Abstract
The thioether-functionalized 2-azabutadienes (ArS)2C=C(H)-N=CPh2 (L1 Ar = Ph, L2 Ar = p-Tol) ligate to [Mn(CO)5Br] to form the octahedral five-membered S, N-chelate complexes fac-[MnBr(CO)3{(ArS)2C=C(H)-N=CPh2] (1 Ar = Ph; 2 Ar = p-Tol), whose crystal structures have been solved by X-ray diffraction. Complex 1 crystallizes in the non-centrosymmetric orthorhombic space group P212121, whereas 2 crystallizes in the triclinic space group P. The secondary interactions occurring in the packing have also been assessed by an Atoms in Molecules (AIM) topological analysis.
1. Introduction
2-Azabutadienes R2C=C(H)-N=CR′2 (R, R′ = Hal, alkyl, aryl) represent an interesting class of unsaturated compounds since they bear a π-conjugated C=C and C=N array in their scaffold, conferring them a rich potential both in organic chemistry and as ligands in coordination chemistry [1,2,3,4,5,6]. The simplest compound, H2C=C(H)-N=CH2, has been the object of several theoretical studies and its occurrence has been spectroscopically evidenced in the atmosphere of Titan [7]. Our research groups are notably working on the design and the reactivity of Cl2C=C(H)-N=CAr2 via 1,3-dipolar cycloaddition [8] and have demonstrated that the chlorine atoms therein can be readily substituted by thiolates NaSR, producing thioether-functionalized 2-azabutadienes (RS)2C=C(H)-N=CAr2 [9,10,11]. These latter compounds are valuable chelating ligands in coordination chemistry, since they can be chelated as S,N donors to a variety of transition metals and have been recently crystallographically characterized for [CdI2{(iPrS)2C=C(H)-N=CPh2}], [HgI2{(iPrS)2C=C(H)-N=CPh2}], and the dinuclear Cu(I) complex [LCu(μ2-I2)CuL] (L = iPrS)2C=C(H)-N=CPh2) [12]. In the context of our interest in the photophysical properties of transition metal complexes, we have recently prepared an extended series of octahedral fac-[(OC)3ReX{(RS)2C=C(H)-N=CAr2}] (X = Cl, Br, I, triflate) complexes [13]. In continuation of this project, we have prepared a series of related Mn(I) complexes fac-[(OC)3MnBr{(RS)2C=C(H)-N=CPh2}] and present in this communication their synthesis and crystal structures. Note that in contrast to the numerous [(OC)3MnX(N∩N)] complexes bearing chelating N,N donor ligands, notably of the α-diimine type [14], only three entries are found in the CSD data base (CSD release on 1 January 2025) [15] for related [(OC)3MnBr(S∩N)] complexes, namely bromo-tricarbonyl-(2-(phenylsulfanyl)-N-[2-(phenylsulfanyl)ethyl]ethan-1-amine)manganese A (CSD refcode DIXLEE), tricarbonyl-bromo-(2-(phenylthiomethyl)pyridine-N,S)manganese B (CSD refcode IHIMIV) and bromotris(carbonyl)-{2-(ethylsulfanyl)-N-[(quinolin-4-yl)methyl]ethan-1-amine}manganese C (CSD refcode DOKXAG) (Scheme 1). Furthermore, there are two hits for dicarbonyl complexes of the type [(OC)2MnX{(S∩N∩N)}] such as bromo-dicarbonyl-(N-(2-(methylsulfanyl)phenyl)-1-(pyridin-2-yl) ethanimine)-manganese D (CSD refcode RODKAY) and bromo-dicarbonyl-(1-(6-(4-fluorophenyl)pyridin-2-yl)-N-(2-(methylsulfanyl)phenyl)methanimine)-manganese E (CSD refcode RODKIG), depicted in Scheme 1 [16,17,18,19]. Very recently, the crystal structure of the chelate complex fac-[Mn(CO)3Br{C5H4N-2-NH(P(=S)Ph2)}] F with a diphenylphosphinesulfide function as the donor has also been reported [20]. Note that there are no entries for any chloro- or iodo manganese S,N-complexes. Interestingly, compounds A and C are also biologically active. For related crystallographically characterized [(OC)3ReBr{(S∩N)] chelates, see references [21,22].
