3.2.3. Ilacuris Pascoe, 1865
Ilacuris Pascoe, 1865: 425 [
8]. Pascoe, 1871: 202 [
9] (in key, as Zygopinae); Gemminger, 1871: 2594 [
10] (catalog, as Zygopinae); Masters, 1886: 682 [
11] (catalog, as Zygopinae); Dalla Torre et al., 1932: 25 [
13] (catalog, as Pissodini); Hustache, 1934: 57 [
12] (catalog, as Zygopinae); Wiley & Shanahan, 1973: 373 [
17] (range extension into Papua New Guinea and natural history); Zimmerman, 1994: 694 [
14] (in Molytinae); Alonso-Zarazaga & Lyal, 1999: 207 [
5] (catalog, transfer to Molytinae: Pissodini); Setliff, 2007: 296 [
3] (catalog); Mecke et al., 2005 [
16] (natural history); Lyal, 2014: 529, 556 [
15] (natural history and transfer to Molytinae: Orthorhinini); Pullen et al., 2014: 283 [
2] (as Molytinae: Orthorhinini).
Jlacuris; Heller, 1893: 47 [
29] (incorrect subsequent spelling).
Illacuris; Masters, 1886: 682 [
11]; Setliff, 2007: 296 [
3]; Lyal, 2014: 556 [
15] (incorrect subsequent spelling).
Type species:Ilacuris laticollis Pascoe, 1865, by monotypy.
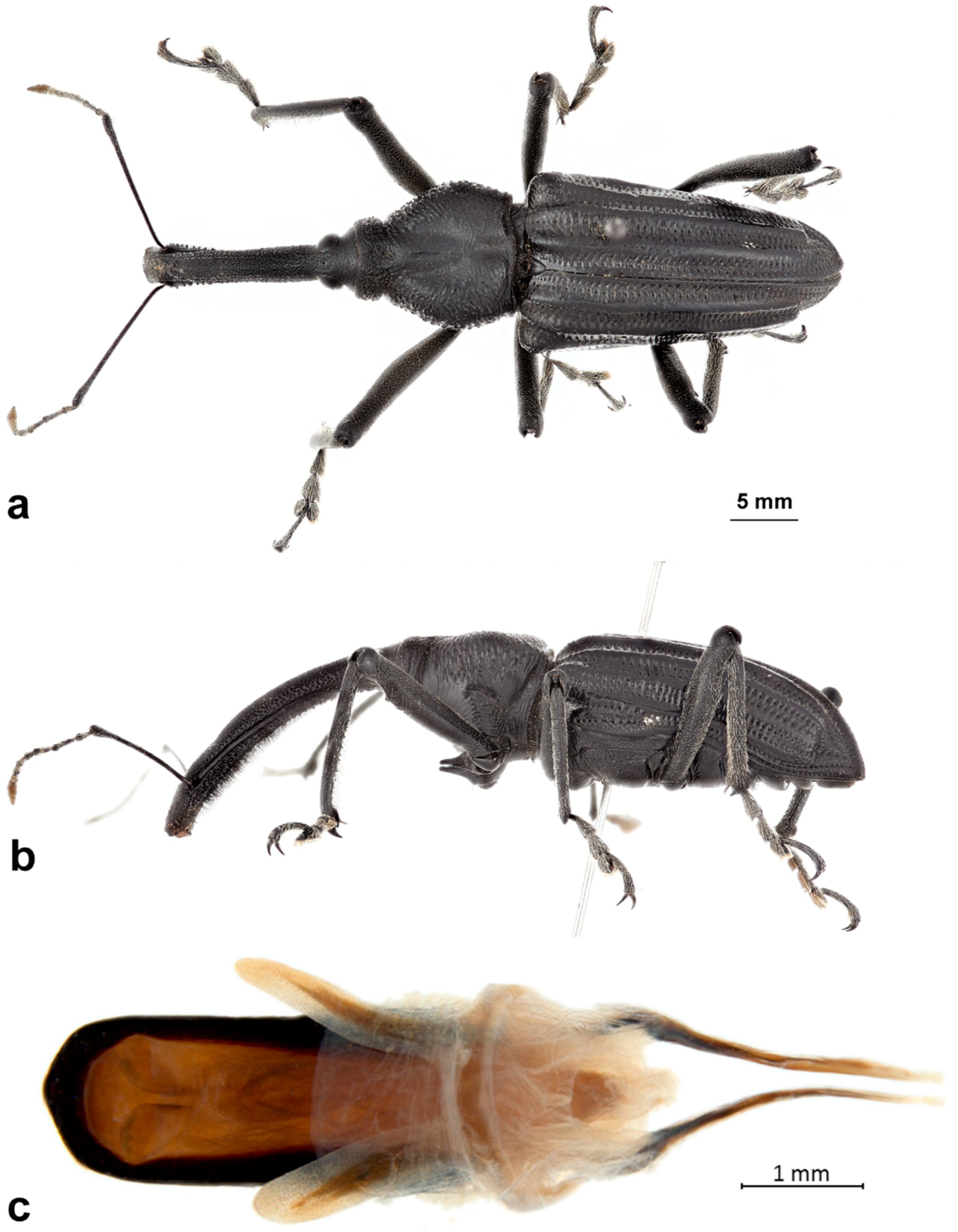
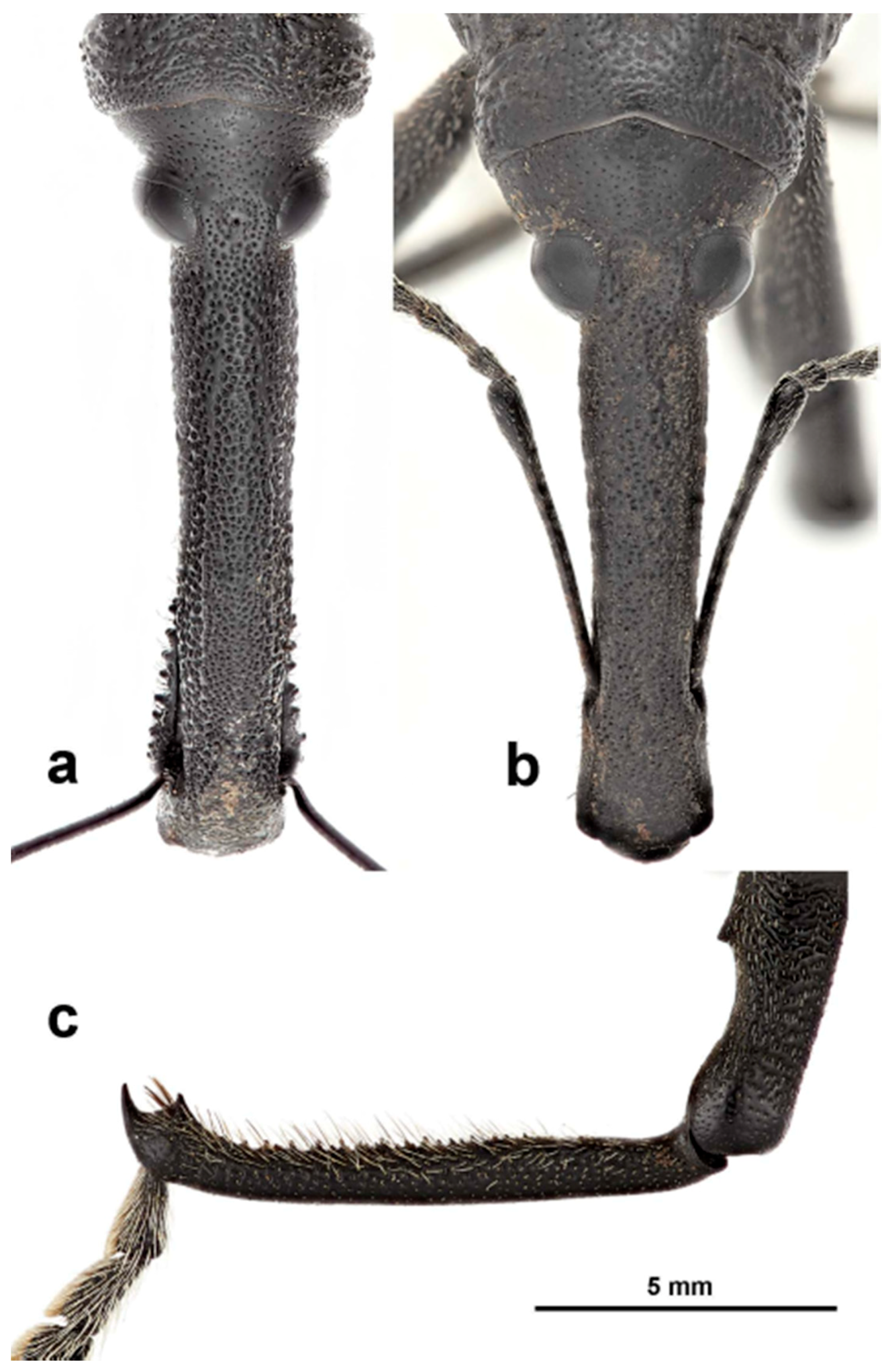
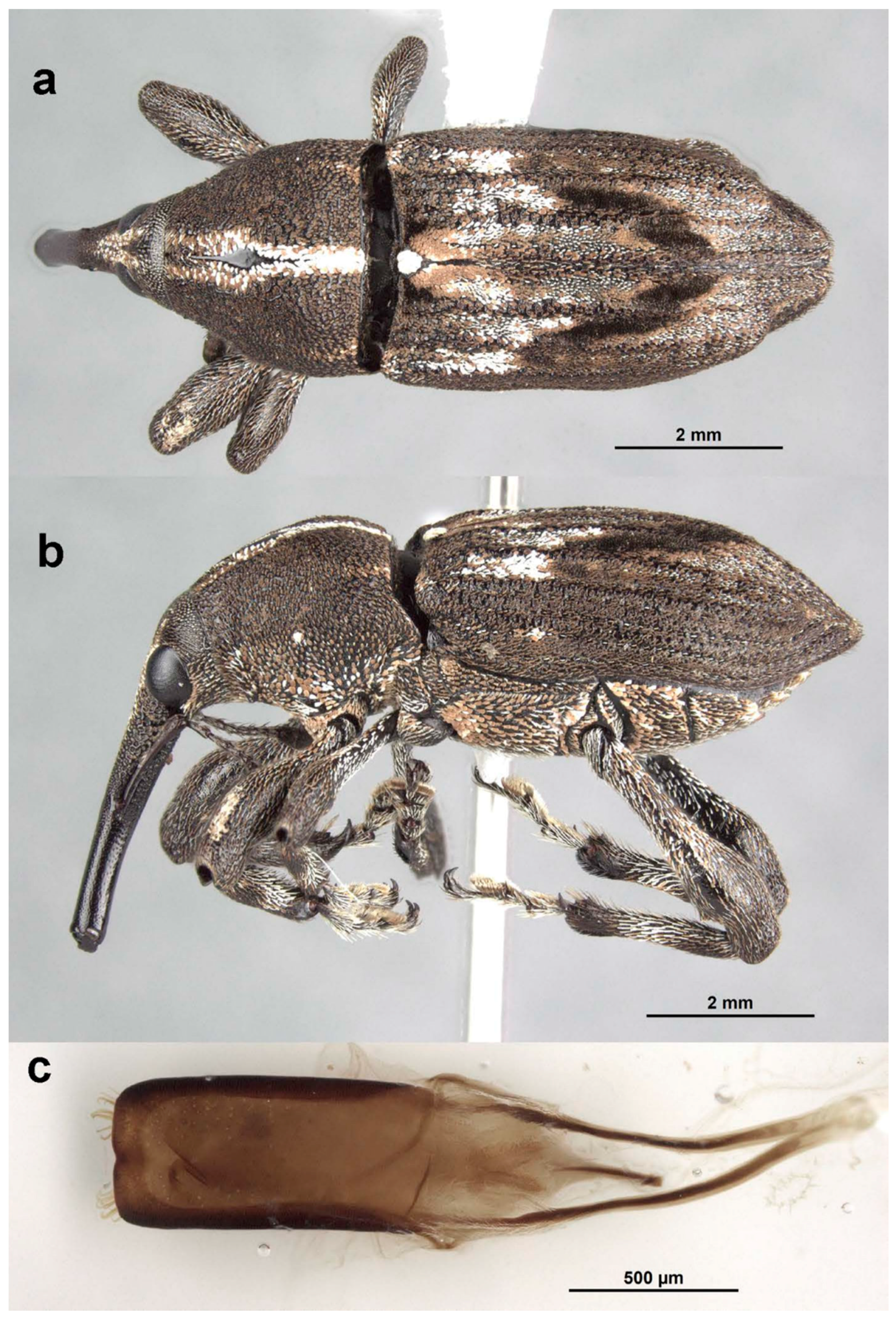
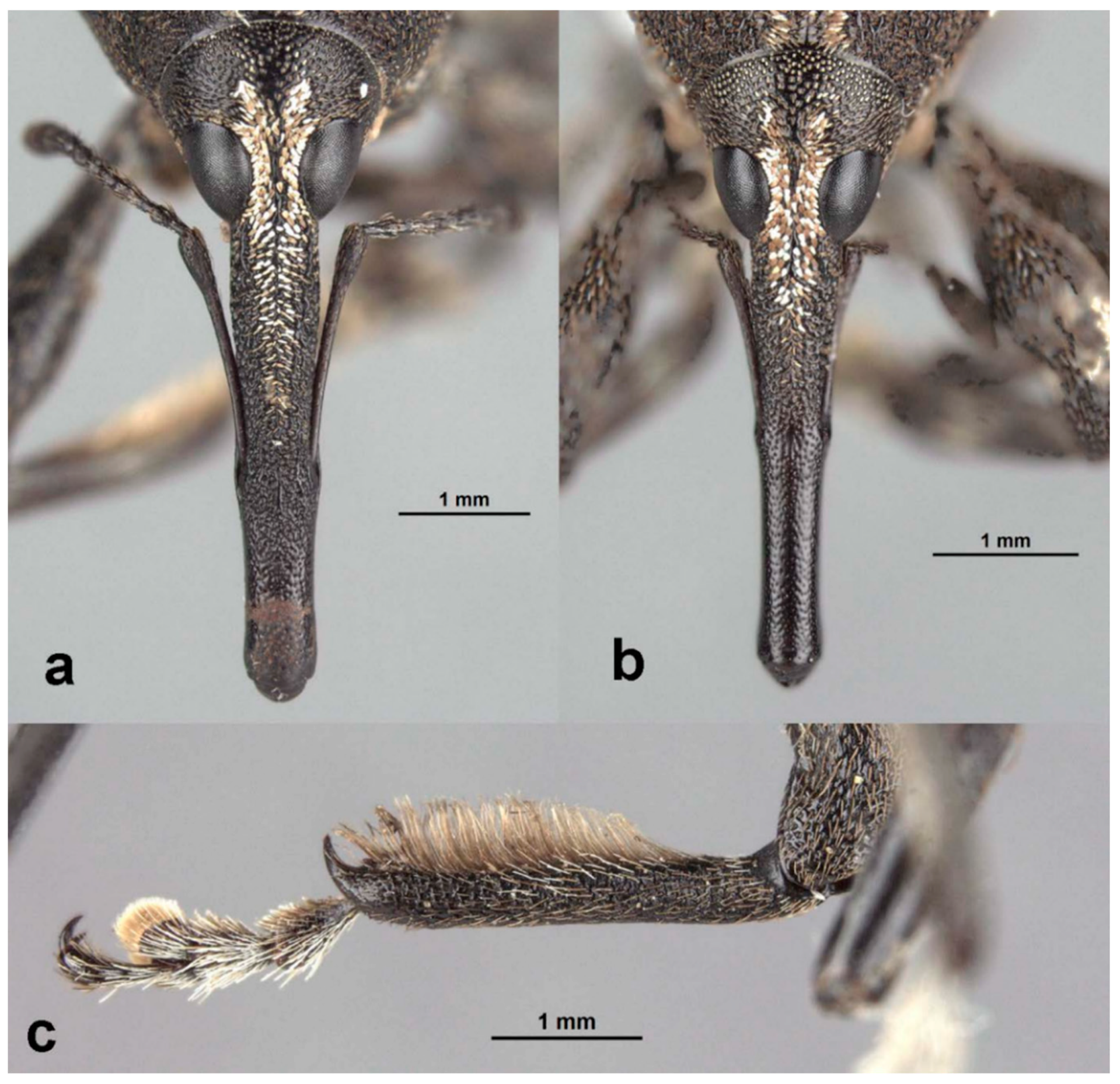
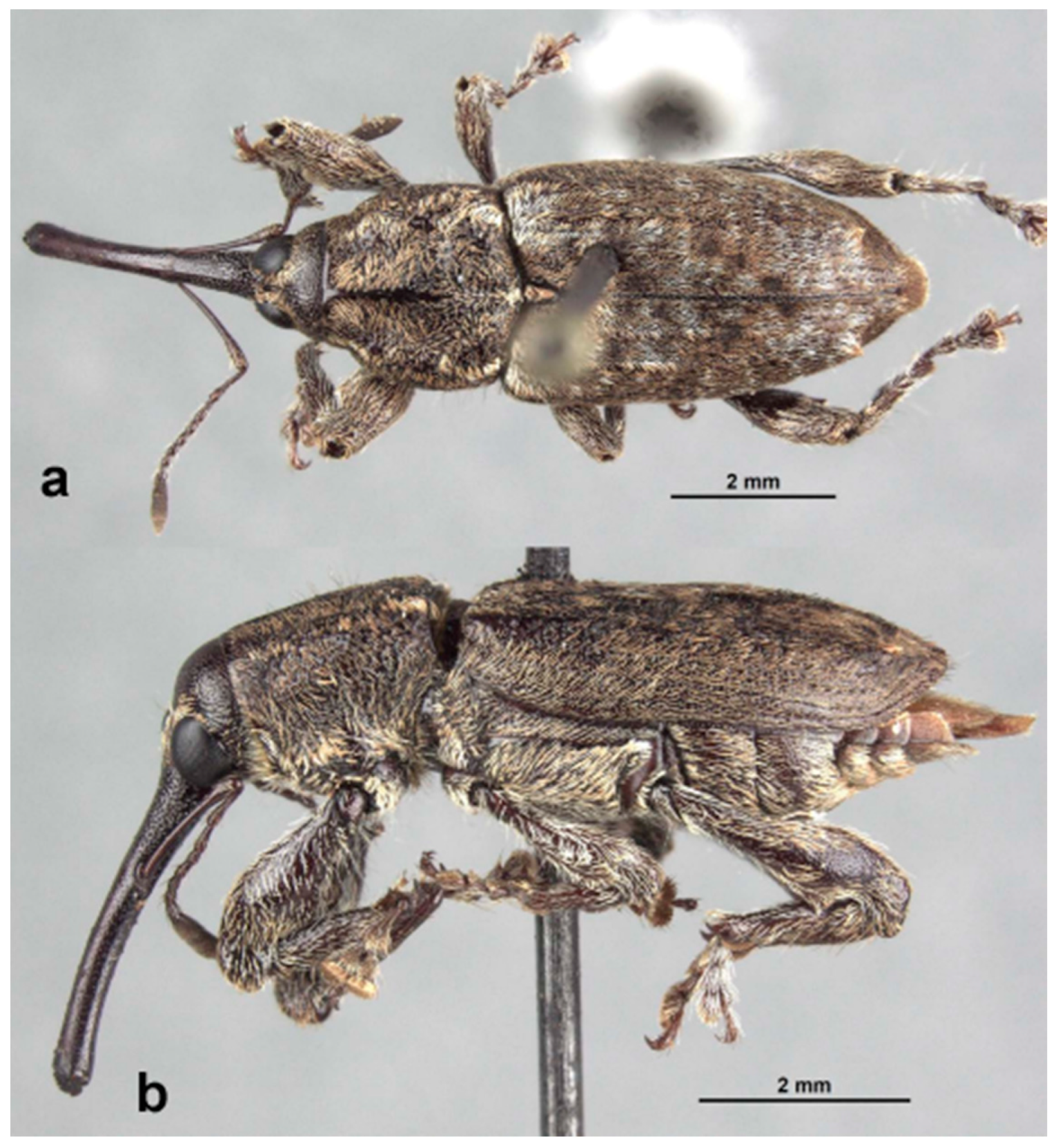
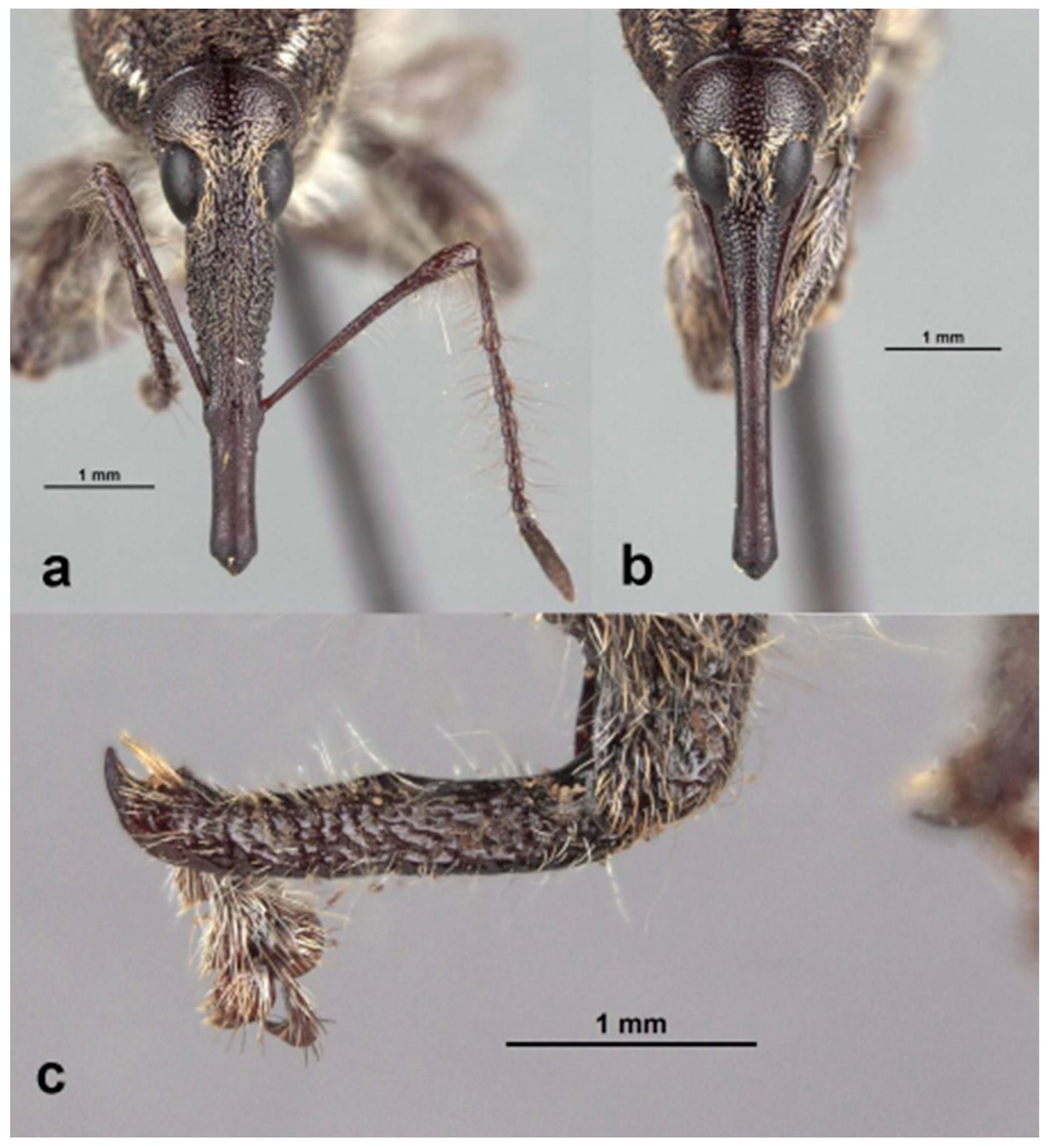
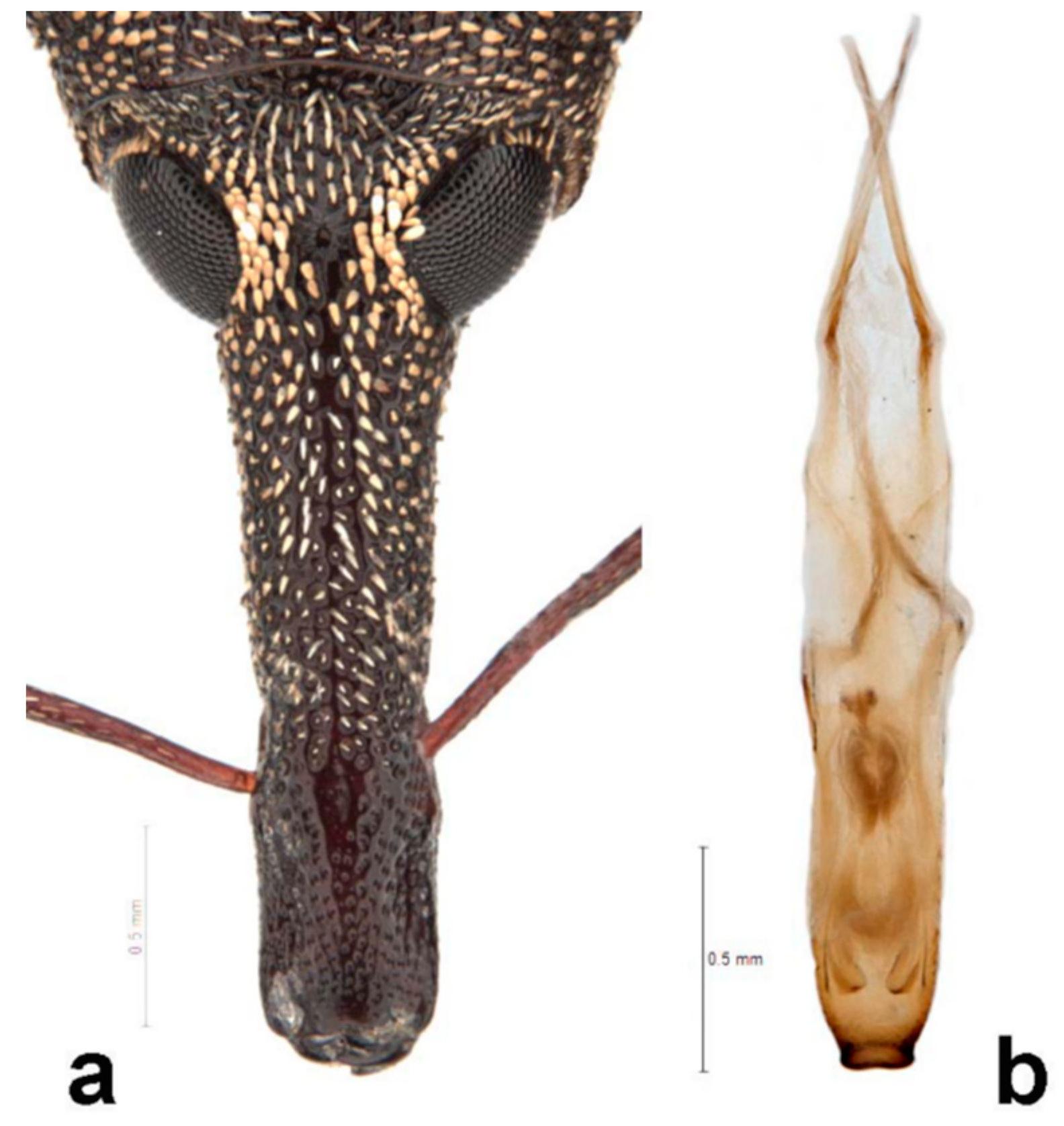
Redescription: Medium-sized (8.5–14.5 mm); elongate oval; prothorax trapezoidal in dorsal view, widest subbasally, base as wide as base of elytra; elytra parallel-sided to declivity in dorsal view, tapering toward apex. Integument dark brown to black; covered with small, appressed to recumbent, tan to brown scales and sparsely distributed, capitate, pale scales; dorsum with