Assemblage and Species Threshold Responses to Environmental and Disturbance Gradients Shape Bat Diversity in Disturbed Cave Landscapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Environmental and Human Disturbance Gradients
2.3. Bat Sampling
2.4. Ecological and Morphological Traits
2.4.1. Foraging Ecology
2.4.2. Cave Dependence and Hunting
2.5. Data Analysis
2.5.1. Species-Specific Responses to Gradients
2.5.2. Identification of Indicator Species
2.5.3. Assemblage-Level Responses to Gradients
2.5.4. Comparison of Ecological Traits
3. Results
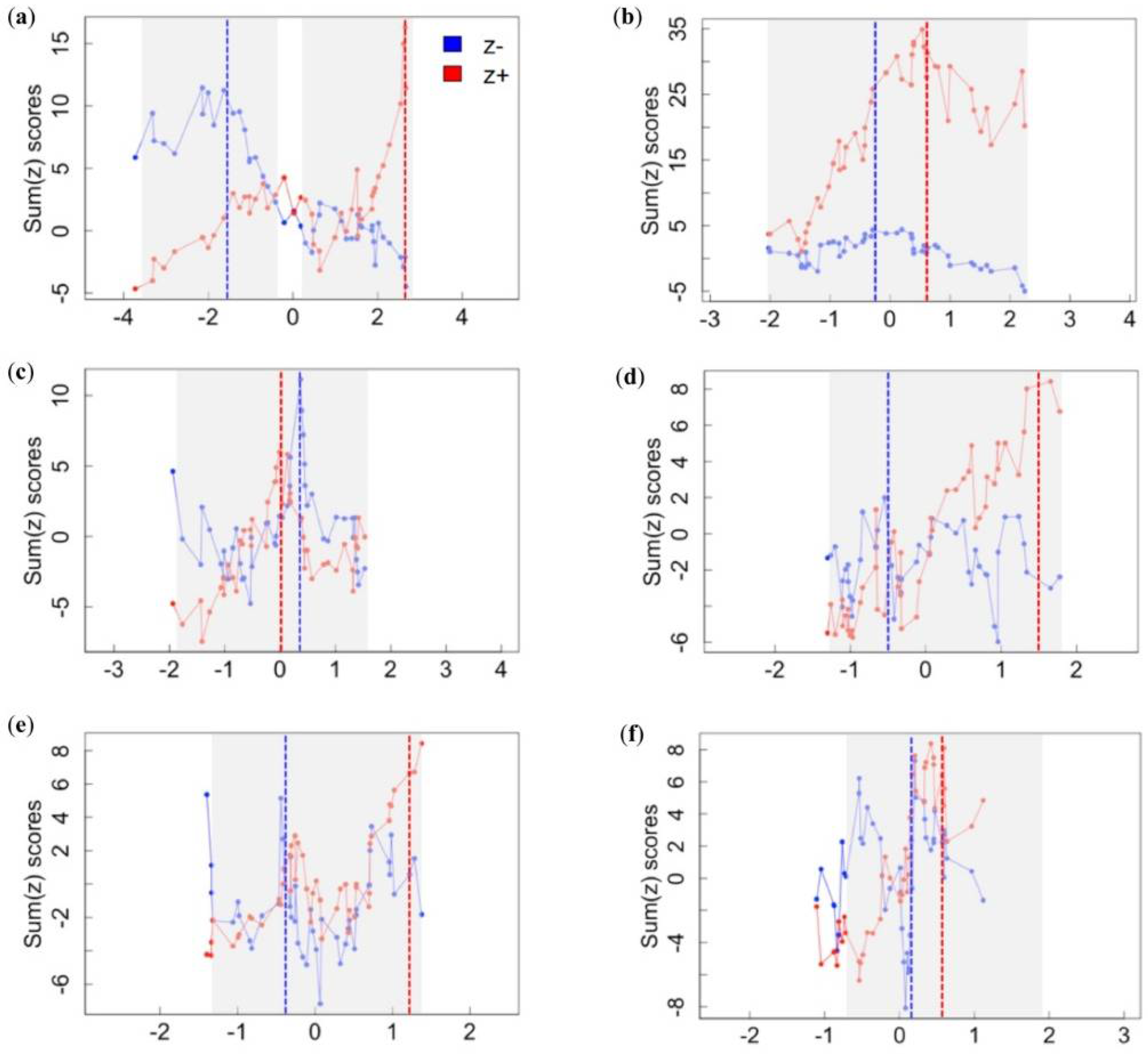
3.1. Assemblage-Level Responses to Gradients
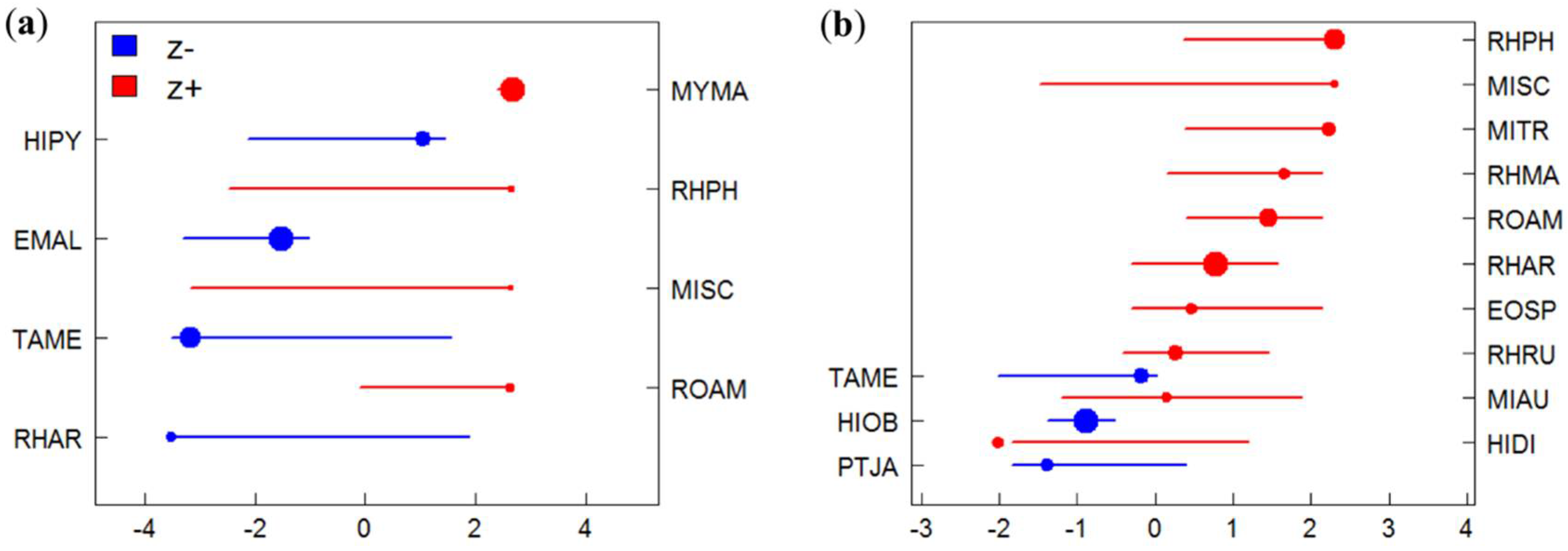
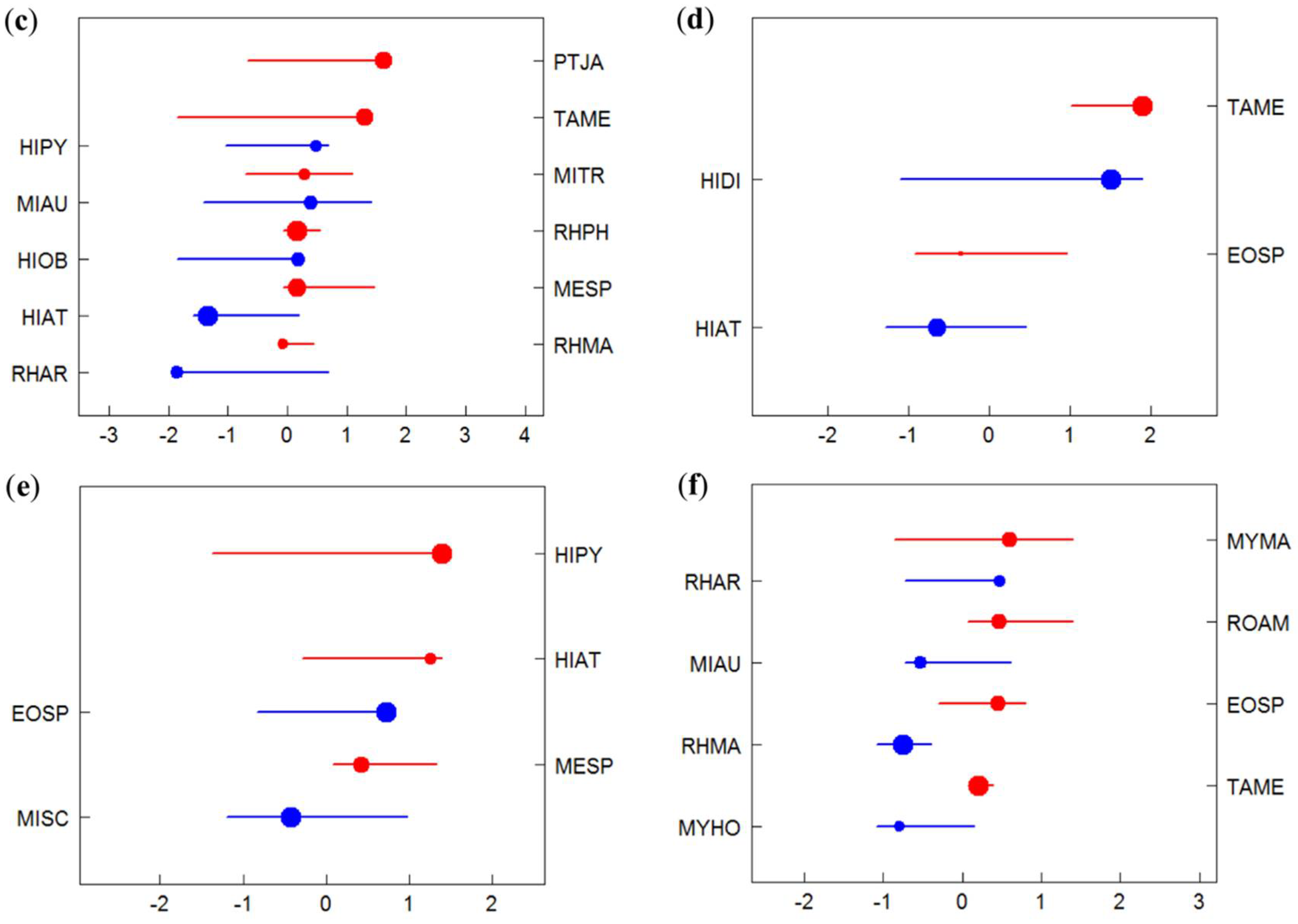
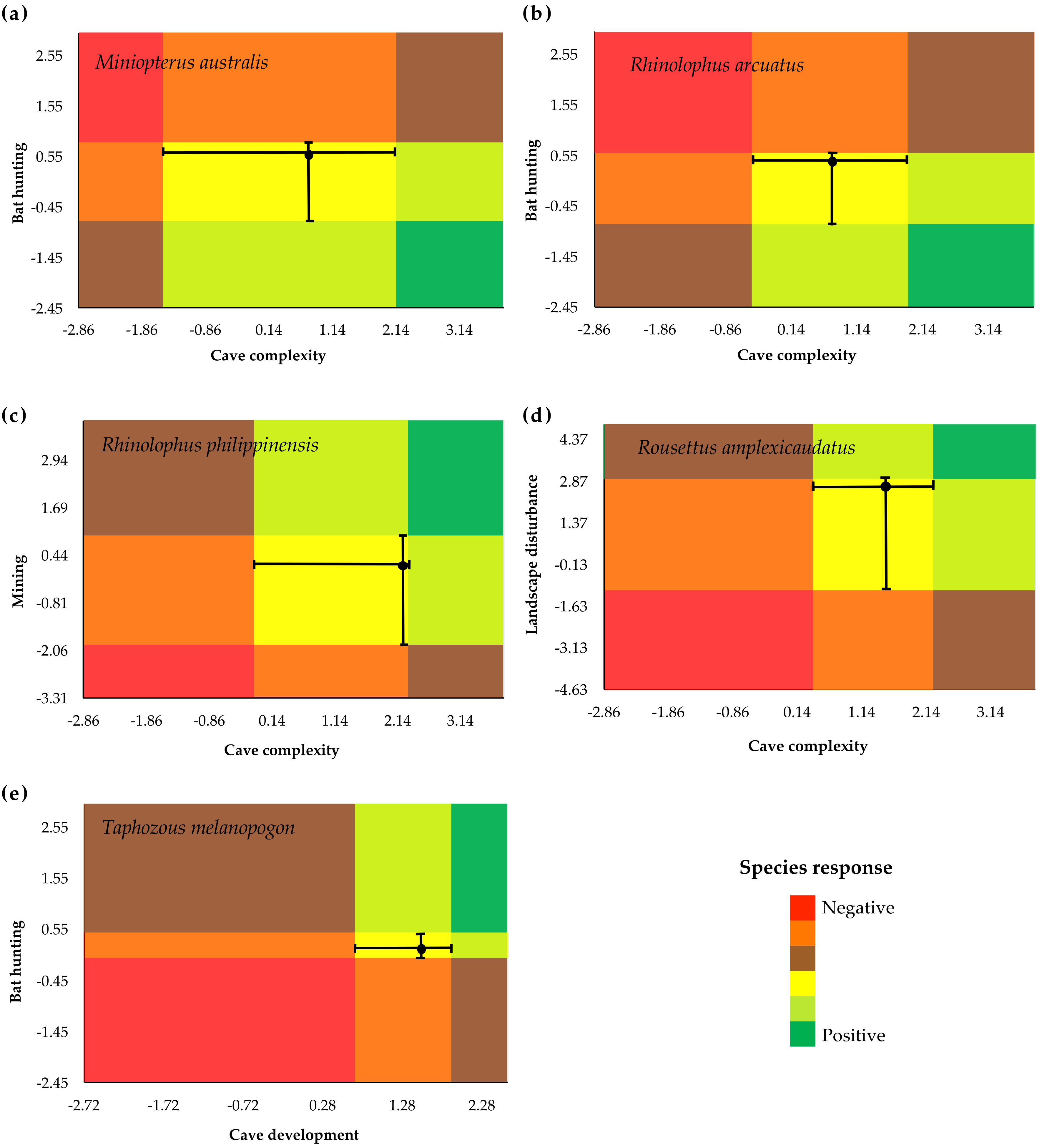
3.2. Species-Specific Responses to Gradients
3.3. Identification of Indicator Species
3.4. Comparison of Ecological Traits
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Groffman, P.M.; Baron, J.S.; Blett, T.; Gold, A.J.; Goodman, I.; Gunderson, L.H.; Levinson, B.M.; Palmer, M.A.; Paerl, H.W.; Peterson, G.D.; et al. Ecological thresholds: The key to successful environmental management or an important concept with no practical application? Ecosystems 2006, 9, 1–13. [Google Scholar] [CrossRef]
- Luck, G.W. An introduction to ecological thresholds. Biol. Conserv. 2005, 124, 299–300. [Google Scholar] [CrossRef]
- Huggett, A.J. The concept and utility of “ecological thresholds” in biodiversity conservation. Biol. Conserv. 2005, 124, 301–310. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.R.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Rompré, G.; Boucher, Y.; Bélanger, L.; Côté, S.; Robinson, W.D. Conserving biodiversity in managed forest landscapes: The use of critical thresholds for habitat. For. Chron. 2010, 86, 589–596. [Google Scholar] [CrossRef]
- Johnson, C.J. Identifying ecological thresholds for regulating human activity: Effective conservation or wishful thinking? Biol. Conserv. 2013, 168, 57–65. [Google Scholar] [CrossRef]
- King, R.S.; Baker, M.E. Considerations for analyzing ecological community thresholds in response to anthropogenic environmental gradients. J. N. Am. Benthol. Soc. 2010, 29, 998–1008. [Google Scholar] [CrossRef]
- Zavaleta, E.; Pasari, J.; Moore, J.; Hernández, D.; Suttle, K.B.; Wilmers, C.C. Ecosystem responses to community disassembly. Ann. N. Y. Acad. Sci. 2009, 1162, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E.; King, R.S. A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods Ecol. Evol. 2010, 1, 25–37. [Google Scholar] [CrossRef]