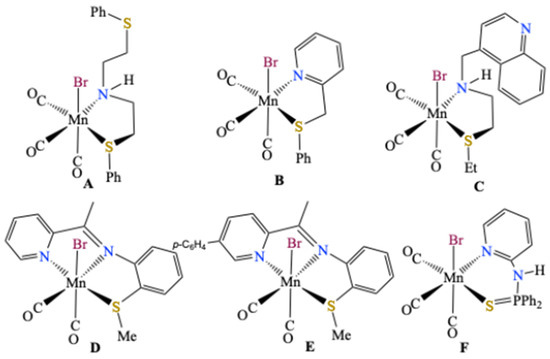
Scheme 1.
Examples of structural characterized [MnBr(CO)3S∩N] and [(OC)2MnBr{(S∩N∩N)] chelate complexes [16,17,18,19,20].
2. Results and Discussion
Series of complexes fac-[MnBr(CO)3{(ArS)2C=C(H)-N=CPh2}] 1 and 2 was obtained via treatment of [Mn(CO)5Br] with an equimolar amount of the corresponding azabutadiene ligand L in refluxing chloroform solution, as shown in Scheme 2. Upon cooling, small red crystals of air-stable 1 and 2 were isolated.
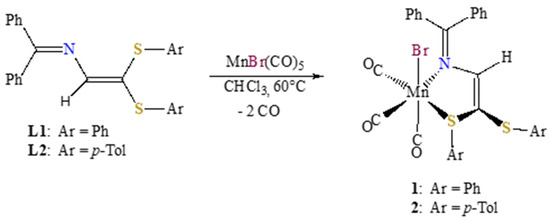
Scheme 2.
Synthesis of the S,N-chelate complexes 1 and 2.
The IR spectra of the isolated products recorded in CH2Cl2, shown in Figure S1, are very distinctive in the carbonyl region. The infrared spectrum of 1 reveals three intense carbonyl vibrations at 2031 (vs), 1959 (s) and 1923 (s) cm−1, a characteristic pattern for a fac-arrangement of the CO ligands. The 1H NMR spectrum of 1 is not informative, since the resonance of the vinylic C=C(H), which appears quite distinct in the region at about δ 6.9–7.1 for the free ligands, is superposed by the aromatic signals. In the 1H NMR spectrum of 2 recorded in CDCl3, two distinct resonances attributed to methyl groups of the coordinated and uncoordinated p-tolyl substituents are found at δ 2.37 and 2.32 ppm (see Supporting Material).
In addition to the spectroscopic characterization of 1 in solution, the complexes were also unambiguously analyzed using X-ray diffraction performed at 115 K. Overall, the structures are very reminiscent of those of their Re(I) counterparts fac-[(OC)3ReBr{(ArS)2C=C(H)-N=CArPh2}] [13]. Complex 1 crystallizes in the orthorhombic space group P212121, containing a molecule of dichloromethane, whereas complex 2 crystallizes in the triclinic space group P.
Both complexes studied here exhibit a fac-octahedral geometry, in which the facial carbonyl groups, one bidentate S,N–azabutadiene chelate and one bromide form the coordination sphere around the central Mn (I) atom. The second SAr substituent is dangling and does not participate in bonding. The molecular structures are presented in Figure 1 and Figure 2 for 1 and 2, respectively. In order to allow a direct comparison of the molecular geometries, the enantiomer S is shown for 2. Complexation of L2 on Mn(I) does not alter the C=N and C=C bond lengths much with respect to those reported for the free ligand (1.321(3) vs. 1.2959(17) and 1.333(3) vs. 1.3515(18) Å) [10]. The mean Mn-Br bond length of 1 and 2 (2.5301(4) Å) fits with those reported for complexes A (2.5288(4) Å) and B (2.5382(5) Å) shown in Scheme 1. Also, the Mn-SPh distance of 1 (2.3454(7) Å) matches with those reported for complexes A and B (2.3748(6) and 2.3449(6) Å) [16,17].