various white patches, maculae or vittae and patches of fuscous scales. Scales becoming longer and more hair-like on venter and legs. Eyes large, outline hemispherical, dorsally elevated, separated by slightly less than width of rostrum at base. Rostrum subequal to or longer than pronotum in both sexes; straight to feebly curved, circular in cross-section; tapering slightly to apex; basal half dorsally covered with sparse hair-like scales, apical half bare, venter bare in female, granulate or finely hirsute in male. Antennae inserted at apical one-third to two-fifths of rostrum in males and near middle of rostrum in females; scrobes sharply defined, running towards anteroventral angle of eye; scapes subcylindrical, clavate towards apex, reaching anterior margin of eye in repose in males, not so in females; funicles 7-segmented, segments 1 and 2 each at least twice as long as 3, 3–7 subequal in length, 7 pilose and closely appressed to but distinct from club; clubs elongate, transversely 4-segmented. Pronotum with glabrous dorsomedian carina from apex to slightly past middle, lateral portions of basal margins produced posteriad over elytral bases laterally, anterolateral angles tapered to apex in dorsal view; Elytra with base weakly sinuate, scutellar shield externally visible, large, cordate, densely squamose; 10 complete striae of small, shallow punctures each bearing a single scale similar to those on adjacent interstriae; declivital callus indistinct, low, rounded. Legs elongate, inner margins of femora with small subapical angulation or small tooth; inner margin of protibiae with patch of long golden hairs throughout apical half of length in