- Caro, T.M.; O’Doherty, G. On the use of surrogate species in conservation biology. Conserv. Biol. 1999, 13, 805–814. [Google Scholar] [CrossRef]
- Williams, N.N.M.; Crone, E.E.E.; T’ai, H.; Roulston, T.H.; Minckley, R.L.; Packer, L.; Potts, S.G. Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 2010, 143, 2280–2291. [Google Scholar] [CrossRef]
- Purvis, A.; Gittleman, J.L.; Cowlishaw, G.; Mace, G.M. Predicting extinction risk in declining species. Proc. R. Soc. B Biol. Sci. 2000, 267, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Purvis, A.; Gittleman, J.L. Biological correlates of extinction risk in bats. Am. Nat. 2003, 161, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, K.; Voigt, C.C. Zoonotic viruses and conservation of bats. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 263–292. ISBN 978-3-319-25218-6. [Google Scholar]
- Furey, N.M.; Racey, P.A. Conservation ecology of cave bats. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 463–500. [Google Scholar]
- Phelps, K.; Jose, R.; Labonite, M.; Kingston, T. Correlates of cave-roosting bat diversity as an effective tool to identify priority caves. Biol. Conserv. 2016, 201, 201–209. [Google Scholar] [CrossRef]
- Medellin, R.A.; Wiederholt, R.; Lopez-Hoffman, L. Conservation relevance of bat caves for biodiversity and ecosystem services. Biol. Conserv. 2017, 1–6. [Google Scholar] [CrossRef]
- Tanalgo, K.C.; Tabora, J.A.G.; Hughes, A.C. Bat cave vulnerability index (BCVI): A holistic rapid assessment tool to identify priorities for effective cave conservation in the tropics. Ecol. Indic. 2018, 89, 852–860. [Google Scholar] [CrossRef]
- Schmieder, D.A.; Kingston, T.; Hashim, R.; Siemers, B.M. Sensory constraints on prey detection performance in an ensemble of vespertilionid understorey rain forest bats. Funct. Ecol. 2012, 26, 1043–1053. [Google Scholar] [CrossRef]
- Senawi, J.; Schmieder, D.; Siemers, B.; Kingston, T. Beyond size—Morphological predictors of bite force in a diverse insectivorous bat assemblage from Malaysia. Funct. Ecol. 2015, 29, 1411–1420. [Google Scholar] [CrossRef]
- Kingston, T.; Francis, C.C.M.; Akbar, Z.; Kunz, T.H. Species richness in an insectivorous bat assemblage from Malaysia. J. Trop. Ecol. 2003, 19, 1–12. [Google Scholar] [CrossRef]
- Kingston, T. Response of Bat Diversity to Forest Disturbance in Southeast Asia: Insights from Long-Term Research in Malaysia; Adams, R.A., Pedersen, S.C., Eds.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-7396-1. [Google Scholar]