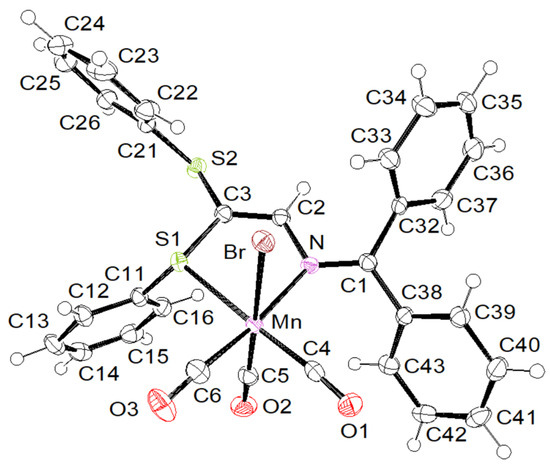
Figure 1.
ORTEP of the molecular structure of 1 at the 50% probability level.
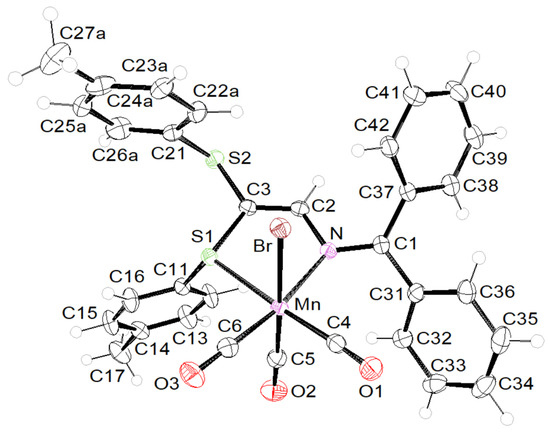
Figure 2.
ORTEP of the molecular structure (enantiomer S) of 2 at the 50% probability level.
The most relevant bond lengths around the manganese atom and within the azabutadiene linkage together with selected bond angles observed for the two complexes are presented in Table 1.

Table 1.
Selected bond lengths (Å) and angles (°) in complexes 1 and 2.
We state that these metric parameters vary very little from one complex to another, indicating that the change in the ligand L does not significantly influence the molecular dimensions. On the other hand, the noncovalent interactions in the packing are different. They are discussed below.
Supramolecular Features
The system of noncovalent interactions giving rise to a supramolecular network is very rich in the crystal structure of 1.
The most frequently encountered mode of noncovalent interactions in 1 is hydrogen bonding. It occurs between the C-H···Br, C-H···O and C-H···Cl (from solvent CH2Cl2 molecule) donor···acceptor couples and is partially depicted in Figure 3. The bromine atom is involved in three H-bonds with one solvent molecule and two other adjacent complex molecules. Furthermore, the oxygen atoms of three carbonyl groups are bound to hydrogen atoms of three different complex molecules. The solvent molecule behaves as a donor in the interaction with bromine and as an acceptor in the interaction with a CH group making part of a phenyl ring. These features evidently lead to the formation of a 3D supramolecular network. The lengths of the noncovalent interactions are gathered in Table S1 in the Supplementary Material.
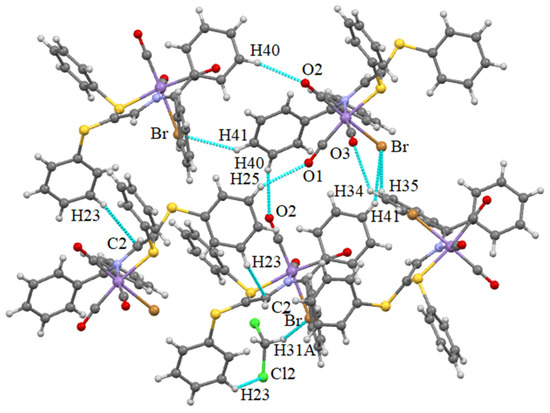
Figure 3.
Intermolecular hydrogen bonds occurring in the structure of 1.