male, hairs lacking in female. Aedeagus with penis subequal in width throughout length, very slightly emarginate at apex at middle, apex with a number of elongate setae on each side of emargination, body slightly shorter than apodemes, internal sac with fine spicules and vaguely defined apical sclerite complex. Female not dissected.
Comments: Sexual dimorphism in two of the three known species (third species known only from a single male) is most evident in the position of the antennal insertions on the rostrum and in the sculpture and form of the rostrum.
Distribution: The genus is known from northern New South Wales northwards into Queensland in Australia as well as the central highlands of Papua New Guinea.
Natural history: Adults of two species,
I. laticollis and
I. papuana, have been collected on, or reared from, species of
Araucaria (label data, [
16]). It is likely that the third species,
I. suttoni, is similarly associated as it was mixed with a series of
I. laticollis presumed to have been collected from
Araucaria at Rivertree, New South Wales.
Ilacuris laticollis Pascoe, 1865
Ilacuris laticollis Pascoe, 1865: 425 [
8]; Pascoe, 1871: 202 [
9] (in key, in Zygopinae) and plate XVII (illustrated); Dalla Torre et al., 1932: 25 [
13] (catalog, in Pissodini); Hustache, 1934: 57 [
12] (catalog, in Zygopinae); Zimmerman, 1994: 694 [
14] (in Molytinae) and Figure 371 on page 561 (wing-folding pads illustrated); Alonso-Zarazaga & Lyal, 1999: 207 [
5] (catalog, in Molytinae: Pissodini); Mecke et al., 2005 [
16] (natural history); Lyal, 2014: 529 [
15] (natural history, in Molytinae: Orthorhinini); Pullen et al., 2014: 283 [
2] (in Molytinae: Orthorhinini).
