | 2 | 1,2 |
| Bistorta vivipara | 7 | 2 | 3 | 5 | 1,2 |
| Brachypodium genuense | 8 | 6 | 5 | 4 | 1,2 |
| Bromopsis erecta s.l. | 8 | 5 | 3 | 3 | 1,2 |
| Campanula scheuchzeri s.l. | 8 | 2 | 5 | 0 | 1,2 |
| Carduus chrysacanthus | 11 | 4 | 2 | 1 | 1,2 |
| Carex kitaibeliana | 10 | 4 | 2 | 1 | 1,2 |
| Carex macrolepis | 6 | 5 | 3 | 2 | 1,2 |
| Carlina acaulis subsp. caulescens | 7 | 5 | 4 | 2 | 1 |
| Carum heldreichii | 7 | 4 | 3 | 2 | 1,2 |
| Cephalanthera longifolia | 4 | 5 | 3 | 3 | 1,2 |
| Cerastium arvense s.l. | 8 | 4 | 4 | 4 | 1,2 |
| Cerastium tomentosum | 11 | 6 | 3 | 1 | 1 |
| Clinopodium alpinum s.l. | 9 | 4 | 5 | 2 | 1,2 |
| Crepis aurea subsp. glabrescens | 9 | 2 | 5 | 7 | 1,2 |
| Cynoglossum magellense | 10 | 4 | 3 | 3 | 1,2 |
| Daphne mezereum | 4 | 5 | 5 | 5 | 1,2 |
| Dianthus longicaulis | 8 | 7 | 3 | 4 | 1 |
| Doronicum columnae | 6 | 4 | 6 | 6 | 1,2 |
| Doronicum orientale | 6 | 4 | 6 | 6 | 2 |
| Dryas octopetala subsp. octopetala | 7 | 2 | 7 | 3 | 1,2 |
| Edraianthus graminifolius subsp. graminifolius | 10 | 3 | 3 | 2 | 1,2 |
| Epipactis atrorubens | 8 | 6 | 4 | 4 | 1,2 |
| Epipactis helleborine | 3 | 5 | 5 | 5 | 1 |
| Erysimum pseudorhaeticum | 9 | 7 | 2 | 3 | 1,2 |
| Euphrasia salisburgensis | 7 | 4 | 5 | 4 | 1 |
| Festuca circummediterranea | 11 | 6 | 1 | 2 | 2 |
| Festuca rubra s.l. | 8 | 4 | 4 | 3 | 1,2 |
| Festuca violacea subsp. italica | 8 | 2 | 4 | 4 | 1,2 |
| Galium anisophyllon | 9 | 3 | 3 | 2 | 1,2 |
| Galium corrudifolium | 11 | 8 | 2 | 2 | 1 |
| Gentiana dinarica | 9 | 3 | 4 | 2 | 1,2 |
| Gentiana lutea subsp. lutea | 8 | 4 | 4 | 2 | 2 |
| Globularia meridionalis | 11 | 3 | 2 | 1 | 1,2 |
| Gymnadenia conopsea | 8 | 4 | 4 | 3 | 1 |
| Helianthemum apenninum subsp. apenninum | 9 | 7 | 2 | 2 | 1,2 |
| Helianthemum nummularium subsp. grandiflorum | 9 | 4 | 4 | 2 | 1,2 |
| Helianthemum oelandicum subsp. alpestre | 9 | 4 | 3 | 2 | 1 |
| Helianthemum oelandicum subsp. incanum | 9 | 4 | 3 | 2 | 1,2 |
| Helictochloa praetutiana subsp. praetutiana | 8 | 4 | 6 | 3 | 1,2 |
| Hepatica nobilis | 4 | 6 | 4 | 4 | 1,2 |
| Hieracium bifidum subsp. stenolepis | 8 | 6 | 4 | 2 | 1,2 |
| Hieracium murorum s.l. | 4 | 4 | 5 | 2 | 1,2 |
| Hieracium pietrae | 9 | 2 | 4 | 2 | 2 |
| Hippocrepis comosa subsp. comosa | 9 | 7 | 2 | 2 | 1,2 |
| Hypericum richeri subsp. richeri | 6 | 6 | 6 | 4 | 1,2 |
| Juniperus communis | 8 | 2 | 4 | 4 | 1,2 |
| Koeleria splendens | 11 | 7 | 3 | 1 | 1 |
| Leontodon hispidus subsp. hispidus | 8 | 3 | 4 | 3 | 1,2 |
| Leucanthemum heterophyllum | 7 | 4 | 3 | 2 | 1,2 |
| Leucanthemum tridactylites | 9 | 4 | 3 | 2 | 1,2 |
| Leucopoa dimorpha | 11 | 4 | 2 | 1 | 1,2 |
| Linum capitatum subsp. serrulatum | 9 | 4 | 3 | 1 | 1,2 |
| Lotus corniculatus subsp. corniculatus | 7 | 5 | 4 | 2 | 1,2 |
| Luzula multiflora subsp. multiflora | 7 | 3 | 6 | 3 | 1 |
| Luzula sylvatica subsp. sieberi | 4 | 4 | 6 | 3 | 1,2 |
| Luzula sylvatica subsp. sicula | 4 | 4 | 6 | 5 | 1,2 |
| Moneses uniflora | 4 | 4 | 5 | 2 | 1,2 |
| Myosotis graui | 9 | 4 | 4 | 2 | 1,2 |
| Onobrychis viciifolia | 8 | 7 | 3 | 3 | 1 |
| Oreojuncus monanthos | 9 | 3 | 2 | 2 | 2 |
| Orthilia secunda | 4 | 5 | 5 | 2 | 1,2 |