There is also a loose contact of 2.86 Å between the aromatic hydrogen atom H23 of a SPh group and the C2 atom of the azabutadiene linkage, 0.04 Å shorter than the sum of VdW radii. The corresponding CH···π interactions are depicted in Figure 4.
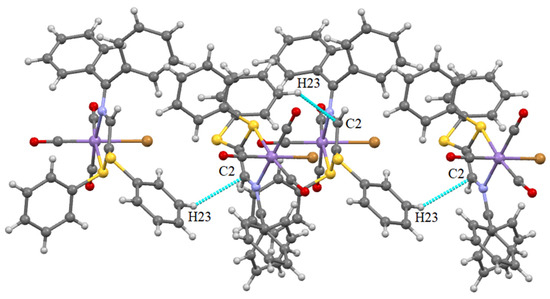
Figure 4.
C-H···π interactions present in the structure of 1.
Another type of interaction involving the π systems is also evidenced between a phenyl cycle of the SPh group (atom C14) bound to the metal with one phenyl group of the imine part (atoms C34 and C35) of the neighbor molecule as shown in Figure 5.
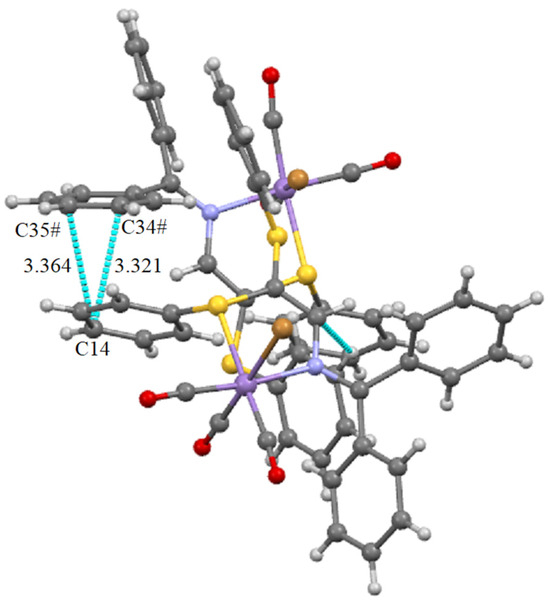
Figure 5.
Potential π···π interaction between two phenyl rings of neighbor molecules in the structure of 1. Symmetry operation # = −1/2 + x, 1/2 − y, 1 − z.
In order to verify the presence of π···π interactions, a topological analysis of electron density in the concerned region of the crystal was carried out on the model of 1, in which its innocent part for Ph···Ph π···π interaction containing the BrMn(CO)3 fragment was neglected. The Quantum Theory of Atoms in Molecules (AIM) was applied for this analysis [23,24]. The molecular graph resulting from this analysis is presented in Figure 6. It reveals the presence of only one bond-critical point corresponding to a C···C interaction at the distance of 3.321 Å. The topological analysis also revealed the presence of S···HC hydrogen bonds and of CH···π interaction involving the C22 ortho carbon atom of the phenyl ring. The CH···S hydrogen bonds are very weak as revealed by the long S···H distances, longer than the sum of van der Waals radii and small electron densities at the bond critical points.
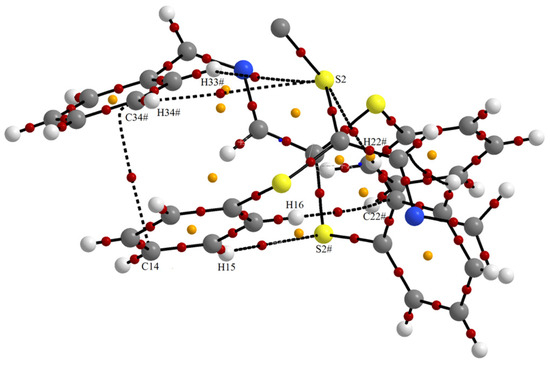
Figure 6.
Molecular graph of the model of the dimer formed through π–π interaction in the structure of 1. Bond-critical points are in red and ring-critical points are in yellow. Symmetry operation #: −1/2 + x, 1/2 − y, 1 − z.