Illacuris laticollis (Pascoe): Masters, 1886: 682 [
11]; Wiley & Shanahan, 1973: 373 [
17]; Setliff, 2007: 296 [
3]; Lyal, 2014: 556 [
15] (incorrect subsequent spelling). All previously published records of
I. laticollis from Papua New Guinea likely to refer to
I. papuana n. sp.; see [
3,
17].
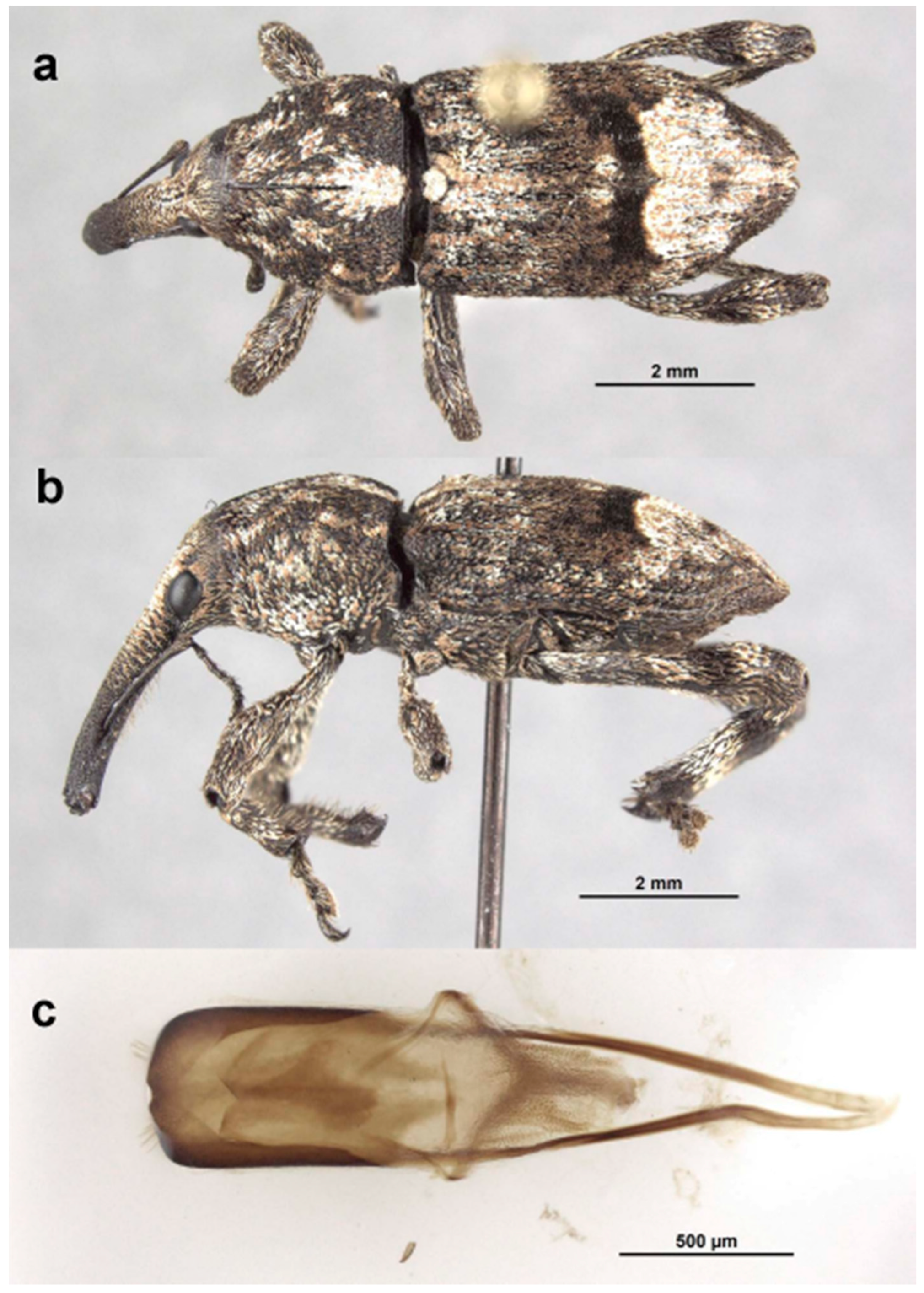
Redescription. Male. Body length 10.9–14.5 mm. Body width 4.6–6.1 mm. Dorsal surface with dispersed to dense, variously colored, appressed scales; no erect or suberect hairs present. Rostrum with antennal insertions at about apical two-fifths of length, ventral surface of rostrum finely granulate from just before base to antennal insertions; in dorsal view with area behind antennal insertions not dilated or much wider than area before antennal insertions, width greatest near base. Antennae with funicle segment 2 slightly longer than 1. Pronotum with distinct, narrow, sharp median carina in anterior half, culminating in elongate narrow, fusiform callosity at about midlength, then posteriorly continued as a low, less distinct, elevated line to about posterior quarter. Median carina bordered laterally by area of pale brown or white scales contrasting with darker scales of rest of pronotal disk; area laterally of pale median fascia irregularly impressed in a broad sinuate furrow deepest laterally of median raised callosity, shallower anteriorly and posteriorly; scales in furrow distinctly metallic gold in sheen and color (in clean specimens); area laterally of furrow with similarly impressed patch of golden scales just in front of midlength towards lateral margin; anterolateral margins in dorsal view tapered evenly to apex. Elytra with odd interstriae (sutural, 3, 5, 7 and 9) distinctly elevated and variously granulate throughout most of their length, except 3 interrupted at about basal two-fifths. Dorsal fascia of dark brown to black scales “V-shaped”; apical quarter of elytra with dispersed scales somewhat paler. Pro- and mesofemora with inner margin with indistinct slight subapical angulation; inner margin of metafemora with moderately large, distinct subapical tooth. Inner margin of protibiae with short row of sparse but distinct, elongate, golden hairs in apical two-fifths. Abdomen with ventrite 1 concave medially. Aedeagus with penis widest in apical half, apex slightly medially emarginate, with a few setae at outer apical corners, body slightly shorter than apodemes; internal sac with a vague apical armature composed of two faint, inwardly convergent elongate-narrow sclerites. Female. Body length 9.5–11.8 mm. Body width 3.8–5.1 mm. As for male, except antennal insertions at about midlength, ventral surface of rostrum smooth from base to antennal insertions; in dorsal view with area behind antennal insertions not dilated or much wider than area before antennal insertions; width is greatest near base. Abdomen with ventrite 1 flat to slightly evenly convex. Genitalia not examined.
Specimens examined. AUSTRALIA: Queensland. Mackay [−21°09′ 149°11′], 1912 (1 male, SAMA). Yeppoon [−23°08′ 150°44′], Dec. 1960 (1 female, ANIC). Barrimoon [−24°40′ 151°19′], 22.i.1944, (2 males, 1 female, ANIC). Wide Bay [−25°52′ 153°07′] (1 male, 1 female, SAMA). Yarraman [−26°51′ 151°59′], 8.xi.1939, A. Hanson (1 male, ANIC). Yarraman State Forest, Forest Drive, 650 m, 26°49′57” S, 151°57′55” E, 19.i.2000, R. S. Anderson, beating Araucaria cunninghamii (1 female, CMNC). Blackbutt [−26°53′ 152°06′], 9.i.1913 (1 female, ANIC). Brisbane [−27°28′ 153°01′], 17.iii.1913, H. Hacker (1 male SAMA). Brisbane [−27°28′ 153°01′] (1 male, ANIC; 2 males, SAMA). Moreton Bay [Brisbane, −27°28′ 153°01′], 29061 (1 male, ANIC). Pine Mountain [−27°33′ 152°43′] (1 male, ANIC). Stanthorpe [−28°40′ 151°56′], E. Sutton (3 males, ANIC; 2 males, MVM; 8 males, 5 females, SAMA) [probably from Rivertree, N.S.W.; see note above about this locality]. Fletcher [−28°46′ 151°51′], E. Sutton (2 males, 5 females, ANIC; 2 males, 1 female, MVM) [probably from Rivertree, N.S.W.; see note above about this locality]. Queensland (1 male, 1 female, SAMA). Imbil [−26°28′ 152°41′], 28.v.1959, F. McDonald (2 males, MVM, UQB). Imbil State Forest [−26.6185 152.6149], 25–28.v.1959, I. C. Yeo (4 males, QMB). New South Wales. Rivertree [−28°38′ 152°18′], E. Sutton (5 males, 1 female, ANIC). Rivertree [−28°38′ 152°18′], 21.vii.1932, E. Sutton (1 male, ANIC). Rivertree [−28.6260 152.2450], 290 m, N. Sutton [no date, likely from Araucaria (see note above about this locality)] (23 males, 12 females, CMNC, GPSC). No data, E. Sutton collection [probably from Rivertree, N.S.W.] (2 males, 1 female, ANIC). Alstonville, −28°50′, 153°26′, 10.viii.1971, J. O’Grady (1 male, ANIC). Nana Glen, −30°08′ 153°01′, 14.ii.1925 (1 male, ANIC). Dorrigo [−30°20′ 152°43′] (1 female, SAMA).