| Pedicularis elegans | 9 | 3 | 3 | 2 | 1,2 |
| Phyteuma orbiculare | 8 | 3 | 5 | 2 | 1,2 |
| Petrosedum rupestre | 7 | 5 | 2 | 1 | 1 |
| Picris hieracioides subsp. hieracioides | 8 | 7 | 4 | 4 | 1,2 |
| Pilosella officinarum | 8 | 6 | 3 | 2 | 1,2 |
| Pinus mugo subsp. mugo | 8 | 3 | 2 | 3 | 1,2 |
| Plantago atrata subsp. atrata | 5 | 4 | 4 | 5 | 1,2 |
| Poa alpina subsp. alpina | 7 | 3 | 5 | 6 | 1,2 |
| Polygala alpestris subsp. alpestris | 8 | 2 | 4 | 2 | 1,2 |
| Polygala chamaebuxus | 6 | 4 | 3 | 3 | 1 |
| Potentilla crantzii subsp. crantzii | 9 | 2 | 5 | 0 | 1,2 |
| Prenanthes purpurea | 4 | 4 | 5 | 5 | 1,2 |
| Pulsatilla alpina subsp. millefoliata | 8 | 3 | 5 | 3 | 1,2 |
| Ranunculus brevifolius | 11 | 4 | 3 | 2 | 1 |
| Ranunculus breyninus | 9 | 3 | 4 | 3 | 1,2 |
| Ranunculus pollinensis | 11 | 3 | 3 | 1 | 1,2 |
| Ranunculus thora | 8 | 3 | 3 | 3 | 1,2 |
| Robertia taraxacoides | 11 | 5 | 4 | 1 | 2 |
| Rosa pendulina | 7 | 4 | 5 | 5 | 1 |
| Rumex nebroides | 8 | 7 | 4 | 4 | 1,2 |
| Sabulina verna subsp. verna | 9 | 7 | 2 | 0 | 1 |
| Salix retusa | 7 | 2 | 6 | 4 | 2 |
| Scabiosa columbaria s.l. | 8 | 5 | 4 | 2 | 1,2 |
| Scabiosa silenifolia | 11 | 4 | 3 | 2 | 1 |
| Senecio doronicum subsp. orientalis | 8 | 2 | 5 | 3 | 1,2 |
| Senecio squalidus subsp. rupestris | 7 | 4 | 4 | 5 | 1 |
| Sesleria juncifolia subsp. juncifolia | 10 | 4 | 2 | 4 | 1,2 |
| Sesleria nitida subsp. nitida | 11 | 5 | 2 | 1 | 1,2 |
| Silene acaulis subsp. bryoides | 9 | 1 | 5 | 1 | 1,2 |
| Silene multicaulis subsp. multicaulis | 11 | 4 | 2 | 2 | 1,2 |
| Thymus praecox subsp. polytrichus | 8 | 6 | 2 | 2 | 1,2 |
| Trifolium pratense s.l. | 7 | 6 | 4 | 3 | 1,2 |
| Trinia dalechampii | 10 | 4 | 3 | 2 | 1,2 |
| Urtica dioica subsp. dioica | 7 | 8 | 6 | 8 | 1,2 |
| Valeriana montana | 8 | 4 | 5 | 2 | 1,2 |
| Veronica aphylla subsp. aphylla | 8 | 2 | 5 | 2 | 2 |
| Viola eugeniae s.l. | 11 | 4 | 2 | 1 | 1,2 |
References
- Chelli, S.; Wellstein, C.; Campetella, G.; Canullo, R.; Tonin, R.; Zerbe, S.; Gerdol, R. Climate change response of vegetation across climatic gradients in Italy. Clim. Res. 2017, 71, 249–262. [Google Scholar] [CrossRef]
- Epstein, H.E.; Isla, M.S.; Donald, A.W. Recent dynamics of arctic and sub-arctic vegetation. Environ. Res. 2013, 8, 015040. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate change impacts in alpine environments. Geogr. Compass 2010, 4, 1133–1153. [Google Scholar] [CrossRef]
- Rogora, M.; Buzzi, F.; Dresti, C.; Leoni, B.; Lepori, F.; Mosello, R.; Patelli, M.; Salmaso, N. Climatic effects on vertical mixing and deep-water oxygen content in the subalpine lakes in Italy. Hydrobiologia 2018, 1–18. [Google Scholar] [CrossRef]
- Erschbamer, B.; Kiebacher, T.; Mallaun, M.; Unterluggauer, P. Short-term signals of climate change along an altitudinal gradient in the South Alps. Plant Ecol. 2009, 202, 79–89. [Google Scholar] [CrossRef]
- Erschbamer, B.; Unterluggauer, P.; Winkler, E.; Mallaun, M. Changes in plant species diversity revealed by long-term monitoring on mountain summits in the Dolomites (Northern Italy). Preslia 2011, 83, 387–401. [Google Scholar]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.; et al. Recent plant diversity changes on Europe’s mountain summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; with 47 Tables; Springer Science & Business Media: Berlin, Germany, 2003; ISBN 978-3-642-18970-8. [Google Scholar]