As secondary contacts, CH···Br, CH···O and also CH···S hydrogen bonds are present in the structure of 2. Instead of the π···π interaction detected in 1, a CH-π one is observed in 2. They are shown in Figure 7 and Figure 8.
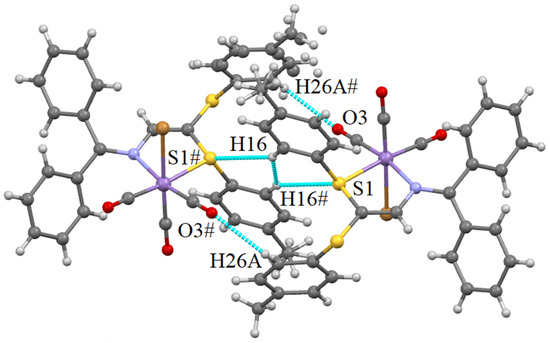
Figure 7.
CH···S and CH···O hydrogen bonds and H···H VdW contacts occurring in the structure of 2. Selected distances (Å): S1···H16# 2.977 (−0.023), H16···H16# 2.196 (−0.204). Shortening with respect to the sum of VdW radii is given in brackets. Symmetry code: # −x, 1 − y, 2 − z.
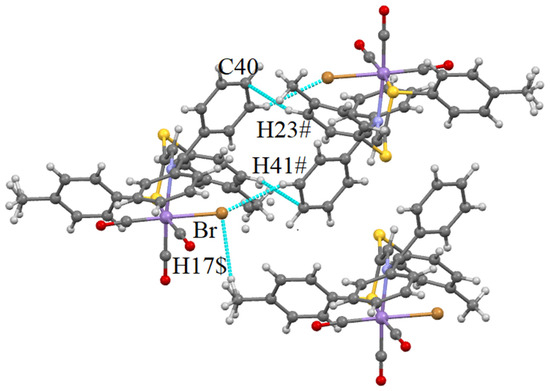
Figure 8.
CH···Br and CH···π interactions occurring in the structure of 2. Distances (Å): Br–H41# 2.943 (−0.107), Br–H17$ 2.962 (−0.088), C40–H23# 2.895 (−0.005). Shortening with respect to the sum of VdW radii is given in brackets. Symmetry codes: # −x, 1 − y, 2 − z; $ x, −1 + y, z.
3. Materials and Methods
[Mn(CO)5Br] was synthesized as previously reported [25]. The reactions were performed under an argon atmosphere. The 1H spectra were recorded on a Bruker AC 300 (Bruker, Wissembourg, France) at 300 MHz, using CDCl3 as the solvent. Infrared spectra were recorded on a Vertex 70 spectrometer (Bruker, Wissembourg, France) using a Platinum ATR accessory equipped with a diamond crystal or on a Bruker Alpha II IRTF (Bruker, Wissembourg, France) spectrometer in solution.
Synthesis of 1 and 2: To a degassed solution of MnBr(CO)5 (155 mg, 0.5 mmol) in CHCl3 (5 mL), we added ligands L1 or L2 (0.55 mmol). The resulting mixture was gently refluxed for 36 h and the reaction was followed by IR spectroscopy. The volume of red solution was reduced to ca. 2 mL under reduced pressure. After several days, layering with hexane at 5 °C yielded red needles. X-ray-suitable crystals of 1 were obtained by a second recrystallisation from CH2Cl2/heptane. Yield: 69%. Anal. Calc. for C30H21BrMnNO3S2 × CH2Cl2 (M.W. = 727.40 g.mol−1): C, 51.19; H, 3.19; N, 1.93; S, 8.82%. Found: C, 50.99; H, 3.10; N, 1.80, S, 8.68%. 1H-NMR (CDCl3) at 298 K: δ 7.12–7.94 (m, CH Ar and vinylic H) ppm. 2: 1H-NMR (CDCl3) at 298 K: δ 7.02–7.83 (m, CH Ar and vinylic H), 2.37 (tol-Me), 2.32 (tol-Me) ppm. Yield: 66%. Anal. Calc. for C32H25BrMnNO3S2 (M.W. = 670.53 g.mol−1): C, 57.32; H, 3.76; N, 2.09; S, 9.56%. Found: C, 57.49; H, 3.96; N, 1.90; S, 9.68%.