Specimen data recorded from other institutions (not examined): AUSTRALIA: Queensland. Yarraman [26°51.001′ S, 151°59.015′ E], 1.xii.1938, L. T. Carron (1, QMB). Yarraman, 21.xi.1936, R. Brimblecombe (1, QDPC). Brisbane [27°28.002′ S, 152°59.985′ E], R. Illidge (1, QMB). Brisbane, 14.x.1968, R. Yule, Araucaria cunninghamii (1, QDPC). Brisbane, 17.iii.1913, H. Hacker (1, QMB). Killarney [28°20.002′ S, 152°18.011′ E], 1.xi.1901 (1, QMB). Gatton [−27.566 152.277], 1.x.1937, C. S. Andrew (1, QMB). East Nanango [−26.666 152.039], 14.x.1967, R. Yule, ex hoop pine (2, QMB). Imbil, viii.1936, R. Brimblecombe, Araucaria cunninghamii (3, QDP). Imbil, 30.x.1937, R. Brimblecombe (1, QDPC). Imbil, 30.xi.1971, R. Yule, Araucaria cunninghamii (4, QDPC). Imbil, 16.xii.1971, R. Yule, Araucaria cunninghamii (5, QDPC). Imbil, 8.xi.1972, R. Yule, Araucaria cunninghamii (2, QDPC). Imbil State Forest, 24.vi.1969, N. W. Heather (1, QDPC). Imbil State Forest, 18.v.1970, N. W. Heather (1, QDPC). Imbil State Forest, 30.vi.1981, R. Gould, Araucaria cunninghamii (1, QDPC). Benarkin, 17.iv.1933, H. Hacker (3, QDPC). Mount Tamborine, no date (3, QDPC). Jimmy’s Scrub, 17.ix.1971, R. Yule, Araucaria cunninghamii (4, QDPC). Jimmys Scrub, 9.xii.1971, R. Yule, Araucaria cunninghamii (4, QDPC). Kandanga, 3.iv.1975, R. J. Rabbits, Araucaria cunninghamii (5, QDPC). Gympie, Nut Farm, 13.xi.1968, N. W. Heather, Araucaria cunninghamii (1, QDPC). Canungra, 2.xi.1977, DeBaar & Wylie (1, QDPC). Amamoor, 18.x.1974, DeBaar & Wylie, Araucaria cunninghamii (1, QDPC). Compartment 6, Mossman Logging Area, Bulburin State Forest, iv.2016, M. Ramsden, Araucaria cunninghamii (1, QDPC). Glen Witheren, 28°2′48” S, 153°7′12” East, main scrub, 15.iv.2001, G. Monteith, ex hoop pine log (1, QMB). Mt. Ipswich, 1867, W. Hart (4, QMB). New South Wales. Rivertree, various dates (23, QMB).
Natural history: Adults have been collected on, and reared from,
Araucaria cunninghamii (Hoop Pine) (label data, [
16]). Geoff Williams reared a number of specimens from
Araucaria logs collected at Reserve Creek Road, Murwillumbah, Queensland (
Figure 5).
Ilacuris papuana Anderson & Setliff, new species
Ilacuris laticollis Pascoe
sensu Wylie & Shanahan, 1973 [
17], in Papua New Guinea.
Description: Male. Body length 8.5–14.2 mm. Body width 3.1–5.2 mm. Dorsal surface with dispersed to dense, variously colored, appressed scales, no erect or suberect hairs present. Rostrum with antennal insertions at about apical two-fifths of length, ventral surface of rostrum finely granulate from just before base to antennal insertions; in dorsal view with area behind antennal insertions not dilated or much wider than area before antennal insertions, width greatest in front of base. Antennae with funicle segment 2 subequal in length to 1. Pronotum with distinct, narrow, sharp median carina in anterior half, culminating in elongate fusiform callosity at about midlength, then posteriorly continued as a low, less distinct, elevated line to about posterior quarter. Median carina bordered laterally by an area of broad, pale brown or white scales contrasting with much smaller, darker scales of the rest of the pronotal disk. Area laterally of pale median fascia continuing to flanks not impressed (except for at most a small very shallow spot laterally of median callosity); scales small, tan throughout rest of disk; flanks with a very small but distinct spot of larger white scales at about midlength; anterolateral margins in dorsal view tapered evenly to apex. Elytra with alternate interstriae (3, 5 and less so 7) slightly elevated and variously granulate throughout basal three-quarters of their length. Dorsal fascia of dark brown to black scales “V-shaped”; apical portion of elytra with dispersed scales somewhat paler in color. Pro- and mesofemora with inner margin lacking subapical angulation or tooth; inner margin of metafemora with small, distinct subapical tooth. Inner margin of protibiae with a row of dense but distinct elongate, golden hairs in apical half to three-fifths. Abdomen with ventrite 1 concave medially. Aedeagus with penis widest at about midlength, apex slightly medially emarginate, with a few setae across the apical margin laterally of emargination, body slightly shorter than apodemes; internal sac with a vague apical armature composed of two faint, inwardly convergent elongate-narrow sclerites. Female. Body length 8.5–14.0 mm. Body width 3.4–5.4 mm. As for male, except antennal insertions at about midlength of rostrum, ventral surface of rostrum smooth from base to antennal insertion; in dorsal view with area behind antennal insertions not dilated or much wider than area before antennal insertions, width greatest near base. Abdomen with ventrite 1 flat to slightly evenly convex. Female genitalia not examined.
Specimens examined: Holotype, male (CMNC), labelled PAPUA NEW GUINEA: Morobe, Wau (18 km N.W.), 950 m, 11.ii.2000, 7°15′46″ S 146°39′54″ E, R. S. Anderson, Araucaria cunninghamii logs, RSA2000-044X. Paratypes, PAPUA NEW GUINEA: Morobe, Wau (18 km N.W.), 950 m, 11.ii.2000, 7°15′46″ S 146°39′54″ E, R. S. Anderson, Araucaria cunninghamii logs, RSA2000-044X (1 female, CMNC). 4.ii.2000, RSA2000-031X (2 females, CMNC). Wau Ecology Institute, 1200 m, 7 20′24″ S 146 42′17″ E, 5.ii.2000, R.S. Anderson, on Araucaria, RSA2000-034X (1 female, CMNC). Wau, 17.v.1962, J. Sedlacek (3 males, 2 females, BPBM). Wau, 16.x.1961, J. Sedlacek (1 female, BPBM). Wau, 1050 m, 16.ix.1961, J. & M. Sedlacek (1 female, ANIC). Wau, 5.ix.1961, J. Sedlacek, klinki pine (2 males, 5 females, BPBM). Wau, 1050 m, 18.xi.1961, J. Sedlacek (1 female, BPBM). Wau, 1200 m, 15.xi.1961, J. Sedlacek (2 males, BPBM). Wau, 1150 m, 16.ix.1961, J. Sedlacek (3 males, 1 female, BPBM). Wau, 1200 m, 3.xii.1961, J. Sedlacek (1 male, 1 female, BPBM). Wau, 8.v.1973, J. L. Gressitt (2 males, BPBM). Wau, 1200–1250 m, v.1966, J.L. Gressitt (1 male, BPBM). Wau, 2.ix.1966, G.A. Samuelson, Araucaria (3 males, BPBM). Wau, Nakata Ridge, 1830 m, 18.xii.1963, J. Sedlacek (1 male, BPBM). Wau, 20.xii.1963 (1 male, BPBM). N.E. Wau, Mount Missim, 1150 m, 21.iii.1964, J. Sedlacek (10 males, BPBM). Mount Missim, 1600 m, 17.iii.1966, J. L. Gressitt (4 males, BPBM). Mount Missim, 1150 m, 11.iv.1964, J. Sedlacek (2 males, 1 female, BPBM). Mount Missim, 1250 m, 14.vii.1971, J. Sedlacek (1 female, BPBM). Bululo, i-ii.1972, J. Sedlacek (2 males, CWOB). Asiki, i-ii.1972, J. Sedlacek (2 males, CWOB). 18 km N.E. Okapa, 1300 m, 2.vi.1967, G. A. Samuelson (1 female, BPBM). Bulldog Road, km 14, south of Edie Creek, 2405 m, 4–10.vii.1964, G. R. Wilkes (1 male, BPBM). S.E. Popondetta, 25 m, v.1956, G. Lippert (1 female, BPBM). N.E. Kainantu, 1560 m, 6.vi.1967, G.A. Samuelson, on Araucaria cunninghamii (6 males, 1 female, BPBM). No data (1 male, BPBM). Taun Creek logging area, 920 m, 07°15′06″ S, 146°37′35″ E, 13.vii.1999, P. Bouchard (2 males, QMB). “Papua New Guinea”, ii.1963, W. Rosenberg (2 males, FSCA). Bulolo, on log, 6.i.1970, L. Radunz (1 female, ANIC). Gadsup, ix.1972, H. Ohlmus (1 male, 4 females, ANIC). Gadsup, ix.1973, H. Ohlmus (2 males, ANIC).
Natural history: Adults have been collected on Araucaria cunninghamii (Hoop Pine) and A. hunsteinii (Klinki Pine) in the vicinity of the Wau Ecology Institute, Papua New Guinea.
Derivation of name: The species name is an adjective formed from part of the name of the country of Papua New Guinea.
Ilacuris suttoni Anderson & Setliff, new species
Description: Male. Body length 9.2 mm. Body width 3.7 mm. Dorsal surface with dispersed to dense, variously colored, broad appressed scales; no erect or suberect hairs present. Rostrum with antennal insertions at about apical one-third, ventral surface finely granulate from just anteriorly of base to antennal insertions and with long fine golden hairs, densest and longest just below antennal insertions; in dorsal view with area behind antennal insertions not dilated, not wider than area before insertions, width greatest at antennal insertions; dorsally carinate from antennal insertions to near base. Antenna with funicle segments 1 and 2 subequal in length. Pronotum with distinct, narrow, sharp median carina in anterior three-quarters; area next to median carina continuing to flanks not impressed; scales mostly white to tan throughout rest of disk except area basally of carina at middle with patch of dense, white to pale brown broad scales in front of scutellar shield; anterolateral angles subapically subquadrate. Elytra with interstria 3 slightly elevated throughout basal half of its length. Mid-dorsal fascia of dark brown to black scales transverse; apical portion of elytra with dense white and pale brown scales immediately behind transverse dark fascia, scales dispersed, somewhat paler towards elytral apex. Pro- and mesofemora with inner margin with small subapical angulation; inner margin of metafemora with small, distinct subapical tooth. Inner margin of protibiae with a row of sparse, elongate, golden hairs in apical three-quarters. Abdomen with ventrite 1 convex medially. Aedeagus with penis widest in apical half, apex somewhat sharply, medially emarginate, with a few setae at middle of apical margin next to emargination, body shorter than apodemes; internal sac with a vague apical sclerite composed of fine dense spicules. Female. Not known.
Specimens examined. Holotype, male: “AUSTRALIA: N.S.W., Rivertree [−28.6260 152.2450], 290 m, N. Sutton” (ANIC). See note above about this locality.
Derivation of name. This species is named after Edmund (“Ned”) Sutton of Fletcher, Queensland, Australia, who collected the only known specimen. Mr. Sutton was one of few people successful in collecting numbers of the large pine weevil Eurhamphus fasciculatus. Parts of his collection are in some North American museums, but the bulk of his collection is now housed in the Queensland Museum.
Natural history. It is assumed that this unique specimen, which was mixed with a long series of I. laticollis labelled the same, was collected at Rivertree, New South Wales, where Sutton is known to have collected beetles associated with Araucaria.
3.2.4. Kuschelorhinus Anderson & Setliff, New Genus
Type species:Kuschelorhinus hirsutus Anderson & Setliff, new species.
