1. Introduction
Climate changes have considerably affected biota in the past which is best studied for the Quaternary glacial-interglacial alternations during the last 2.5 million years. There is no unanimity on their pace in the past [
1], but the velocity of the ongoing climate change is considered as extremely fast [
2].
It has been causing significant shifts in the flora and vegetation of the mountain ecosystems all over the world during the last decades. This particularly concerns the upper elevations, where thermal conditions are most crucial for determining the floristic composition of plant communities, as well as the numbers and viability of populations of the specialized species adapted to these harsh environments [
3].
That trend totally refers to the Ukrainian Carpathians, which are medium-high mountains where the alpine zone (above 1800 m a.s.l.) comprises only about 0.1% of the area [
4] and may completely disappear by 2050 [
5]. Alpine species occur mainly in the highest massifs: the Chornohora, Svydovets, Marmarosh, and Chyvchyny Mts (
Figure 1). There is no subnival zone in the Ukrainian Carpathians, as only a few summits slightly exceed 2000 m a.s.l. Thus, most cryophilic species (for instance,
Cerastium uniflorum,
Gentiana frigida,
Gentianella tenella,
Ranunculus glacialis, and
Silene acaulis), which are typical for the highest massifs in the Polish, Slovak, and Romanian Carpathians, are missing in Ukraine. However, other cold-adapted oreophytes that, according to European compendia on ecological values of plants [
6,
7,
8], are characteristic for the upper alpine zone, do occur in the Ukrainian Carpathians or at least have rather recent reliable historical records there (e.g.,
Agrostis rupestris,
Callianthemum coriandrifolium,
Cerastium cerastoides,
Gentiana nivalis,
Luzula spicata,
Oreochloa disticha,
Salix herbacea,
Saussurea alpina,
Saxifraga bryoides,
S. carpatica).
Localities of these cryophiles are restricted mainly to the highest sites of the Ukrainian Carpathians, which, however, refer to the lower limits of the species’ altitudinal ranges as it follows from their distribution in other European mountains. Thus, responding to climate change in the Ukrainian Carpathians, most cryophilic species are unable to retreat upslope, which is possible in the higher mountains. Therefore, they are prone to decline and extinction that is particularly worrying for the endemics as it has also been admitted for comparatively low ranges of the outer Alps [
9]. In addition to their ecological marginality (i.e., occurrence at the lower limit of the altitudinal range), the alpine species are also geographically peripheral in the Ukrainian Carpathians, where they are confined to the north-eastern edge of their distribution in Central Europe due to location of the target region on the outer side of the Carpathian arc projecting into the vast lowland territories lying between European and Asian high-mountain ranges (
Figure 1). Consequently, extinction of cryophilic species in scarce outlying high-mountain “environmental islands” in the region results in noticeable shrinkage of their geographical ranges and biodiversity loss on the all-European scale [
10]. Therefore, small alpine patches in the Ukrainian Carpathians are very convenient for monitoring population dynamics of the species whose viability significantly depends on the changing thermal conditions.
The goal of this study was to assess and track the population dynamics of such species with regard to their habitat restriction. In comparison with the studies on shifts in the species’ distribution and abundance, which have been taking place in the alpine habitats in different mountain systems during the last decades [
10,
11,
12,
13], monitoring of population parameters of the climate-sensitive species enables the detection of finer changes and trends in their performance. The results of this research are obtained on populations of the model species representing particular types of the alpine habitats and are aimed at better explaining the ongoing species’ dynamics in the context of previously revealed long-term changes in their distribution in the Ukrainian Carpathians [
10].