- Cannone, N.; Sgorbati, S.; Guglielmin, M. Unexpected impacts of climate change on alpine vegetation. Front. Ecol. Environ. 2007, 5, 360–364. [Google Scholar] [CrossRef]
- Holzinger, B.; Hulber, K.; Camenisch, M.; Grabherr, G. Changes in plant species richness over the last century in the eastern Swiss Alps: Elevational gradient, bedrock effects and migration rates. Plant Ecol. 2008, 195, 179–196. [Google Scholar] [CrossRef]
- Parolo, G.; Graziano, R. Upward migration of vascular plants following a climate warming trend in the Alps. Basic Appl. Ecol. 2008, 9, 100–107. [Google Scholar] [CrossRef]
- Britton, A.J.; Beale, C.M.; Towers, W.; Hewison, R.L. Biodiversity gains and losses: Evidence for homogenisation of Scottish alpine vegetation. Biol. Conserv. 2009, 142, 1728–1739. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Long-term monitoring of mountain peaks in the Alps. In Biomonitoring: General and Applied Aspects on Regional and Global Scales; Springer: Dordrecht, The Netherlands, 2001; pp. 153–177. ISBN 978-90-481-5621-4. [Google Scholar] [CrossRef]
- Engler, R.; Randin, C.; Thuiller, W.; Dullinger, S.; Zimmermann, N.E.; Araùjo, M.B.; Pearman, P.B.; Le Lay, G.; Piedallu, C.; Albert, C.H.; et al. 21st climate change threatened European mountain flora. Glob. Chang. Biol. 2011, 17, 2330–2341. [Google Scholar] [CrossRef]
- Matteodo, M.; Wipf, S.; Stöckli, V.; Rixen, C.; Vittoz, P. Elevation gradient of successful plant traits for colonizing alpine summits under climate change. Environ. Res. Lett. 2013, 8, 024043. [Google Scholar] [CrossRef]
- Evangelista, A.; Frate, L.; Carranza, M.L.; Attorre, F.; Pelino, G.; Stanisci, A. Changes in composition, ecology and structure of high-mountain vegetation: A re-visitation study over 42 years. AoB Plants 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Kullman, L. Alpine flora dynamics–A critical review of responses to climate change in the Swedish Scandes since the early 1950s. Nord. J. Bot. 2010, 28, 398–408. [Google Scholar] [CrossRef]
- Walther, G.R.; Beißner, S.; Burga, C.A. Trends in the upward shift of alpine plants. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Reiter, K.; Klettner, C.; Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Glob. Chang. Biol. 2007, 13, 147–156. [Google Scholar] [CrossRef]
- Kelly, A.E.; Goulden, M.L. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 11823–11826. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; De Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Kazakis, G.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Guisan, A.; Vittoz, P.; Wohlgemuth, T.; Zimmermann, N.E.; Dullinger, S.; Pauli, H.; Willner, W.; Svenning, J.C. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 2010, 33, 295–303. [Google Scholar] [CrossRef]
- Breshears, D.D.; Huxman, T.E.; Adams, H.D.; Zou, C.B.; Davison, J.E. Vegetation synchronously leans upslope as climate warms. Proc. Natl. Acad. Sci. USA 2008, 105, 11591–11592. [Google Scholar] [CrossRef] [PubMed]