- Furey, N.M.; Racey, P.A. Can wing morphology inform conservation priorities for Southeast Asian cave bats? Biotropica 2016, 48, 545–556. [Google Scholar] [CrossRef]
- Mildenstein, T.; Tanshi, I.; Racey, P.A. Exploitation of bats for bushmeat and medicine. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer International Publishing: Cham, Switzerland, 2016; pp. 325–375. [Google Scholar]
- Urich, P.; Day, M.; Lynagh, F. Policy and practice in karst landscape protection: Bohol, the Philippines. Geogr. J. 2001, 167, 305–323. [Google Scholar] [CrossRef]
- Ellis, B.M. Cave surveys. In The Science of Speleology; Ford, T.D., Cullingford, C.H.D., Eds.; Academic Press: London, UK, 1976; pp. 213–266. [Google Scholar]
- Brunet, A.K.; Medellín, R.A. The species-area relationship in bat assemblages of tropical caves. J. Mammal. 2001, 82, 1114–1122. [Google Scholar] [CrossRef]
- Arita, H.T. The conservation of cave-roosting bats in Yucatan, Mexico. Biol. Conserv. 1996, 76, 177–185. [Google Scholar] [CrossRef]
- Harlow, L.L. The Essence of Multivariate Thinking: Basic Themes and Methods, 2nd ed.; Routledge: New York, NY, USA, 2014; ISBN 041487372X. [Google Scholar]
- Ingle, N.R.; Heaney, L.R. A key to the bats of the Philippine Islands. Fieldiana Zool. 1992, 69, 1–44. [Google Scholar] [CrossRef]
- Sikes, R.S.; Gannon, W.L. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Heaney, L.R.; Dolar, M.L.; Balete, D.S.; Esselstyn, J.A.; Rickart, E.A.; Sedlock, J.L. Synopsis of Philippine mammals. Available online: http://archive.fieldmuseum.org/philippine_mammals/index.html (accessed on 10 March 2016).
- Francis, C.M. A Guide to the Mammals of Southeast Asia; Princeton University Press: Princeton, NJ, USA, 2008; ISBN 978-0691135519. [Google Scholar]
- Norberg, U.M.; Rayner, J.M.V. Ecological morphology and flight in bats (Mammalia; Chiroptera): Wing adaptations, flight performance, foraging strategy and echolocation. Philos. Trans. R. Soc. B Biol. Sci. 1987, 316, 335–427. [Google Scholar] [CrossRef]
- Denzinger, A.; Schnitzler, H.-U. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front. Physiol. 2013, 4, 164. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Tomaschewski, I. Chemical composition of leaves consumed by Ptenochirus jagori (Pteropodidae). Mamm. Biol. Z. Säugetierkd. 2003, 68, 112–115. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Available online: http://www.iucnredlist.org (accessed on 10 February 2016).
- Sedlock, J.L.; Jose, R.P.; Vogt, J.M.; Paguntalan, L.M.J.; Cariño, A.B. A survey of bats in a karst landscape in the central Philippines. Acta Chiropterol. 2014, 16, 197–211. [Google Scholar] [CrossRef]