The X-ray data collections were performed on a Nonius Kappa CCD using the Collect program [26]. The data were reduced with Denzo software [27] without absorption correction. The missing absorption corrections were partially compensated by the data scaling procedure in the data reduction with the Scalpack program [28]. The structures were solved with SIR92 [27]. The refinements of the structures were performed with SHELXL [28,29,30]. All non-hydrogen atoms were refined with anisotropic temperature factors. The hydrogen atoms were placed in calculated positions and refined in an isotropic riding model. A disorder of one tolyl substituent, consisting of a slight twist of a six-membered C6 ring between two sites, is observed in the structure of 2. The site occupancies were refined to 0.52 and 0.48. The intermolecular interactions were explored with the MERCURY program [31] and the molecular structures were plotted with ORTEP3 [31]. Crystal data and refinement details are gathered in Table 2.

Table 2.
Crystal data, data collection and structure refinement for 1 and 2.
Data were collected using graphite-monochromated MoKα radiation λ = 0.71073 Å and were deposited at the Cambridge Crystallographic Data Centre as 2457097 (1) and 2457098 (2) (Supplementary Materials). The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/getstructures (accessed on 26 June 2025).
The wave functions for AIM topological analyses were calculated with GW09-W package [32,33]. The 6-311G (d,p) standard basis set was applied for all, except for the manganese atom. Since the AIM theory should preferentially use the all-electron and not the ECP (effective core potential) basis sets, the double zeta polarized DZP-DKH basis sets of Jörge were chosen for the Mn atom [34]. Because we were interested in comparative interpretation of metric parameters found in the structures, these calculations were performed on the molecular geometries really present in the crystal structures of 1 and 2. Topological parameters were calculated using the AIMALL packages [35]. The calculations were performed either at the Centre de Calculs de Université de Bourgogne (DNUM) for AIMALL or on a local PC for wave functions.
4. Conclusions
We have demonstrated that the addition of the thioether-functionalized azabutadienes L1 and L2 affords in a carbonyl substitution reaction the structurally characterized octahedral S,N-chelated complexes 1 and 2, in which only one of the SAr groups is ligated to the metal. The propensity of the second non-coordinated SAr group for binding to other soft metal ions such as Cu(I), Ag(I) or Hg(II) may allow the assembly of heterometallic complexes in on-going studies.
Supplementary Materials
CIF file, Check-CIF report, IR Spectra of 1 and 2. Table S1. Noncovalent interactions (NCIs) in the structure of 1. Figure S1. IR spectrum in CH2Cl2 at room temperature for 1 in the carbonyl region. Figure S2. IR spectrum in CH2Cl2 at room temperature for 2. Figure S3. 1H NMR spectrum of compound 2 in CDCl3 at room temperature.
Author Contributions
R.K. and M.K. prepared the compounds; Y.R. collected the X-ray data and solved the structures; M.M.K. performed the quantum calculations; M.M.K., A.K. and M.K. designed the study, analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Data Availability Statement
The X-ray data are available at the CCDC as stated in the paper.