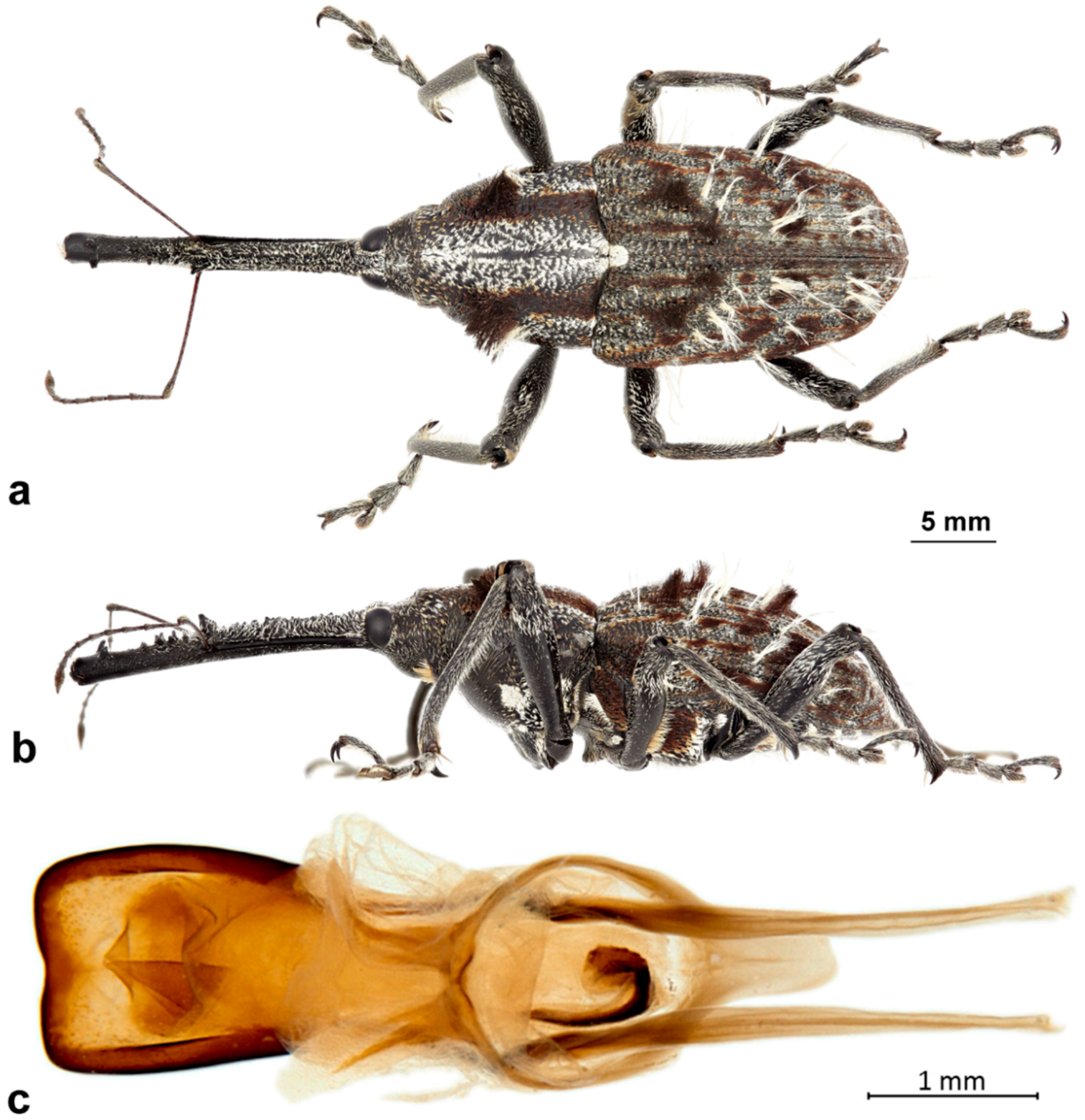
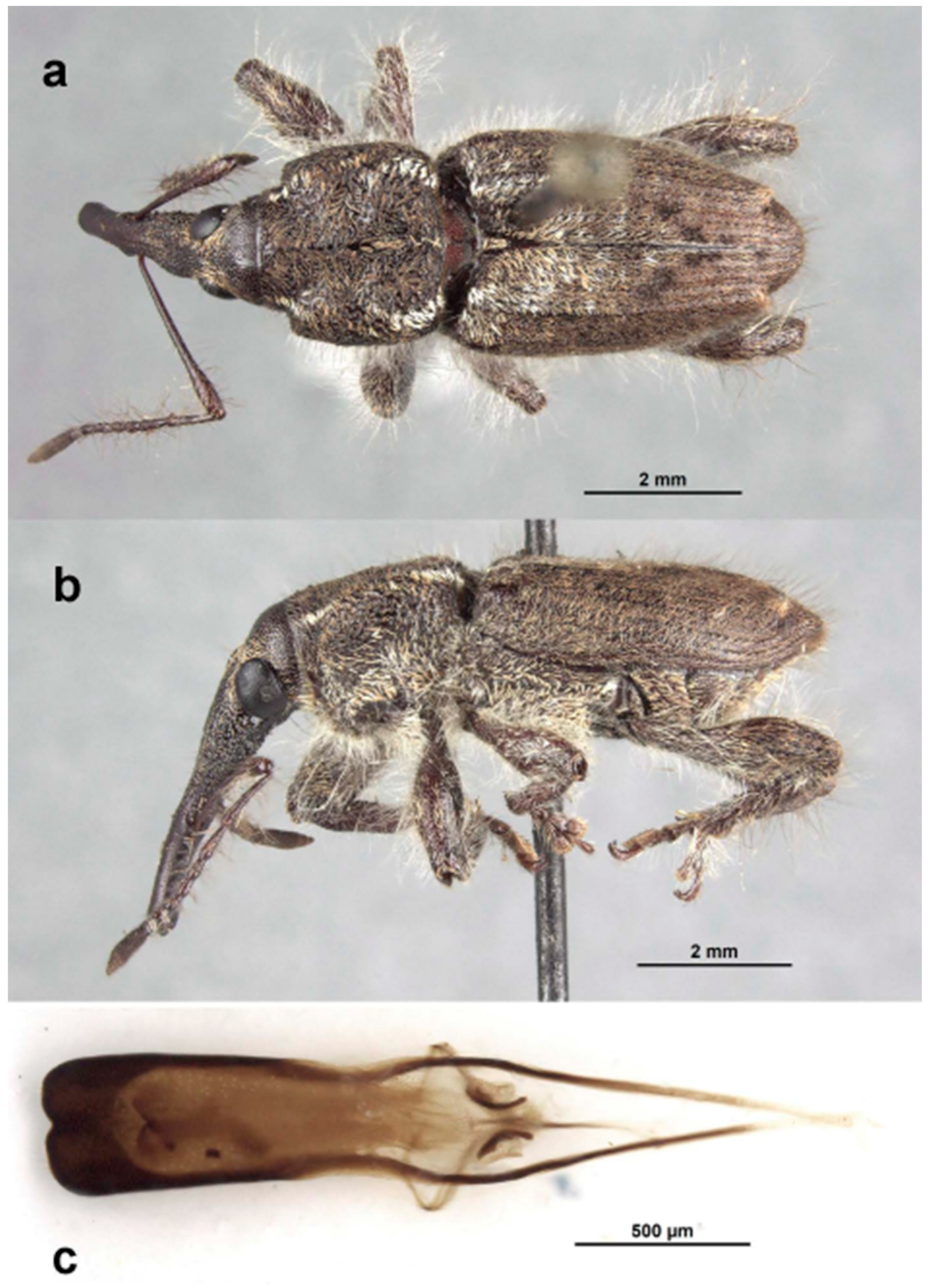
Description. Medium-sized (7.9–9.8 mm); elongate oval; prothorax trapezoidal in dorsal view, widest subbasally, maximum width equal to maximum width of elytra, slightly less so in female; elytra subparallel-sided to about apical third, tapering towards apex. Integument dark brown to black; covered with small, appressed to recumbent, tan to brown, elongate-narrow hair-like scales; dorsum with few patches of white or fuscous hair-like scales. Male with dense covering of elongate, very fine, wispy hairs covering almost entirety of surface of body and appendages; female with such hairs limited to pronotum. Eyes large, outline subcircular, slightly elevated, separated by less than width of rostrum at base in male, less so in female. Rostrum longer than pronotum in both sexes; straight (male) to slightly curved (female), circular in cross-section; tapering slightly to apex; basal half dorsally covered with sparse hair-like scales in male, almost bare in female, apical half bare, venter moderately coarsely granulate in basal portion in male, bare in female. Antennae inserted slightly before midlength of rostrum in male, slightly behind midlength in female; scrobes sharply defined, running towards anteroventral angle of eye; scapes subcylindrical, clavate towards apex, reaching posterior margin of eye in repose in male, about middle of eye in female; funicles 7-segmented, segments 1 and 2 each two to three times longer than 3 in male, slightly less so in female, 3 slightly longer than 4, 4–7 subequal in length, 7 with appressed white scales, closely appressed to but distinct from club; clubs elongate, transversely 4-segmented. Pronotum with glabrous dorsomedian elevated ridge from apex to slightly past midlength, lateral portions of basal margins not produced posteriad over elytral bases laterally, anterolateral corners in dorsal view acutely angulate in male, tapered to apex in female. Elytra with base weakly sinuate, scutellar shield externally visible, elongate-triangular, densely squamose; with 10 complete striae of small, shallow punctures; declivital callus distinct, tuberculate, with apical tuft of elongate-narrow scales. Legs elongate; femora with inner margin distinctly toothed, tooth in male large and recurved on profemora, slightly smaller on meso- and metafemora, in female slightly smaller on all femora; inner margin of protibiae expanded and flanged at about midlength, more so in male than female. Aedeagus with penis widest at apex, emarginate at apex at middle, apex lacking setae, body about as long as apodemes, internal sac with distinct apical armature of two elongate, narrow curved sclerites. Female not dissected.
Distribution: The genus is only known from the central highlands of Papua New Guinea (a record from New Britain requires confirmation).
Comments: Sexual dimorphism in the single known specie is extreme and evident in the antennal insertions on the rostrum, the sculpture and form of the rostrum (swollen and granulate basally in the male) and mostly notably in the dense covering of long, fine, wispy hairs over the entire body surface (including appendages) in the male. Kuschelorhinus is closely related to Ilacuris but is here given generic status because of the extreme sexual dimorphism, the form of the aedeagus, the very different vestiture lacking any broad scales, the lack of long setae along the inner margin of the front tibiae in males and the presence of distinct elytral declivital calli.