- Mickleburgh, S.P.; Hutson, A.M.; Racey, P.A. Old World Fruit Bats: An Action Plan for Their Conservation; IUCN: Grand, Switzerland, 1992; ISBN 2831700558. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Dinno, A. dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums 2016, R Package Version 1.3.2. Available online: http://CRAN.R-project.org/package=dunn.test (accessed on May 2016).
- R Core Team. R: A Language and Environment for Statistical Computing 2017; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Doran, N.E.N.; Kiernan, K.; Swain, R.; Richardson, A.M.M. Hickmania troglodytes, the Tasmanian cave spider, and its potential role in cave management. J. Insect Conserv. 1999, 3, 257–262. [Google Scholar] [CrossRef]
- Pellegrini, T.G.; Sales, L.P.; Anguiar, P.; Ferreira, R.L. Linking spatial scale dependence of land-use descriptors and invertebrate cave community composition. Subterr. Biol. 2016, 18, 17–38. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Fischer, J.; Cunningham, R.B. Native vegetation cover thresholds associated with species responses. Biol. Conserv. 2005, 124, 311–316. [Google Scholar] [CrossRef]
- Suarez-Rubio, M.; Wilson, S.; Leimgruber, P.; Lookingbill, T. Threshold responses of forest birds to landscape changes around exurban development. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gómez, E.S.; Moreno, C.E.; García-Morales, R.; Zuria, I.; Sánchez-Rojas, G.; Briones-Salas, M. Deforestation thresholds for phyllostomid bat populations in tropical landscapes in the Huasteca region, Mexico. Trop. Conserv. Sci. 2015, 8, 646–661. [Google Scholar] [CrossRef]
- Berger, E.; Haase, P.; Oetken, M.; Sundermann, A. Field data reveal low critical chemical concentrations for river benthic invertebrates. Sci. Total Environ. 2016, 544, 864–873. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Legendre, P.; Wiser, S.K.; Brotons, L. Using species combinations in indicator value analyses. Methods Ecol. Evol. 2012, 3, 973–982. [Google Scholar] [CrossRef]
- Vandewalle, M.; de Bello, F.; Berg, M.P.; Bolger, T.; Dolédec, S.; Dubs, F.; Feld, C.K.; Harrington, R.; Harrison, P.A.; Lavorel, S.; et al. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodivers. Conserv. 2010, 19, 2921–2947. [Google Scholar] [CrossRef]
- Meyer, C.F.J.; Fründ, J.; Lizano, W.P.; Kalko, E.K.V. Ecological correlates of vulnerability to fragmentation in Neotropical bats. J. Appl. Ecol. 2007, 45, 381–391. [Google Scholar] [CrossRef]