- Cannone, N.; Pignatti, S. Ecological responses of plant species and communities to climate warming: Upward shift or range filling processes? Clim. Chang. 2014, 123, 201–214. [Google Scholar] [CrossRef]
- Bodin, J.; Badeau, V.; Bruno, E.; Cluzeau, C.; Moisselin, J.-M.; Walther, G.-R.; Dupouey, J.-L. Shifts of forest species along an elevational gradient in Southeast France: Climate change or stand maturation? J. Veg. Sci. 2013, 24, 269–283. [Google Scholar] [CrossRef]
- Dullinger, S.; Dirnböck, T.; Grabherr, G. Patterns of shrub invasion into high mountain grasslands of the northern calcareous Alps, Austria. Arct. Antarct. Alp. Res. 2003, 35, 434–441. [Google Scholar] [CrossRef]
- Palombo, C.; Chirici, G.; Marchetti, M.; Tognetti, R. Is land abandonment affecting forest dynamics at high elevation in Mediterranean mountains more than climate change? Plant Biosyst. 2013, 147, 1–11. [Google Scholar] [CrossRef]
- Matějka, K.; Málková, J. Long-term dynamics of plant communities in subalpine and alpine zone of the Eastern Giant Mts. Opera Corcon. 2010, 47 (Suppl. 1), 123–138. [Google Scholar]
- Solár, J. Effect of climate change on mountain pine distribution in western Tatra Mountains. In Climate Change-Realities, Impacts Over Ice Cap, Sea Level and Risks; Singh, B.R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 438–458. [Google Scholar] [CrossRef]
- Dirnböck, T.; Dullinger, S.; Grabherr, G. A regional impact assessment of climate and land-use change on alpine vegetation. J. Biogeogr. 2003, 30, 401–417. [Google Scholar] [CrossRef]
- Campagnaro, T.; Frate, L.; Carranza, M.L.; Sizia, T. Multi-Scale Detection of Spatial Pattern Change. In Alpine Cultural Landscapes: Implications for Biodiversity Conservation. Ecol. Indic. 2017, 74, 147–159. [Google Scholar] [CrossRef]
- Dai, L.; Palombo, C.; Van Gils, H.; Rossiter, D.G.; Tognetti, R.; Luo, G. Pinus mugo Krummholz Dynamics during Concomitant Change in Pastoralism and Climate in the Central Apennines. Mt. Res. Dev. 2017, 37, 75–86. [Google Scholar] [CrossRef]
- Wild, J.; Winkler, E. Krummholz and grassland coexistence above the forest-line in the Krkonoše Mountains: Grid-based model of shrub dynamics. Ecol. Model. 2008, 213, 293–307. [Google Scholar] [CrossRef]
- Stanisci, A. High-mountain dwarf shrublands in Abruzzo National Park and Majella massif: Preliminary results. Fitosociologia 1994, 26, 81–92. [Google Scholar]
- Stanisci, A. Gli arbusteti altomontani dell’Appennino centrale e meridionale. Fitosociologia 1997, 34, 3–46. [Google Scholar]
- Biondi, E.; Blasi, C.; Burrascano, S.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Galdenzi, D.; Gigante, D.; Lasen, C.; Spampinato, G.; et al. Manuale Italiano di Interpretazione Degli Habitat (Direttiva 92/43/CEE). 2010. Available online: http://vnr.unipg.it/habitat/cerca.do (accessed on 10 January 2018).
- Feoli, E. Heath species and heathlands of Italy: An analysis of their relationships under the perspective of climate change based on the description of habitats used for the project “Carta della Natura” (Italian Map of Nature). Ecol. Quest. 2010, 12, 161–170. [Google Scholar] [CrossRef]