2. Materials and Methods
The archives of the daily records of the high-mountain Pozhyzhevska Meteorological Station (1450 m a.s.l.) in the Chornohora Mts, Ukrainian Carpathians [
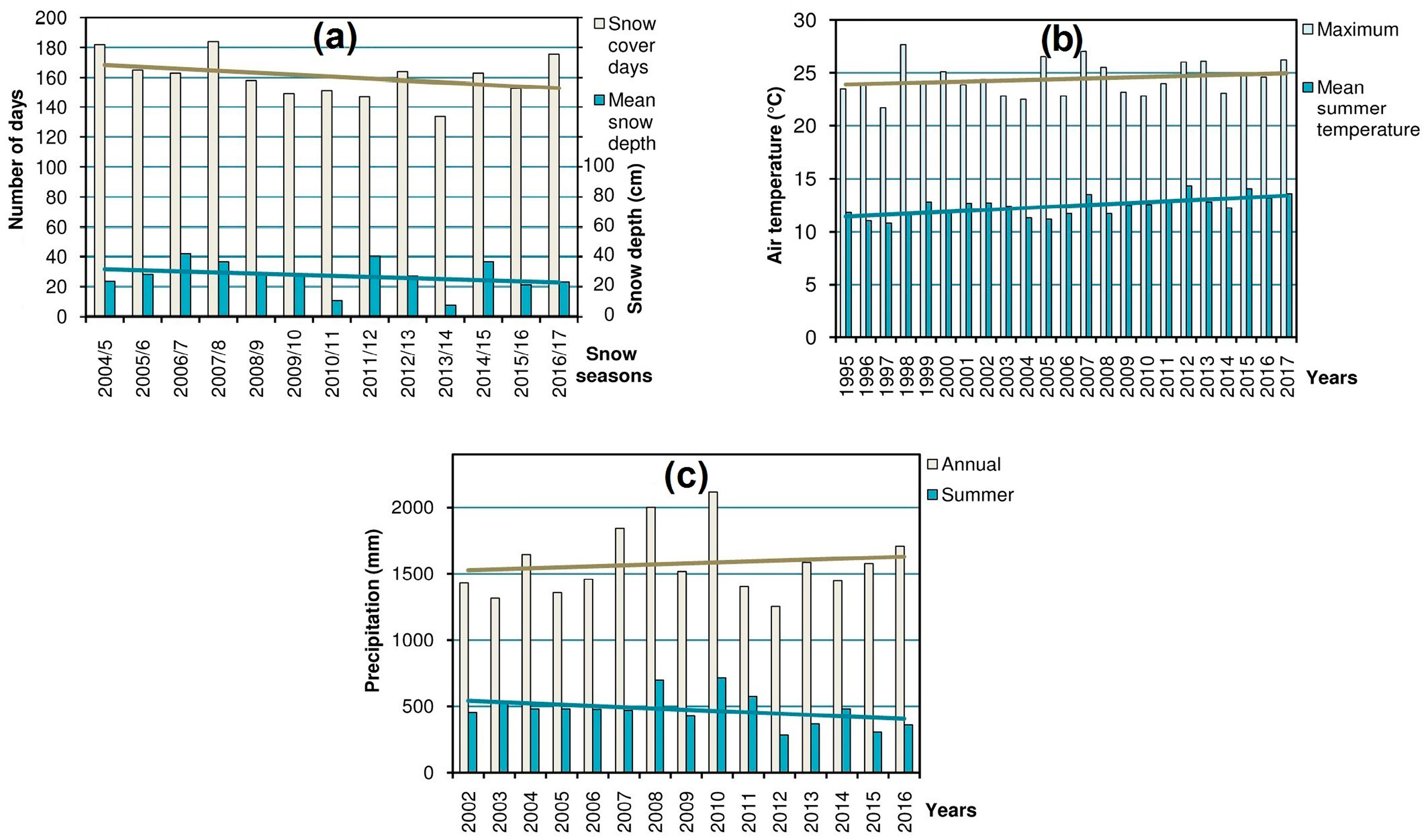
14] (in the region where most of the research was carried out) were processed in order to obtain the yearly values of the main variables: snow cover (duration and mean depth), summer air temperature (mean and maximum), and precipitation (annual and summer), which characterize the high-mountain climate and, consequently, shape the alpine biota [
15,
16]. Corresponding charts were built to reveal the trends possibly referring to climate change (
Figure 2) which could help to explain the dynamics of populations and habitats of the studied species.