- Farneda, F.Z.; Rocha, R.; López-Baucells, A.; Groenenberg, M.; Silva, I.; Palmeirim, J.M.; Bobrowiec, P.E.D.; Meyer, C.F.J. Trait-related responses to habitat fragmentation in Amazonian bats. J. Appl. Ecol. 2015, 52, 1381–1391. [Google Scholar] [CrossRef]
- Frank, H.K.; Frishkoff, L.O.; Mendenhall, C.D.; Daily, G.C.; Hadly, E.A. Phylogeny, traits, and biodiversity of a Neotropical bat assemblage: Close relatives show similar responses to local deforestation. Am. Nat. 2017, 190, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Hanspach, J.; Fischer, J.; Ikin, K.; Stott, J.; Law, B.S. Using trait-based filtering as a predictive framework for conservation: A case study of bats on farms in southeastern Australia. J. Appl. Ecol. 2012, 49, 842–850. [Google Scholar] [CrossRef]
- Tababa, R.; Dagunan, M.A.; Dejano, B.; De La Cruz, S.; Gutierrez, B.; Alcala, E.; Averia, L. Preliminary results of the cave bat assessment conducted at Central Negros, Philippines. LCC Dev. Educ. J. Multidiscip. Res. 2012, 1, 85–99. [Google Scholar]
- Tanalgo, K.C.; Tabora, J.A.G. Cave-dwelling bats (Mammalia: Chiroptera) and conservation concerns in South central Mindanao, Philippines. J. Threat. Taxa 2015, 7, 8185. [Google Scholar] [CrossRef]
- Nurul-Ain, E.; Rosli, H.; Kingston, T. Resource availability and roosting ecology shape reproductive phenology of rain forest insectivorous bats. Biotropica 2017, 49, 382–394. [Google Scholar] [CrossRef]
- Foley, M.M.; Martone, R.G.; Fox, M.D.; Kappel, C.V.; Mease, L.A.; Erickson, A.L.; Halpern, B.S.; Selkoe, K.A.; Taylor, P.; Scarborough, C. Using ecological thresholds to inform resource management: Current options and future possibilities. Front. Mar. Sci. 2015, 2, 95. [Google Scholar] [CrossRef]
- Kelly, R.P.; Erickson, A.L.; Mease, L.A.; Battista, W.; Kittinger, J.N.; Fujita, R. Embracing thresholds for better environmental management. Philos. Trans. R. Soc. B Biol. Sci. 2014, 370, 20130276. [Google Scholar] [CrossRef]
- Muylaert, R.L.; Stevens, R.D.; Ribeiro, M.C. Threshold effect of habitat loss on bat richness in cerrado-forest landscapes. Ecol. Appl. 2016, 26, 1854–1867. [Google Scholar] [CrossRef] [PubMed]
- Gnaspini, P.; Trajano, E. Guano communities in tropical caves. In Ecosystems of the World: Subterranean Ecosystems; Wilkens, H., Culver, D.C., Humphrey, W.F., Eds.; Elsevier: New York, NY, USA, 2000; pp. 251–268. ISBN 0444822992. [Google Scholar]
- Kunz, T.H.; de Torrez, E.B.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]

| Factors | Method | Description | Landscape Disturbance | Cave Complexity | Mining | Cave Development | Resource Extraction | Hunting |