- Ricotta, C.; Carranza, M.L.; Avena, G.; Blasi, C. Quantitative comparison of the diversity of landscapes with actual vs. potential natural vegetation. Appl. Veg. Sci. 2000, 3, 157–162. [Google Scholar] [CrossRef]
- Carranza, M.L.; Acosta, A.; Ricotta, C. Analyzing landscape diversity in time: The use of Renyi’s generalized entropy function. Ecol. Indic. 2007, 7, 505–510. [Google Scholar] [CrossRef]
- Malavasi, M.; Carranza, M.L.; Moravec, D.; Cutini, M. Reforestation dynamics after land abandonment: A trajectory analysis in Mediterranean mountains. Reg. Environ. Chang. 2018. [Google Scholar] [CrossRef]
- Kleijn, D.; Rundlo, F.M.; Scheper, J.; Smith, H.G.; Tscharntke, T. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 2011, 26, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, B. Short-term changes in key plant communities of Central Apennines (Italy). Acta Bot. Gallica 2005, 152, 545–561. [Google Scholar] [CrossRef]
- Fernández-Calzado, R.; Molero Mesa, J. Changes in the summit flora of a Mediterranean mountain (Sierra Nevada, Spain) as a possible effect of climate change. Lazaroa 2013, 34, 65–75. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Gavilán, R.G.; Escudero, A.; Iriondo, J.M.; Fernández-González, F. Decline of dry grassland specialists in Mediterranean high-mountain communities influenced by recent climate warming. J. Veg. Sci. 2014, 25, 1394–1404. [Google Scholar] [CrossRef]
- Koch, B.; Edwards, P.J.; Blanckenhorn, W.U.; Walter, T.; Hofer, G. Shrub encroachment affects the diversity of plants, butterflies, and grasshoppers on two Swiss subalpine pastures. Arct. Antarct. Alp. Res. 2015, 47, 345–357. [Google Scholar] [CrossRef]
- Elsen, P.R.; Monahan, W.B.; Merenlender, A.M. Global patterns of protection of elevational gradients in mountain ranges. Proc. Natl. Acad. Sci. USA 2018, 201720141. [Google Scholar] [CrossRef] [PubMed]
- Stӧckli, V.; Wipf, S.; Nilsson, C.; Rixen, C. Using historical plant surveys to track biodiversity on mountain summits. Plant Ecol. Divers. 2011, 4, 415–425. [Google Scholar] [CrossRef]
- Evangelista, A.; Frate, L.; Stinca, A.; Carranza, M.L.; Stanisci, A. VIOLA—The vegetation database of the central Apennines: Structure, current status and usefulness for monitoring EU habitats. Plant Soc. 2016, 53, 47–58. [Google Scholar] [CrossRef]
- Frate, L.; Carranza, M.L.; Evangelista, A.; Stinca, A.; Schaminée, J.H.J.; Stanisci, A. Climate and land use change impacts on Mediterranean high-mountain vegetation in the Apennines since the 1950s. Plant Ecol. Divers. 2018, 18, 85–96. [Google Scholar] [CrossRef]
- Raunkiær, C.C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: Oxford, UK, 1934. [Google Scholar]
- Pignatti, S. Ecologia Vegetale; UTET: Torino, Italia, 1995; ISBN 8802046700. [Google Scholar]
- Ellenberg, H. Indicator Values of Vascular Plants in Central Europe. Scr. Geobot. 1974, 9, 7–122. [Google Scholar]
- Calderaro, C.; Palombo, C.; Fracasso, R.; Tognetti, R.; Marchetti, M. Dinamiche di vegetazione di Pino Mugo e Faggio nell’ecotono della treeline in risposta ai cambiamenti climatici e di uso del suolo sul Massiccio della Majella. In Atti del Secondo Congresso Internazionale di Selvicoltura; Accademia Italiana di Scienze Forestali: Firenze, Italy, 2015; Volume 1, pp. 194–202. ISBN 978-88-87553-21-5. [Google Scholar]