The species were considered as rare according to the compendium compiled by Malinovski et al. [
17]. Almost all of them are included in the “Red Data Book of Ukraine” [
18]. While selecting target species, special attention was paid to the high-mountain plants that proved to be susceptible to global warming in the other mountain systems of Europe [
12,
15].
The target species were classified according to their habitat preferences, i.e., occurrence predominantly in the following types of habitats: snowbeds, screes, rocks, ridges, swards, tall-herb communities, dwarf-shrub heaths, springs, and mires [
19,
20].
Snowbeds harbor chionophilous communities (class Salicetea herbaceae) with low-statured, poorly competitive cryophilic species adapted to short (2–2.5 month) vegetative periods and confined to mossy or barren patches, for instance, Cerastium cerastoides, Salix herbacea, Saxifraga carpatica, and Veronica alpina.
Alpine gravel screes are formed by erosion due to snowpack shift on steep slopes or hollows. These scarce in the Ukrainian Carpathians localities are almost deprived of vegetation and provide suitable habitats for low-competitive glareophytic cold-dwelling r-strategists that need barren microsites, such as Cardaminopsis neglecta, Leontodon pseudotaraxaci, Oxyria digyna, Saxifraga oppositifolia, and Trifolium badium, as well as more thermophilic Biscutella laevigata and Rumex scutatus, which are restricted mostly to communities that belong to the alliance Androsacion alpinae.
Rocky localities represent different saxicolous communities where the following high-mountain rare species occur: Agrostis rupestris, Antennaria carpatica, Carex rupestris, Draba siliquosa, Erigeron alpinus, E. atticus, Hedysarum hedysaroides, Lloydia serotina, Primula minima, Saussurea alpina, and Thlaspi dacicum.
The ridge habitats are also characterized by the rocky substrate. Their hypsometric position depends on the altitude of a particular mountain range, where they are restricted to its uppermost unsheltered sites exposed to the lowest temperatures. Such localities may provide microrefugia for the most specialized cryophilic species (for instance, Dryas octopetala, Gentiana nivalis, Luzula spicata, Oreochloa disticha, Saxifraga bryoides, Veronica aphylla, and V. bellidioides).
The
swards are low-grass tussock communities on rather flat areas [
20]. The following rare species occur there:
Callianthemum coriandrifolium,
Polygonum viviparum,
Saxifraga adscendens,
Senecio carpathicus, and
Thlaspi kovatsii.Tall-herb and tall-grass communities of the class Mulgedio-Aconitetea are more common in the subalpine zone. They harbor some rare taxa (Achillea lingulata, Aconitum moldavicum subsp. hosteanum, Bupleurum longifolium subsp. vapincense, Campanula serrata, Centaurea kotschyana, Delphinium elatum subsp. nacladense, Dianthus speciosus, Festuca carpatica, Gentiana punctata, Heracleum carpaticum, H. palmatum, and Pedicularis hacquetii) which occur mostly on moist humus-rich soils in small depressions and grooves between rocks or at their bottom.
Alpine and subalpine dwarf-shrub heaths belong to the class Loiseleurio-Vaccinietea. They are dominated by common (Vaccinium myrtillus, V. gaultherioides, V. vitis-idaea) or rare (Loiseleuria procumbens, Rhododendron myrtifolium) ericaceous species.
The rare species which occur in the mires, springs, and riparian sites are Carex bicolor, Epilobium anagallidifolium, Pedicularis oederi, P. verticillata, Saxifraga aizoides, and Swertia perennis subsp. alpestris.