|---|---|---|---|---|---|---|---|---|
| Available roosting area | Cave surveys | Upper half of total surface area (m2) of a cave available to roosting bats, calculated following published methods and equations [27]. | 0.10 [0.01–0.22] | 0.50 [0.16–0.51] | −0.04 [−0.46–−0.02] | 0.11 [0.01–0.38] | −0.26 [−0.38–−0.02] | −0.12 [−0.35–−0.02] |
| Structural variability | Total cave length divided by the greatest length between two survey stations [28]. Single tunnel caves have a structural variability of 1.0, with an increasing value indicating greater complexity in chamber and passageways. | 0.21 [0.08–0.28] | 0.39 [0.08–0.45] | −0.19 [−0.41–−0.02] | −0.09 [−0.40–−0.01] | −0.23 [−0.37–−0.01] | 0.06 [0.01–0.35] | |
| Number of entrances | Total number of cave entrances, including vertical entrances. | 0.13 [0.11–0.21] | 0.37 [0.09–0.45] | −0.16 [−0.42–−0.01] | 0.08 [0.01–0.36] | −0.14 [−0.43–−0.02] | 0.07 [0.02–0.58] | |
| Temperature range | Difference between maximum and minimum temperature values (°C) recorded at survey stations using a hand-held weather station. | 0.11 [0.01–0.22] | 0.35 [0.04–0.48] | −0.35 [−0.52–−0.03] | 0.17 [0.02–0.49] | 0.27 [0.02–0.45] | −0.07 [−0.45–−0.01] | |
| Humidity range | Difference between maximum and minimum humidity values (%) recorded as above. | 0.10 [0.02–0.19] | 0.05 [0.02–0.41] | −0.47 [−0.54–−0.06] | −0.13 [−0.53–−0.02] | 0.50 [0.03–0.57] | −0.18 [−0.54–−0.02] | |
| Non-forested habitat | Google Earth images & visual observations in 1-km radius | Percentage of non-forested habitat calculated using ImageJ analysis software (https://imagej.nih.gov). | 0.35 [0.32–0.38] | −0.16 [−0.25–−0.02] | −0.09 [−0.23–−0.01] | −0.05 [−0.20–−0.01] | −0.01 [−0.25–−0.01] | 0.02 [0.01–0.28] |
| Urbanization | Number of human residents. | 0.35 [0.30–0.38] | −0.17 [−0.27–−0.02] | −0.15 [−0.27–−0.02] | −0.06 [−0.24–−0.01] | 0.17 [0.01–0.28] | 0.10 [0.01–0.29] | |
| Distance to village | Euclidean distance (km) to the center of the nearest village/neighborhood and point of access (e.g., foot trail, road) to the cave. | 0.33 [0.27–0.37] | −0.25 [−0.34–−0.06] | −0.14 [−0.30–−0.01] | −0.09 [−0.23–−0.02] | −0.04 [−0.22–−0.02] | −0.06 [−0.26–−0.02] | |
| Distance to access | 0.21 [0.12–0.29] | 0.13 [0.02–0.40] | 0.41 [0.03–0.46] | −0.06 [−0.44–−0.02] | 0.10 [0.01–0.44] | −0.42 [−0.46–−0.12] | ||
| Protected area | Scored binarily: 1 (cave lies within boundaries of protected area), or 0 (not within boundaries). | 0.28 [0.21–0.32] | −0.01 [−0.27–0.02] | 0.02 [0.01–0.41] | 0.48 [0.04–0.49] | 0.06 [0.01–0.43] | 0.15 [0.02–0.39] | |