- Van Gils, H.A.M.J.; Conti, F.; Ciaschetti, G.; Westinga, E. Fine resolution distribution modelling of endemics in Majella National Park, Central Italy. Plant Biosyst. 2012, 146 (Suppl. 1), 276–287. [Google Scholar] [CrossRef]
- Stanisci, A.; Pelino, G.; Blasi, C. Vascular plant diversity and climate change in the alpine belt of the central Apennines (Italy). Biodivers. Conserv. 2005, 14, 1301–1318. [Google Scholar] [CrossRef]
- Stanisci, A.; Evangelista, A.; Frate, L.; Stinca, A.; Carranza, M.L. VIOLA—Database of High Mountain Vegetation of Central Apennines. Phytocoenologia 2016, 46, 231–232. [Google Scholar] [CrossRef]
- Bazzichelli, G.; Furnari, F. Ricerche sulla flora e sulla vegetazione di altitudine nel Parco Nazionale d’Abruzzo. Pubbl. Ist. Bot. Univ. Catania 1970, 2, 1–41. [Google Scholar]
- Bonin, G. Contribution à la connaissance de la végétation des montagnes de l’Apennin centro-méridional. Ph.D. Thesis, Aix-Marseille University, Provence, France, 1978. [Google Scholar]
- Stanisci, A.; Frate, L.; Morra Di Cella, U.; Pelino, G.; Petey, M.; Siniscalco, C.; Carranza, M.L. Short-term signals of climate change in Italian summit vegetation: Observations at two GLORIA sites. Plant Biosyst. 2016, 150, 227–235. [Google Scholar] [CrossRef]
- .Theurillat, J.P.; Guisan, A. Impact of climate change on vegetation in the European Alps: A review. Clim. Chang. 2001, 50, 77–109. [Google Scholar] [CrossRef]
- Chytrý, M.; Tichý, L.; Hennekens, S.M.; Schaminée, J.H. Assessing vegetation change using vegetation-plot databases: A risky business. Appl. Veg. Sci. 2014, 17, 32–41. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd ed.; Springer: Wien, Austria; New York, NY, USA, 1964; ISBN 978-3-662-02056-2. [Google Scholar]
- Westhoff, V.; Van Der Maarel, E. The Braun-Blanquet Approach. In Classification of Plant Communities. Classification of Plant Communities; Whittaker, R.H., Ed.; Springer: Dordrecht, The Netherlands, 1978; ISBN 978-94-009-9183-5. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, D.H.; Webb, D.A. 1964–1980. Flora Europea, Reissue ed.; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0521153706. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Reissue ed.; Cambridge University Press: Cambridge, UK, 1983; ISBN 978-0521224932. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Milano, Italia, 1982; p. 2302. ISBN 9788820623128. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole-New Business Media: Milano, Italia, 2017; ISBN 8850652429. [Google Scholar]
- Pignatti, S. Valori di bioindicazione delle piante vascolari della Flora d’Italia. Braun-Blanq. 2005, 39, 3–97. [Google Scholar]
- Jongman, R.H.; Braak, C.J.F. Data Analysis in Community and Landscape Ecology; Cambridge University Press: New York, NY, USA, 1987; ISBN 90-220-0908-4. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 857–871. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.0-10. 2013. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 January 2018).