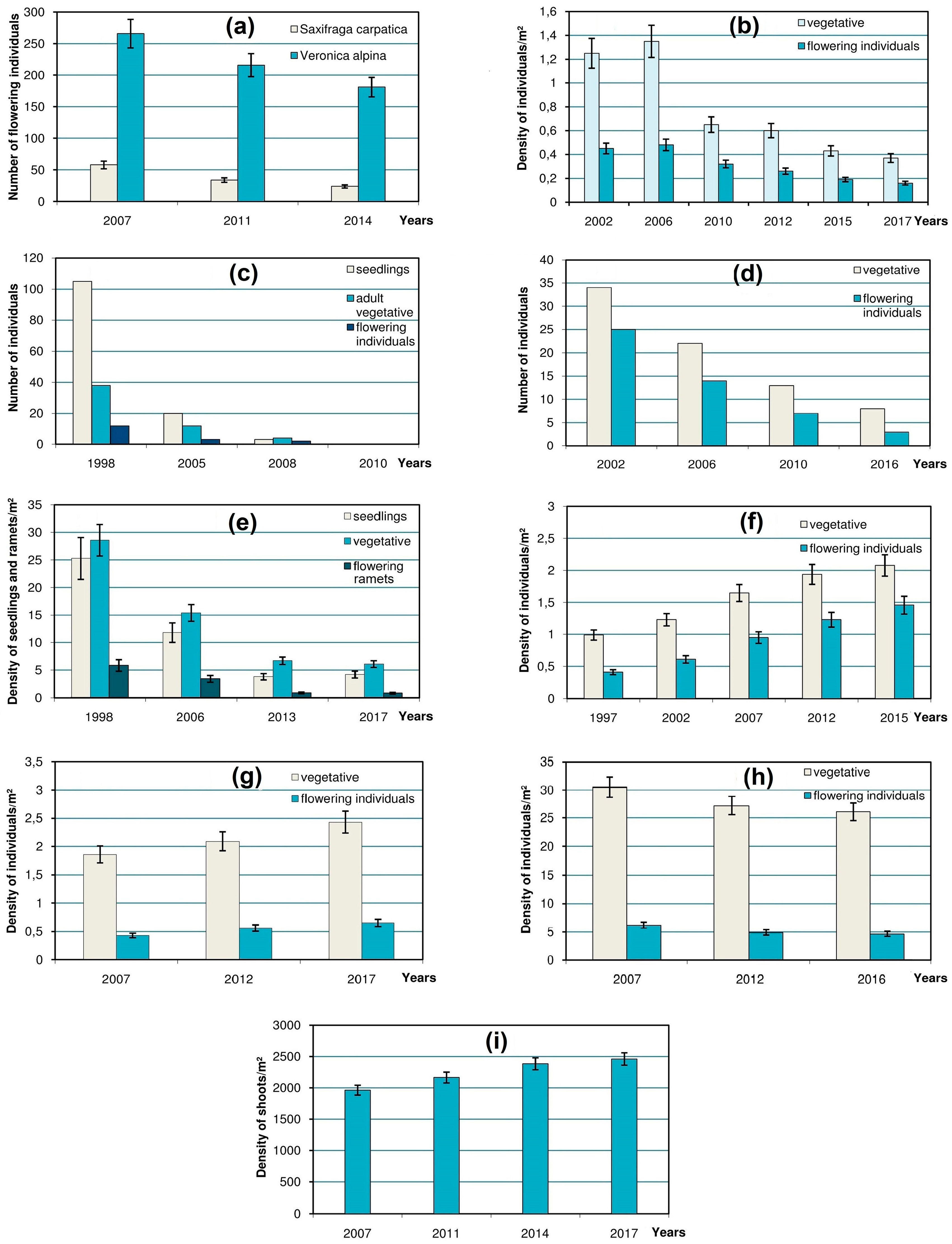
One or two case study species were chosen to represent each type of these habitats. Trends in population size of 10 selected species were monitored between 1997 and 2017 on permanent plots (
Figure 3). Periods between their resurveys might be uneven and dependent on the opportunity to visit a locality. Location and size of the permanent plots are listed in
Table 1. The area and spatial dispatch of permanent plots depended on the population spatial characteristics and homogeneity of the species dispersal and were designed in order to obtain adequate data variation [
21]. The density of adult individuals or ramets (depending on the life form) was estimated on 10.0 × 0.5, 12.5 × 0.5, or 20 × 1 m line transects or rectangular plots subdivided into 0.25 or 1.0 m
2 quadrats. Sample size in each studied locality numbered at least 20 quadrats. If the properties of substratum (e.g., solid rock, gravel scree) or snowpack shift did not allow for the establishment of permanent transects, then firm marking rods were pegged to fix the quadrat frames during next resurveys [
22].