| Road development | Most developed roadway (e.g., highway, dirt lane) observed in 1-km radius of cave entrance. | −0.07 [−0.19–−0.01] | 0.28 [0.03–0.43] | 0.25 [0.03–0.50] | −0.47 [−0.62–−0.03] | 0.36 [0.02–0.56] | −0.26 [−0.50–−0.02] | |
| Mining | Visual observations & resident interviews | Percentage of the cave area affected by a specific disturbance type: mining for limestone and/or phosphate, modification of the cave structure to accommodate humans (e.g., tourists, miners), dumping of household waste (e.g., trash, sewage) and vandalism/graffiti. | 0.17 [0.05–0.26] | 0.19 [0.02–0.35] | 0.25 [0.02–0.42] | −0.20 [−0.49–−0.02] | 0.19 [0.02–0.57] | 0.58 [−0.59–−0.03] |
| Cave modification | 0.27 [0.20–0.31] | 0.05 [0.01–0.31] | 0.30 [0.02–0.43] | −0.09 [−0.41–−0.01] | 0.10 [0.01–0.46] | 0.37 [−0.46–−0.02] | ||
| Household waste | 0.28 [0.20–0.32] | 0.15 [0.02–0.32] | 0.24 [0.02–0.35] | 0.23 [0.01–0.37] | 0.00 [0.01–0.31] | −0.07 [−0.34–−0.01] | ||
| Vandalism/graffiti | 0.07 [0.01–0.19] | −0.05 [−0.35–−0.01] | 0.29 [0.03–0.49] | 0.48 [0.04–0.61] | 0.34 [0.02–0.64] | −0.26 [−0.59–−0.08] | ||
| Resource extraction | Proportion of interviewee responses that reported they or other residents visited the cave to extract resources (e.g., guano, swiftlet nests, water) or hunt bats. | −0.29 [−0.33–−0.23] | 0.11 [0.01–0.23] | 0.02 [0.01–0.28] | 0.28 [0.02–0.35] | −0.10 [−0.35–−0.01] | 0.02 [0.01–0.39] | |
| Bat hunting | −0.32 [−0.36–−0.25] | 0.23 [0.03–0.32] | 0.08 [0.01–0.27] | −0.02 [−0.26–−0.01] | 0.15 [0.01–0.30] | 0.16 [0.01–0.34] | ||
| Visitation frequency | Greatest visitation frequency (i.e., daily, weekly, monthly or yearly/never) indicated by two or more interviewees. | −0.26 [−0.31–−0.17] | 0.03 [0.01–0.25] | −0.10 [−0.35–−0.01] | 0.22 [0.02–0.46] | 0.41 [0.02–0.50] | 0.31 [0.02–0.49] |
| Gradient | Response Group | Assemblage Threshold | Confidence Limits | ||||
|---|---|---|---|---|---|---|---|
| 0.05 | 0.10 | 0.50 | 0.90 | 0.95 | |||
| Landscape disturbance | sum(z−) | −1.53 | −3.52 | −3.30 | −2.13 | −1.02 | −0.10 |
| sum(z+) | 2.66 | 0.10 | 0.63 | 2.66 | 2.68 | 2.68 | |
| Cave complexity | sum(z−) | −0.18 | −2.01 | −1.61 | −0.81 | −0.18 | 0.16 |
| sum(z+) | 0.59 | 0.02 | 0.27 | 0.69 | 2.14 | 2.22 | |
| Mining | sum(z−) | 0.38 | −1.85 | −1.85 | 0.08 | 0.47 | 0.68 |
| sum(z+) | 0.01 | −0.24 | −0.09 | 0.18 | 1.47 | 1.63 | |
| Cave development | sum(z−) | −0.50 | −1.28 | −1.23 | −0.65 | 1.01 | 1.27 |
| sum(z+) | 1.50 | −0.02 | 0.18 | 0.86 | 1.72 | 1.89 | |
| Resource extraction | sum(z−) | −0.43 | −1.37 | −1.34 | −0.99 | 0.52 | 0.70 |