- Hammer, Ø; Harper, D.A.; Ryan, P.D. PAST—PAlaeontological STatistics. Zuerich, Switzerland, 2008. Available online: https://www.uv.es/pe/2001_1/past/pastprog/past.pdf (accessed on 10 January 2018).
- Gehrig-Fasel, J.; Guisan, A.; Zimmermann, N.E. Tree line shifts in the Swiss Alps: Climate change or land abandonment? J. Veg. Sci. 2007, 18, 571–582. [Google Scholar] [CrossRef]
- Vitali, A.; Urbinati, C.; Weisberg, P.J.; Urza, A.K.; Garbarino, M. Effects of natural and anthropogenic drivers on land-cover change and treeline dynamics in the Apennines (Italy). J. Veg. Sci. 2018, 29, 189–199. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Gruber, A.; Pauli, H. Patterns and current changes in alpine plant diversity. In Arctic and Alpine Biodiversity: Patterns, Causes and Ecosystem Consequences; Springer: Berlin/Heidelberg, Germany, 1995; pp. 167–181. [Google Scholar] [CrossRef]
- Huelber, K.; Gottfried, M.; Pauli, H.; Reiter, K.; Winkler, M.; Grabherr, G. Phenological responses of snowbed species to snow removal dates in the Central Alps: Implications for climate warming. Arct. Antarct. Alp. Res. 2006, 38, 99–103. [Google Scholar] [CrossRef]
- Vittoz, P.; Bodin, J.; Ungricht, S.; Burga, C.A.; Walther, G.R. One century of vegetation change on Isla Persa, a nunatak in the Bernina massif in the Swiss Alps. J. Veg. Sci. 2008, 19, 671–680. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Bowman, W.D.; Humphries, H.C.; Seastedt, T.R.; Suding, K.N. Changes in alpine vegetation over 21 years: Are patterns across a heterogenous landscape consistent with predictions? Ecosphere 2013, 4, 1–18. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; Springer: Berlin, Germany, 2003; p. 349. ISBN 978-3-642-18969-2. [Google Scholar]
- Scherrer, D.; Körner, C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 2011, 38, 406–416. [Google Scholar] [CrossRef]
- Allegrezza, M.; Corti, G.; Cocco, S.; Pesaresi, S.; Chirico, G.B.; Saracino, A.; Bonanomi, G. Microclimate buffering and fertility island formation during Juniperus communis ontogenesis modulate competition–facilitation balance. J. Veg. Sci. 2016, 27, 616–627. [Google Scholar] [CrossRef]
| Mean Increase % | ||||||
|---|---|---|---|---|---|---|
| Taxon | contr. % | T1 | T2 | L. form | T | V. belt |
| Poa alpina subsp. alpina | 2.649 | 0.351 | 0.595 | H Caesp | 3 | 1,2 |
| Valeriana montana | 2.476 | 0.351 | 0.514 | H Scap | 4 | 1,2 |
| Campanula scheuchzeri s.l. | 2.449 | 0.541 | 0.595 | H Scap | 2 | 2 |
| Leucopoa dimorpha | 2.449 | 0.514 | 0.568 | H Caesp | 4 | 2 |
| Pulsatilla alpina subsp. millefoliata | 2.434 | 0.432 | 0.514 | H Scap | 3 | 2 |