If the density of seedlings of the studied species was significantly higher than in adult individuals, the former parameter was estimated separately on smaller (0.2 × 0.2 m) quadrats [
23].
When appropriate, e.g., in small populations (such as Erigeron atticus or Veronica bellidioides) their complete census was estimated.
In
Figure 3, the error bars in charts show 90% confidence intervals of the mean value of a parameter computed using t statistic for corresponding sample size [
23] by means of the STATISTICA software package.
The study plots were established either in historically undisturbed sites or in the localities where grazing had stopped already in the 1970s, i.e., recent successional shifts have been caused mostly by climate change rather than by post-anthropogenic recovery.
Spatial shifts of the boundary between the patches of
Salix herbacea and
Poa granitica subsp.
disparilis in a shrinking snowbed habitat in the Chornohora Mts (1890 m a.s.l.) were monitored from 2011 to 2017 by means of marker pins using mapping and photopoint method [
23].
Resurveys of the studied populations were performed on similar calendar dates.
The role of gaps in vegetation in the species’ recruitment was defined by observations of occurrence of their seedlings in the open microsites.
Nomenclature for vascular plants follows Mirek et al. [
24], and Malynovski and Kricsfalusy [
25] for phytosociological units.
3. Results
Dynamics of the snow cover, summer air temperature, and precipitation in the Chornohora Mts, Ukrainian Carpathians (
Figure 2) reveals distinct temporal trends of these variables.
In a
snowbed in the Svydovets Mts that has gradually shrunk, a significant decline was marked in the populations of
Saxifraga carpatica and
Veronica alpina (
Figure 3a). Monitoring of the boundary between patches of a prostrate chionophylous dwarf shrub
Salix herbacea and a tussock grass
Poa granitica subsp.
disparilis showed that the latter species has advanced for 4.1 ± 0.4 cm in 6 years (i.e., about 7 mm per year) in a small shrinking snowbed habitat in the Chornohora Mts.
The changes in the
scree-dwellers are illustrated by population dynamics of a case study glareophytic species
Cardaminopsis neglecta in its alpine scree habitat in the Chornohora Mts (
Figure 3b). This once large population has shrunk considerably over the last century [
10] and, despite some fluctuations, has been gradually declining during the 15-year period of its ongoing monitoring.
The dynamics of rare petrophytous species in their
rocky localities was monitored on a small population of a rare high-mountain species
Erigeron atticus on rock ledges in the Chornohora Mts. The monitoring showed its decline and eventual extinction that was confirmed in 2010 (
Figure 3c). The dramatic decrease of seedlings of
E. atticus results from the rapid loss of barren microsites suitable for their recruitment due to the massive spread of
Pinus mugo and
Juniperus communis subsp.
alpina.
The vulnerability of the most specialized cryophilic species in the
ridge habitats demonstrates that the population of
Veronica bellidioides in the Marmarosh Mts (
Figure 3d) is prone to decrease and extinction.
Hygrophytous species which inhabit
mires and
springs are represented by the population of
Swertia perennis subsp.
alpestris on the calcium-rich fen in the Chornohora Mts. Rapid decrease in its density has been monitored for the last 19 years (
Figure 3e). The same declining trend concerns
Pedicularis oederi that now remains only in the vicinity of Mt. Brebeneskul in the Chornohora Mts where it forms a metapopulation, which includes a series of local populations. Some of them, especially those confined to springs, are very unstable because their microhabitats tend to dry up in summer due to climate change. The number of spring-related local populations of
P. oederi has reduced from 7 to 3 since their first assessment in 1997 [
26]. However, local populations confined to peat bogs proved to be more stable and less vulnerable.
Despite the above facts of the negative dynamics of the rare alpine species in the Ukrainian Carpathians, there are also positive examples concerning the size of their populations in the high-mountain zone. This particularly refers to
Achillea lingulata, a case study species which represents
tall-herb communities and exhibits a marked increase in its density (especially of flowering individuals) in the habitat located at the edge of the rocks in the Chornohora Mts (
Figure 3f).