Derivation of genus name: This genus is named in honor of Guillermo ‘Willy’ Kuschel (1918–2017), in recognition of his lifetime of contributions to the study of the Curculionoidea; the name is masculine in gender.
Kuschelorhinus hirsutus Anderson & Setliff, new species
Description. Male. Body length 7.9–9.3 mm. Body width 3.2–3.8 mm. Dorsal surface with dispersed, mostly tan, appressed to recumbent, elongate, narrow hair-like scales; rather dense, white long, wispy erect hairs on dorsum, legs, antennae (except clubs) and venter. Rostrum with antennal insertions at about apical two-fifths of length, ventral surface coarsely granulate from just before base to antennal insertions; in dorsal view with area behind antennal insertions strongly dilated, much wider than area before insertions; width greatest at or before base. Antennae with funicle segment 2 much shorter than 1; 1 and 2 very long, 1 about as long as combined length of 3–5. Pronotum with distinct, narrow median carina in anterior half, culminating in slightly fusiform callosity at about midlength, then posteriorly continued as a low, less distinct line to about posterior quarter. Median carina bordered laterally by a deeply impressed area in anterior half, less deep in posterior half; areas next to impression raised towards pronotal margins, with scattered denser white scales forming a faint lateral vitta; otherwise scales of pronotum uniformly elongate-narrow, fine, tan except for patch of broad white hair-like scales towards each posterolateral corner; anterolateral corners subapically distinctly angled, quadrate. Prosternum strongly tumescent in front of procoxae. Elytra with interstria 3 slightly elevated and variously granulate throughout basal three-quarters of length; dorsal fascia of dark brown to black scales faint, “V-shaped”; apical portion of elytra with uniformly tan, elongate hair-like scales. Pro-, meso- and metafemora with inner margin distinctly toothed, tooth especially large on profemora. Inner margin of protibiae expanded laterally at midlength opposite profemoral tooth. Abdomen with ventrite 1 concave medially, lacking hair-like scales (which uniformly cover ventrites 2–5), with only very fine hairs. Aedeagus with penis apically abruptly, rather deeply emarginate; internal sac with apical sclerites individually distinct, strongly curved outwards. Female. Body length 8.9–9.8 mm. Body width 3.1–3.9 mm. As for male except dorsal and other surfaces lacking long, erect wispy hairs, except for a few scattered long hairs on pronotum. Rostrum with antennal insertions at basal third of length, ventral surface smooth from the base to antennal insertions; in dorsal view with area before antennal insertions not dilated or much wider than area behind insertions, width greatest near base. Pronotum with anterolateral corners subquadrate. Prosternum flat in front of procoxae. Pro-, meso- and metafemora with inner margin only weakly toothed, tooth largest on metafemora. Protibiae with very slight median expansion. Abdomen with ventrite 1 flat to slightly evenly convex, lacking scales, with only fine appressed hairs. Genitalia not examined.
Specimens examined. Holotype, male: “NEW GUINEA: NE / Wau, Morobe Distr. / 1300 m, 1.II.1961 // J. Sedlacek / Collector / BISHOP” (BPBM). Paratypes: Same data (1 male, ANIC). PAPUA NEW GUINEA, Wau, 23.viii.1956, 960 m, E. J. Ford Jr. (4 males, 2 females, BPBM). Wau, 1100–1200 m, i.1966, J. Sedlacek (6 males, 5 females, BPBM, CWOB). Wau, 1200–1300 m, 14–17.i.1963, J. Sedlacek (1 female, BPBM). Wau, 1250 m, 23.i.1963 (1 female, BPBM). Wau, 1300 m, 1.ii.1961, J. Sedlacek (2 males, BPBM). Wau 1200 m, 25–30.ix.1964, J. Sedlacek (1 female, BPBM). Wau 1250 m, 23.i.1964, J. Sedlacek (4 males, 2 females, BPBM). Wau, 1300 m, 1.ii.1961, J. Sedlacek (20 males, 15 females, BMNH, BPBM, CMNC, CWOB, GPSC, USNM). Wau, 1150–1250 m, 1.ii.1963, J. Sedlacek (2 males, 2 females, BPBM). Wau, 1200–1300 m, 14–17.i.1963, J. Sedlacek (19 males, 11 females, BPBM, CMNC). Wau, 23.i.1963, 1250 m, J. Sedlacek (5 females, BPBM). Wau, 1100 m, 30.ix.1961, J. Sedlacek (1 male, BPBM). Wau, 1000–1250 m, 3.iii.1964, J. Sedlacek (7 males, 8 females, BPBM). Wau, 1200 m, 17.viii.1963, J. Sedlacek (1 male, BPBM). Wau, 1200 m, 26–27.ix.1964, J. Sedlacek (1 female, BPBM). Wau, 980–1100 m, 14.viii.1964, J. Sedlacek (1 male, 1 female, BPBM). Wau, 1100 m, 31.i.1963, J. Sedlacek (1 male, BPBM). Wau, 1250 m, 22.vii.1969, J. Sedlacek (1 male, BPBM). Wau, 1200–1300 m, 6–12.iv.1962, J. Sedlacek (1 male, 1 female, BPBM). Wau, 1700–1800 m, 27.ix.1965, J. Sedlacek, malaise trap (1 female, BPBM). Wau, Mount Missim, 11.iv.1964, J. Sedlacek (6 males, 6 females, BPBM). Wau, Hospital Creek, 10.iv.1965, J. Sedlacek (2 females, BPBM). Wau, J. Sedlacek (2 females, BPBM). Bulolo, 900 m, 29.iii.1968, P. Colman (2 males, 2 females, BPBM). Bulolo, 880 m, 14.viii.1956, J. Sedlacek, light trap (1 female, BPBM). Bululo, 1010 m, 28.viii.1956, J. Sedlacek, light trap (1 male, BPBM). Crooked Creek logging area, 920 m, 7°12′56″ S, 146°35′39″ E, 11.vii.1999, P. Bouchard (3 males, 2 females, QMB). Forestry road north of Wau, 21.x.1969, J.E. Tobler (2 male, 2 females, CASC). NEW GUINEA, Bulolo District, 17.xii.1967, B. B. Lowery (1 male, ANIC). NEW GUINEA, Bulolo, 26.xii.1971, H. Ohlmus (1 male, ANIC). Same data but 28.xii.1971 (2 males, ANIC). NEW GUINEA, Wau, Feb. 1973, H. Ohlmus (1 female, ANIC). New Britain: Gazelle Peninsula, Gaulim, 130 m, 28.xi.1962, J. Sedlacek (23 males, 12 females, BPBM, CMNC, CWOB, GPSC). Forest Station Bulolo, 7.x.1976, Ento Crew, ex. Araucaria hunstenii log (1 male, BMNH). Hump Logging Area, Bululo, 4.v.1970, B. Gray, under bark upper stem Araucaria hunstenii (1 female, BMNH).
Natural history. Two adult specimens were collected from Klinki Pine (
Araucaria hunsteinii) in Bulolo, Papua New Guinea. The majority of specimens were collected together with
I. papuana in mixed stands of
Araucaria; however, a long series of specimens collected on New Britain appears to be from a locality where no
Araucaria is known to occur [
30] and may be mislabeled. Label data on a few specimens indicate that they were collected at a light trap.
Derivation of name. This species is named for its distinct, densely hairy males; the name is an adjective.
3.2.5. Notopissodes Zimmerman & Oberprieler, 2014
Notopissodes Zimmerman & Oberprieler in Pullen et al., 2014: 469 [
2].
Type species:Notopissodes pictus Zimmerman & Oberprieler, 2014, by original designation.
Distribution: The genus is known from northern New South Wales northwards to northern Queensland in Australia but appears to be rare.
Natural history.Notopissodes is evidently also associated with Araucariaceae, N. pictus having been reared from Hoop Pine (Araucaria cunninghamii). Its larvae probably develop in the wood or bark of trunks or branches.
Comments.Notopissodes was only recently described, except for its genitalia (see under N. pictus below). Apart from the type species, one other species is known, as described below. Notopissodes differs significantly in its male genitalia from Eurhamphus, Ilacuris, Kuschelorhinus and Vanapa, the penis being narrowly elongate and dorsally closed and the tegmen having a long apodeme, and it is not evidently closely related to these genera.
Notopissodes pictus Zimmerman & Oberprieler, 2014
Notopissodes pictus Zimmerman, 1992: 580, plate 594, Figures 7 and 8 [
31] (not available, no description)
Notopissodes pictus Zimmerman & Oberprieler in Pullen et al., 2014: 470 [
2].
Description of genitalia. Male: aedeagus with body of penis narrow, 5 x longer than wide, subequal in width for four-fifths of length, then slightly narrowing apicad but widening again just before apex, apex broadly, roundly truncate, asetose; temones half as long as body; internal sac with fine spicules, condensed basally into a lyre-shaped field behind a small narrow sclerite with short, flat lateral arms; tegmen dorsally very weakly sclerotized, broad, parameres long and broad, only medially lightly sclerotized; sternite IX very thin (but dissected specimen somewhat teneral), asymmetrical, bladal part consisting of narrow, unequal arms, apodeme long, curved, apically tapered. Female: gonocoxites long, narrow, weakly sclerotized; styli apical, shortly elongate, apically obliquely truncate and with a few long setae; bursa membranous, small; spermatheca crescentic, with globular ramus and elongate collum differentiated, duct short, membranous, inserted on ventral side of bursa, spermathecal gland large, distinctly capitate, with short membranous duct; sternite VIII with bladal part shortly spatulate, broadly truncate, apodeme stout, straight, slightly longer than bladal part.
Specimens examined. See Pullen et al. (2014: 470) [
2] for details of the type series. Additional specimens: Queensland: Canungra Creek, 4 miles s. of Canungra, 3.ii.1973, G. B. Monteith (1 male, QMB); Wrattens Camp via Widgee, 28.iii.1974, G. B. Monteith (1 female, QMB).
Comments. The species is known from Dorrigo in northern New South Wales northwards to Widgee and Mt. Goonaneman in southern Queensland, with an isolated record also from the Paluma Range in northern Queensland.
Natural history. A short series of specimens in the ANIC is labelled as having been reared from Hoop Pine (Araucaria cunninghamii). The larvae presumably develop in the wood or bark of trunks or branches of this tree species.