| Doronicum columnae | 2.304 | 0.162 | 0.432 | G | 4 | 2 |
| Hypericum richeri subsp. richeri | 2.298 | 0.189 | 0.432 | H Scap | 6 | 1,2 |
| Orthilia secunda | 2.252 | 0.297 | 0.378 | C | 5 | 1,2 |
| Hippocrepis comosa subsp. comosa | 2.211 | 0.297 | 0.432 | H Caesp | 7 | 1,2 |
| Thymus praecox subsp. polytrichus | 2.162 | 0.216 | 0.405 | C | 6 | 1 |
| Trifolium pratense subsp. semipurpureum | 1.947 | 0.135 | 0.378 | H Scap | 6 | 1 |
| Helianthemum nummularium subsp. grandiflorum | 1.843 | 0.216 | 0.297 | C | 4 | 2 |
| Ranunculus pollinensis | 1.701 | 0.027 | 0.351 | H Scap | 3 | 2 |
| Carex kitaibeliana | 1.595 | 0.135 | 0.27 | H Caesp | 4 | 1 |
| Mean Decrease % | ||||||
| Hieracium murorum s.l. | 2.341 | 0.405 | 0.351 | H Scap | 4 | 1,2 |
| Silene multicaulis subsp. multicaulis | 2.292 | 0.378 | 0.324 | H Caesp | 4 | 1 |
| Brachypodium genuense | 1.933 | 0.297 | 0.27 | H Caesp | 6 | 2 |
| Phyteuma orbiculare | 1.792 | 0.892 | 0.703 | H Scap | 3 | 1 |
| Ranunculus breyninus | 1.745 | 0.378 | 0 | H Scap | 3 | 1 |
| Epipactis atrorubens | 1.645 | 0.27 | 0. 162 | G | 6 | 1 |
| Gentiana dinarica | 1.556 | 0.243 | 0.189 | H Ros | 3 | 1 |
| Source | Sum of Squares | Df | Mean Square | F. Model | R2 | Pr (> F) |
|---|---|---|---|---|---|---|
| Time | 0.09777 | 1 | 0.097765 | 4.3227 | 0.05327 | 0.003 ** |
| Elevation Range | 0.15007 | 1 | 0.150068 | 6.6352 | 0.08177 | 0.002 ** |
| Interaction | 0.00432 | 1 | 0.004324 | 0.1912 | 0.00236 | 0.920 |
| Residual | 1.58318 | 70 | 0.022617 | 0.86261 | ||
| Total | 1.83534 | 73 | 1.00000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, V.; Carranza, M.L.; Evangelista, A.; Marchetti, M.; Stinca, A.; Stanisci, A. Long-Term Changes in the Composition, Ecology, and Structure of Pinus mugo Scrubs in the Apennines (Italy). Diversity 2018, 10, 70. https://doi.org/10.3390/d10030070
Calabrese V, Carranza ML, Evangelista A, Marchetti M, Stinca A, Stanisci A. Long-Term Changes in the Composition, Ecology, and Structure of Pinus mugo Scrubs in the Apennines (Italy). Diversity. 2018; 10(3):70. https://doi.org/10.3390/d10030070
Chicago/Turabian StyleCalabrese, Valentina, Maria Laura Carranza, Alberto Evangelista, Marco Marchetti, Adriano Stinca, and Angela Stanisci. 2018. "Long-Term Changes in the Composition, Ecology, and Structure of Pinus mugo Scrubs in the Apennines (Italy)" Diversity 10, no. 3: 70. https://doi.org/10.3390/d10030070
APA StyleCalabrese, V., Carranza, M. L., Evangelista, A., Marchetti, M., Stinca, A., & Stanisci, A. (2018). Long-Term Changes in the Composition, Ecology, and Structure of Pinus mugo Scrubs in the Apennines (Italy). Diversity, 10(3), 70. https://doi.org/10.3390/d10030070