Positive dynamics have also been demonstrated by some rare alpine species that occur in the
sward habitats. For instance, the monitored population of
Senecio carpathicus in the Chornohora Mts has been showing a considerable increase during the last decade (
Figure 3g). Despite its low stature, this species is highly competitive even in rather dense low-grass communities (mostly of the alliance
Festuco saxatilis-Seslerion bielzii) and does not need gaps for recruitment. However, other species that can be characterized as gap-demanding (for instance,
Polygonum viviparum,
Saxifraga adscendens,
Thlaspi kovatsii) show their highest density on patches with sparser vegetation cover. One of them,
Polygonum viviparum exhibits some decrease in its alpine locality (
Figure 3h) because of progressive crowding in its grassy habitat during the succession.
The changes in rare species occurring in ericaceous
dwarf-shrub heaths were tracked in a case study of a rare alpine population for the Ukrainian Carpathians alpine cushion dwarf shrub
Loiseleuria procumbens, which forms dense mats within the association
Loiseleurio-Cetrarietum. During the decade of monitoring, its density on the study plot had grown by about 25%, mostly due to covering of the gaps between clones (
Figure 3i). The gaps in the mats of other ericaceous dwarf-shrubs are crucial for the recruitment of some rare herbaceous species. For instance, it was found that barren loci resulting from the frost damage to the mats of
Vaccinium myrtillus and
V. vitis-idaea facilitate the recruitment of
Gentiana punctata. Thus, in 2013, as much as 84.7% of immature individuals in the monitored population of
G. punctata in the community
Vaccinietum myrtilii in the subalpine zone of the Chornohora Mts were restricted to such loci, where their density ranged within 3–8 individuals per m
2.
4. Discussion
The trends in the analyzed climatic parameters (
Figure 2) are confirmed by the comprehensive overview of the dynamics of the main meteorological climatic variables during the last decades all over the Carpathian region [
27] referring to climate change. This implies successional shifts in the alpine ecosystems and populations of oreophytic plant species. Thus, the habitats characterized by massive snowpack (i.e., snowbeds and screes) are doomed to shrinkage due to the decrease in snow cover (
Figure 2a). This entails the decline of the rare species confined to such habitats. The same concerns affect some hygrophytous species. Despite the increasing trend in annual precipitation total, the summer precipitation amount has markedly dropped during last 14 years (
Figure 2c). Redistribution of precipitation in the region at the expense of the summer season was also noticed by Balabukh and Lukianets [
28]. Because of changes in hydrological and thermal conditions, i.e., the rise of temperature (
Figure 2b) and, consequently, evaporation, as well as scarcer summer precipitation, the habitats of the listed above rare hygrophytous species are prone to drying up and shrinkage, which explains negative dynamics of their populations.
Stenotopic cold-demanding species typical of snowbeds, screes and ridges proved to be very vulnerable to climate changes. Some of them belong to specialized life forms, for instance, cushions that accumulate heat [
15,
29], e.g.,
Saxifraga bryoides and
S. oppositifolia. Physiological and morphological adaptations of the cold-specialized species, particularly in the leaves, enable efficient gas exchange in cold environment [
15,
30], but are disadvantageous in the warmer climate and cause overheating and withering of plants that cannot tolerate the increase of temperature above certain levels. Therefore, such cold specialists are vulnerable to warming, particularly the declining cryophilic species restricted to the uppermost barren ridge habitats. Such wind-swept ridges do not accumulate much snow that contributes to harsh thermal conditions which can be tolerated only by highly adapted plants (such as
Luzula spicata,
Saxifraga bryoides,
Veronica bellidioides). In contrast to the other types of habitats, the ridge localities are rather resistant to the climate-induced encroachment of advancing plants; therefore, the shrinkage of vegetation-free patches is not evident there. Thus, the decrease of the cryophilic species in these habitats can be explained mostly by the direct effect of rising temperature rather than by unfavorable phytosociological shifts. Apparently, due to climate change, these cryophiles (whose thermal optimum corresponds to the higher mountains) have reached the limit of their tolerance during summer periods when increasing maximum temperatures (
Figure 2b) become the main adverse factor [
31]. As stated by Dahl [
15], it is maximum summer temperature that shapes the distribution of cold-demanding species at the trailing edge. The declining trend of the only existing in the Ukrainian Carpathians population of a cold-demanding species
Veronica bellidioides (
Figure 3d) proves that it is at the brink of extinction in the region [
32] which is in line with the data from the Sudeten Mts [
33].