| sum(z+) | 1.25 | −0.20 | 0.04 | 1.12 | 1.39 | 1.39 | |
| Bat hunting | sum(z−) | 0.21 | −1.08 | −1.08 | −0.25 | 0.33 | 0.38 |
| sum(z+) | 0.60 | 0.07 | 0.18 | 0.52 | 1.40 | 1.40 | |
| Gradients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Landscape Disturbance | Cave Complexity | Mining | Cave Development | Resource Extraction | Bat Hunting | |||||||
| Categorical traits | χ2 | p-value | χ2 | p-value | χ2 | p-value | χ2 | p-value | χ2 | p-value | χ2 | p-value |
| Endemism | 0.01 | 0.91 | 1.47 | 0.22 | 0.01 | 0.91 | 1.02 | 0.31 | 0.80 | 0.37 | 0.10 | 0.75 |
| Dietary niche | 3.21 | 0.07 | 0.09 | 0.76 | 0.28 | 0.60 | 0.28 | 0.60 | 1.64 | 0.20 | 2.03 | 0.15 |
| Roost dependence | 0.42 | 0.51 | 5.43 | 0.02 | 0.09 | 0.76 | 0.42 | 0.51 | 0.65 | 0.42 | 0.05 | 0.83 |
| Foraging space | 4.85 | 0.09 | 0.18 | 0.91 | 0.57 | 0.75 | 0.57 | 0.75 | 6.38 | 0.04 | 2.11 | 0.35 |
| Colony size | 2.15 | 0.37 | 1.12 | 0.57 | 0.48 | 0.79 | 0.15 | 0.93 | 5.11 | 0.08 | 5.73 | 0.06 |
| Continuous traits | H | p-value | H | p-value | H | p-value | H | p-value | H | p-value | H | p-value |
| Aspect ratio | 0.81 | 0.36 | 0.31 | 0.58 | 0.03 | 0.87 | 0.06 | 0.81 | 1.97 | 0.16 | 0.00 | 1.00 |
| Wing loading | 2.16 | 0.14 | 1.93 | 0.16 | 2.41 | 0.12 | 3.84 | 0.05 | 3.08 | 0.08 | 4.81 | 0.03 |
| Peak frequency | 0.22 | 0.64 | 0.15 | 0.70 | 8.07 | <0.01 | 0.02 | 0.90 | 0.36 | 0.55 | 0.30 | 0.59 |
| Body mass | 4.86 | 0.03 | 0.55 | 0.46 | 3.53 | 0.06 | 1.50 | 0.22 | 2.49 | 0.11 | 4.81 | 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phelps, K.; Jose, R.; Labonite, M.; Kingston, T. Assemblage and Species Threshold Responses to Environmental and Disturbance Gradients Shape Bat Diversity in Disturbed Cave Landscapes. Diversity 2018, 10, 55. https://doi.org/10.3390/d10030055
Phelps K, Jose R, Labonite M, Kingston T. Assemblage and Species Threshold Responses to Environmental and Disturbance Gradients Shape Bat Diversity in Disturbed Cave Landscapes. Diversity. 2018; 10(3):55. https://doi.org/10.3390/d10030055
Chicago/Turabian StylePhelps, Kendra, Reizl Jose, Marina Labonite, and Tigga Kingston. 2018. "Assemblage and Species Threshold Responses to Environmental and Disturbance Gradients Shape Bat Diversity in Disturbed Cave Landscapes" Diversity 10, no. 3: 55. https://doi.org/10.3390/d10030055
APA StylePhelps, K., Jose, R., Labonite, M., & Kingston, T. (2018). Assemblage and Species Threshold Responses to Environmental and Disturbance Gradients Shape Bat Diversity in Disturbed Cave Landscapes. Diversity, 10(3), 55. https://doi.org/10.3390/d10030055