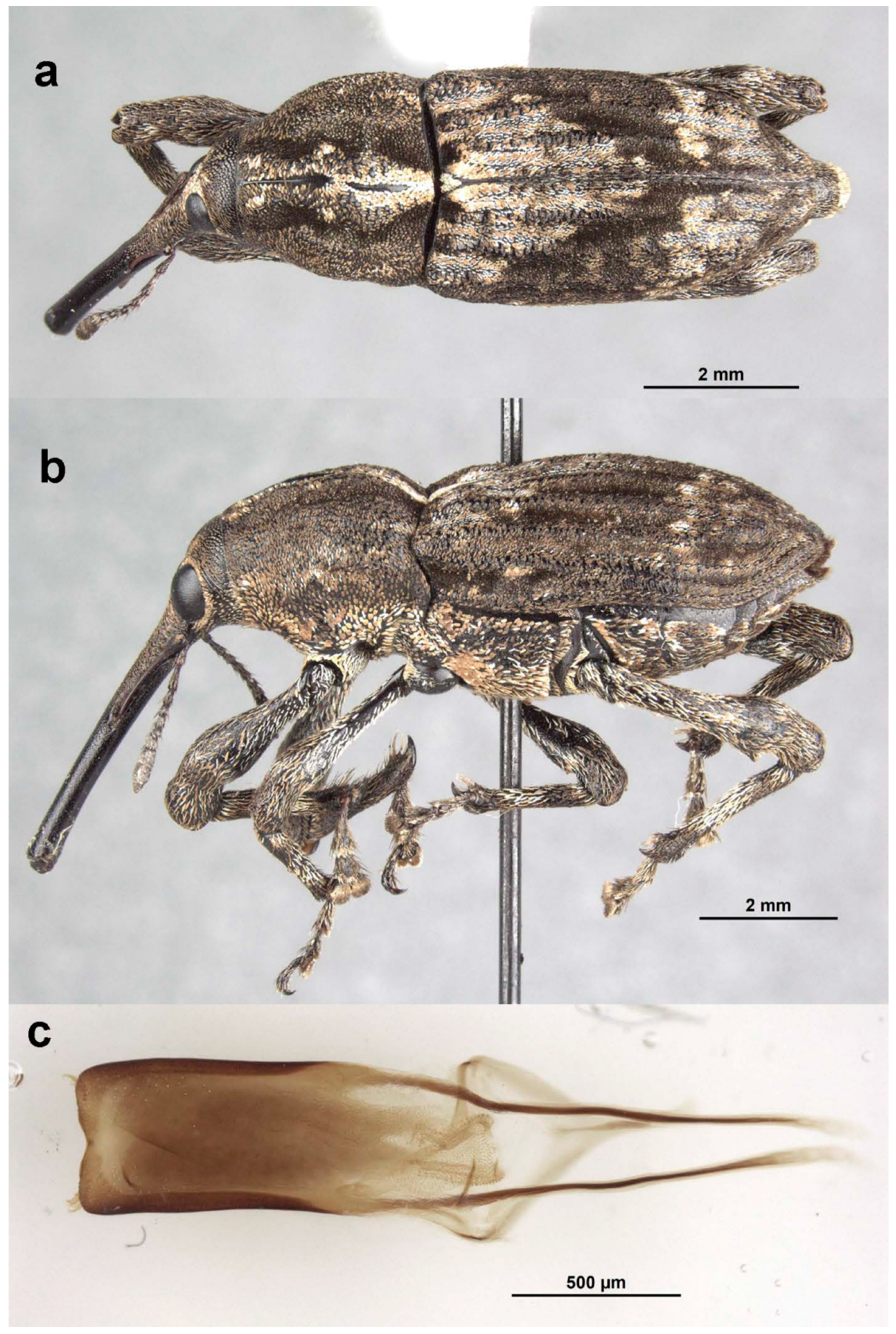
Notopissodes variegatus Oberprieler, new species
Notopissodes sp., Pullen et al., 2014: 470 [
2].
Description Female. Body: length 5.15 mm, width 1.8 mm; rostrum: length 1.4 mm, width at base 0.64 mm, width at antennal insertions 0.35 mm; pronotum: length 1.3 mm, width at middle 1.6 mm; elytra: length 3.4 mm, width across humeri 1.8 mm; antennae: scape length 1.82 mm, length of funicle segment 1 0.2 mm, club length 0.4 mm, club width at middle 0.15 mm. Rostrum as long as pronotum, dorsally behind antennal insertions moderately densely covered with broad, creamy scales; forehead with similar scales adjacent to eyes but denuded around deep median fovea. Pronotum densely covered with large, shallow, open punctures arranged in indistinct circles, interstices between circles narrow and slightly raised, giving surface a granulose appearance, each puncture with a pale hair-scale directed to centre of circles (summit of pronotum, at ca. 0.7 of length). Elytra with interstriae mostly covered with short, broad, subappressed, intermixed white, creamy and black scales, forming an irregular pattern except for yellow and white scales condensed into broad, irregular V stretching from humeri posteromesad onto interstriae 2 and a broad, transverse one on top of declivity stretching onto interstriae 1, interstriae 1–3 on declivity with dense creamy scales. Scutellar shield shorter than wide. For other characters see generic description in Pullen et al. [
2]. Genitalia not examined. Male unknown but probably similar except for slightly shorter rostrum and longer scapes.
Specimens examined. Holotype, female: “Fletcher [−28°46′ 151°51′] / Queensland / E. Sutton” (ANIC).
Comments. This species is only known from a single female, but we deem it appropriate to describe it in the context of this study of all the Araucaria-associated Orthorhinini. It is readily distinguishable from N. pictus by its smaller size and by its different, variegated dorsal scale pattern. Although the single specimen bears an exact locality on its label, it is uncertain whether it was actually collected in Fletcher or rather at a nearby locality where Sutton is known to have collected beetles on hoop pines, such as Rivertree across the border in New South Wales.
Natural history. The species is likely to also live on Hoop Pine (Araucaria cunninghamii) and its larvae to develop in the wood or bark of this tree species.
Derivation of name. The species is named for its variegated color pattern of scales on the elytra; the name is an adjective.
3.2.6. Imbilius Marshall, 1938
Imbilius Marshall, 1938: 9 [
32] (in Hylobiinae: Hylobiina); Zimmerman, 1994: 694 [
14] (transfer to Molytinae); Alonso-Zarazaga & Lyal, 1999: 202 [
5] (catalogue, in Molytinae: Hylobiini); Pullen et al., 2014: 283 [
2] (catalogue, in Molytinae: Orthorhinini).
Type species: Imbilius araucariae Marshall, 1938, by original designation.
Distribution: The genus appears restricted to a small forest area in southern Queensland, Australia, to date known from only two localities and few specimens.
Natural history.Imbilius is associated with Hoop Pine (
Araucaria cunninghamii), several specimens of its single species having been reared from dying or dead trunks of this tree species [
32].
Comments. Marshall’s description of the genus is apt and comprehensive, except for the genitalia (see I. araucariae below).
Imbilius araucariae Marshall, 1938
Imbilius araucariae Marshall, 1938: 9 [
32]; Zimmerman, 1992: 578 [
31] (illustrated in color); Pullen et al., 2014: 283 [
2] (catalogue).
Diagnosis: Body length (exclusive of head and rostrum) 3.8–4.4 mm. Body testaceous, densely covered with small, elongate, truncate, appressed, shiny, greyish-brown (male) to cinnamon-coloured (female) scales (except dorsal anterior two-thirds and venter of rostrum only sparsely setose), lateral side of humeri and posterior pronotal angles with narrow strip of white scales, a few scattered white scales also on elytra, pronotum medially with a pair of small patches of black scales. Rostrum of both sexes slightly shorter than pronotum, weakly downcurved, antennal insertions lateral, in apical third of rostral length. Antennae with funicle 7-segmented, segment 1 as long as 2 + 3, others shorter, slightly transverse; clubs elongate, compact, about 2× wider than funicle. Elytra with interstriae 3 bluntly raised in basal half, interstriae 5 similarly raised from near base to declivity, there forming a blunt flange, 7 and 8 slightly elevated from humeri to declivity; elytral apices produced into separate blunt cones. Femora short, subcylindrical, armed with short tooth on inside at apical third; tibiae stout, shorter than femora, subcylindrical, apically uncinate. Tarsi short and broad; claws divergent, simple but basally swollen. Ventrites 1 and 2 about 3× longer than 3 and 4, at lower level.
Description of genitalia. Male: aedeagus with body of penis narrow, 4.5× longer than wide, subparallel in basal three-quarters of length, at apical quarter slightly constricted, then roundly expanded before strongly tapering to a narrowly acute asetose apex, dorsally closed (sclerotized), ostium flanked by 2 elongate, slanting, sclerotized flanges; temones 0.75× as long as body; internal sac with a pair of elongate, parallel, looped sclerites basally of ostium; tegmen with well sclerotized slanting ring slightly drawn out at parameres, parameres long, narrow, close together, more weakly sclerotized; sternite IX thick, straight, almost symmetrical, bladal part roundly triangular, with thick, forked, sclerotized internal arms, apodeme apically abruptly hooked dorsad. Gonocoxites moderately short, broad, tapering caudad, apically roundly truncate, very weakly sclerotized (specimen teneral); styli apical, moderately long, stout, apically truncate with a few long setae; bursa membranous, large; spermatheca thickly crescentic, basally broad, not differentiated into ramus and collum, duct moderately short, membranous, inserted on ventral side of bursa, spermathecal gland large, shortly elongate, with very short membranous duct; sternite VIII with bladal part spatulate, unsclerotized except for narrow, widely Y-shaped arms, apodeme stout, straight, ca. 1.5× longer than bladal part, apically flared, truncate.
Specimens examined. Imbil State Forest [−25.46° 152.68°], 16.xii.1971, light trap, R. A. Yule (1 male, ANIC); Imbil State Forest, October 1971, ex Araucaria cunninghamii killed by Hyleops glabratus, R. A. Yule (2 females, ANIC); Imbil State Forest, 22.vi.1972, ex Araucaria cunninghamii, R. A. Yule (1 female, ANIC); Amamoor State Forest, 18 km S. Gympie, Dec. 1977, ex Araucaria cunninghamii, M. De Baar (2 females, ANIC).
Comments. The sexes are very similar but the male tends to be darker, less vividly cinnamon-colored. The species is only known from a small area of native forests south of Gympie in southern Queensland and has only been collected a few times.
Natural history. The species was described from six specimens reared from
Araucaria cunninghamii in 1936 by A. Brimblecombe [
32] and a few more reared from the same host several decades later (see above). Its larvae appear to develop in the bark or wood of dead or dying (felled) trees, but nothing has been recorded about its life history.