By contrast, more thermophilic rare tall herbs would benefit from warming that enables their establishment at higher elevations due to longer snowless periods. Tall-herb communities have been expanding lately in the Carpathians [
34,
35].
The important driving factor that affects the rare species is the climate-induced change in vegetation, which is secondary to warming as its consequence [
36]. This factor should be considered separately because its impact involves shifts in interspecific competition in alpine ecosystems. Thus, sparsely vegetated snowbed, scree, or petrophytous habitats of many low-competitive rare alpine plants undergo gradual overgrowth with more thermophilic and rather competitive tussock-forming graminoids, shrubs, or elfin woodland. This may cause shrinkage or even loss of some of their habitats and, consequently, extinction of populations of the rare species which occur there as it happened to the monitored population of
Erigeron atticus (
Figure 3c).
In addition to climate change, present successional processes in the high-mountain zone have a marked post-anthropogenic background resulting from the abandonment of traditional land-use, mostly grazing, which once was very common in the region. These two drivers—climate change and restoration of natural vegetation—usually act simultaneously and synergistically. However, the effect of land-use abandonment on vegetation was most evident during the first decades after cessation of grazing, i.e., in the 1970–80s, while climate change has been gaining in importance lately, especially in the alpine communities where the climatic component is most crucial in shaping the vegetation [
2]. Likewise, the latter factor is more evident in the habitats with scarce vegetation (e.g., ridges, snowbeds, screes), while the consequences of the previous land-use are better traceable in crowded communities (dwarf-shrub heaths, tall-herb patches, swards).
Alpine species are mostly small and low-statured, which is caused by adaptation to the harsh environment but makes them vulnerable and prone to suppression by taller or more expansive ingressing plants. For instance,
Salix herbacea, a prostrate dwarf shrub adapted to short growth seasons ends its aerial development and drops leaves already in late August–early September in its studied shrinking snowbed habitat in the Chornohora Mts. However, due to warming, the temperature still remains rather high there (mainly over +5 °C) in September and partially in October. That enables advancing graminoids (
Festuca picta,
Luzula alpinopilosa,
Poa granitica subsp.
disparilis) to take advantage of the prolonged growth period and succeed in the competition with
S. herbacea that results in gradual displacement of the latter species. This example concerns clonal plants which prevail in the high-mountain zone for their better persistence in the alpine environment [
13,
37].
Infrequent in the alpine flora aclonal plants [
38], which are capable only of seed reproduction, are still more vulnerable, especially short-lived species (
Cardaminopsis neglecta,
Oxyria digyna,
Pedicularis oederi,
P. verticillata,
Saxifraga carpatica,
S. adscendens). Because flower development, seed yield, and germination, as well as seedling establishment, are susceptible to adverse alpine conditions (frost, desiccation, etc.); their populations undergo significant fluctuations and are unstable. They are very sensitive to vegetation change because most need open sites for seed recruitment; however, such suitable microhabitats tend to shrink due to crowding which has been increasing throughout the succession. The vulnerability of short-lived species was also noticed by Eriksson and Jakobsson [
39] and Bekker and Kwak [
40], and their susceptibility to climate change in the alpine ecosystems was claimed by Cannone et al. [
13].
Vegetation-free microsites are crucial for the seed recruitment of many alpine species including long-lived clonal plants, e.g.,
Dryas octopetala,
Salix herbacea [
41], and
Gentiana punctata [
42]. While the persistence of their established clones is provided by vegetative growth, the seed reproduction enables their spread due to encroachment into open uninhabited patches. Such sites become colonized by pioneer and early-successional cold-dwelling species (
Cardaminopsis neglecta,
Cerastium cerastoides,
Saxifraga carpatica,
Veronica alpina) on the screes, late-melting snowbeds or poorly vegetated rocks. As primary climatogenic succession leads to gradual crowding, the area of microsites suitable for recruitment of such species has been diminishing in the course of warming.
One of the causes of the displacement of many rare low-statured alpine species is the expansion of ericaceous dwarf-shrub heaths throughout the alpine zone of the European mountains which has been widely acknowledged lately [
11,
43]. This process is contributed to both by climate change and by land abandonment, and involves upward advance and active spread of the
Vaccinium species (
V. myrtillus,
V. gaultherioides,
V. vitis-idaea), more common at the lower elevations. They form dense mats that, however, are vulnerable to severe frost damage which has been happening more frequently lately because of the climate-driven decrease in snow depth [
44]. Frost-induced vacated gaps in closed heath or grassland vegetation (sometimes up to a few m
2) provide suitable microsites for the seedling establishment of some rare species with various thermal requirements—both restricted to the upper elevations (
Doronicum stiriacum,
Polygonum viviparum,
Salix retusa) or occurring in a wider altitudinal range (
Gentiana lutea,
G. punctata). Such disturbances are also favorable for clonal proliferation of some low-statured poorly competitive rare species (
Diphasiastrum alpinum,
Loiseleuria procumbens,
Selaginella selaginoides).
Unlike the mentioned above
Vaccinium species, another ericaceous dwarf shrub,
Loiseleuria procumbens, benefits from the decrease in snow cover [
44]. This frost-tolerant evergreen cushion plant which inhabits convex windswept snowless sites exhibits positive dynamics in the alpine habitats (
Figure 3i) due to the replacement of its frost-susceptible competitors. However, distributional limit of
L. procumbens in the Chornohora Mts has shifted upward considerably since late 19th century at the expense of the lowermost subalpine populations [
10].
5. Conclusions
Climate change affects the alpine species in two ways: (i) by the direct influence (positive or negative) on their viability; (ii) through alterations in phytosociological relations in the course of the climate-induced succession of vegetation in their localities. These factors are often synergetic, and their effects are hardly distinguishable from each other. Apparently, the denser the vegetation cover, the bigger is the role of interspecific competition. The response of a particular species to warming depends on its ecological (especially thermal) requirements, competitiveness, and position of a habitat within the altitudinal range of the species distribution (upper, optimum, or lower). Consequently, the declining response of the most cryophilic species at the lower (rear) edge of their distribution would totally differ from the spreading dynamics of more thermophilic plants at their upper (leading) limit.
Most vulnerable are the species is confined to the types of habitats that tend to shrink, i.e., snowbeds, alpine screes, barren rocks; uppermost parts of the highest ridges with the harshest climatic conditions and hygrophytous sites (mires and streams). In the Ukrainian Carpathians, the area of the habitats suitable for cold-demanding species is very limited, and they are prone to decline and extinction. However, populations of some other rare species, particularly rather thermophilic tall herbs, have increased lately.
An important factor that limits the viability of populations of many studied species is the availability of open microsites suitable for their seedling recruitment, which tend to reduce in the course of succession. On the other hand, the climate-induced decrease of snow cover often causes frost damage to vegetation that provides gaps available for recruitment of many rare species.
Climate change has a considerable impact on the abundance and distribution of species in the Ukrainian Carpathians, particularly in their high-mountain zone. Decline and extinction of rare cold-demanding species pose a significant threat of biodiversity loss in the region, i.e., on the north-eastern edge of distribution of many alpine species in Central Europe. This especially refers to narrow-range species, for instance,
Cardaminopsis neglecta (
Figure 3b).
Until lately, the impact of climate change on the population dynamics of threatened species has been largely underestimated in the conservation literature concerning the Carpathian region, particularly in the Red Data Book of Ukraine [
18]. Apparently, the role of that factor should be fundamentally reconsidered.