Acknowledgments
We thank S. Adache from SynBioN (Université de Lorraine-CNRS–http://synbion.univ-lorraine.fr/accueil/) for the elemental analyses. Quantum calculations (AIMAll) were performed using HPC resources from DNUM CCUB (Centre de Calcul de l’Université Bourgogne Europe).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baum, M.J.; Scholz, D.; Tataruch, F.; Viehe, H.G. New Syntheses of Amino-Substituted 2-Azabutadienes. Chimia 1975, 29, 514–515. [Google Scholar] [CrossRef]
- Sato, N.; Adachi, J. Studies on pyrazines. 2. Structural assignment of the reaction of alpha-amino-alpha-phenylacetonitrile with chloral or bromal to N-(2,2-dihaloethenyl)-1-imino-1-phenylacetonitriles. J. Org. Chem. 1978, 43, 340–341. [Google Scholar] [CrossRef]
- Macicek, J.; Angelova, O.; Dryanska, V. Structures of 1,1-diphenyl-2-aza-1,3-butadienes. II. 3-Cyano-4-(n-fluorophenyl)-1,1-diphenyl-2-aza-1,3-butadienes (n = 2, 4). Acta Cryst. 1993, C49, 1818–1821. [Google Scholar] [CrossRef]
- King, R.B.; Hodges, K.C. Keteneimmonium and 2-Azabutadiene Complexes from Reactions of α-Chloroenamines with Metal Carbonyl Anions. J. Am. Chem. Soc. 1974, 96, 1263–1264. [Google Scholar] [CrossRef]
- Liu, F.; Yu, Y.; Liebeskind, L.S. N-Substituted Imines by the Copper-Catalyzed N-Imination of Boronic Acids and Organostannanes with O-Acyl Ketoximes. Org. Lett. 2007, 9, 1947–1950. [Google Scholar] [CrossRef]
- Zapata, F.; Caballero, A.; Espinosa, A.; Tarraga, A.; Molina, P. A Selective Chromogenic and Fluorescent Molecular Probe for YbIII Based on a Bichromophoric Azadiene. Europ. J. Inorg. Chem. 2010, 11, 697–703. [Google Scholar] [CrossRef]
- Jiang, N.J.; Melosso, M.; Bizzocchi, L.; Alessandrini, S.; Guillemin, J.-C.; Dore, L.; Puzzarini, C. Spectroscopic and Computational Characterization of 2-Aza-1,3-butadiene, a Molecule of Astrochemical Significance. Phys. Chem. A 2022, 126, 1881–1888. [Google Scholar] [CrossRef]
- Jacquot, S.; Belaissaoui, A.; Schmitt, G.; Laude, B.; Kubicki, M.M.; Blacque, O. Reaction of diphenyldiazomethane with N-methyloxy- and N-ethyloxycarbonyl-N-(2,2,2-trichloroethylidene)amines. Eur. J. Org. Chem. 1999, 1999, 1541–1544. [Google Scholar] [CrossRef]
- Jacquot-Rousseau, S.; Schmitt, G.; Khatyr, A.; Knorr, M.; Kubicki, M.M.; Vigier, E.; Blacque, O. Reactivity of 4,4-Dichloro-1,1-diphenyl-2-azabutadiene Towards Alkoxides and Thiolates: Synthesis of Functionalised π-Conjugated 2-Azabutadienes and Unexpected 1,4-Thiazine Formation. Eur. J. Org. Chem. 2006, 2006, 1555–1562. [Google Scholar] [CrossRef]
- Kinghat, R.; Boudiba, H.; Khatyr, A.; Knorr, M.; Kubicki, M.M. 4,4-Bis(4-methylphenylsulfanyl)-1,1-diphenyl-2-azabuta-1,3-diene. Acta Cryst. 2008, E64, o370. [Google Scholar] [CrossRef]
- Kinghat, R.; Schmitt, G.; Ciamala, K.; Khatyr, A.; Knorr, M.; Jacquot-Rousseau, S.; Rousselin, Y.; Kubicki, M.M. 1,3-Dipolar cycloaddition of diaryldiazomethanes across N-ethoxy-carbonyl-N-(2,2,2-trichloroethylidene)amine and reactivity of the resulting 2-azabutadienes towards thiolates and cyclic amides. Comptes Rendus Chim. 2016, 19, 320–332. [Google Scholar] [CrossRef]
- Kinghat, R.; Khatyr, A.; Knorr, M.; Strohmann, C.; Kubicki, M.M. 4,4-Bis(isopropylthio)-1,1-diphenyl-2-azabuta-1,3-diene Adducts with Cadmium(II), Mercury(II) and Copper(I) Iodides. Crystal, Molecular and Electronic Structures of d10 Transition Metal Chelate Complexes. Chemistry 2024, 6, 62–80. [Google Scholar] [CrossRef]
- Schlachter, A.; Juvenal, F.; Kinghat Tangou, R.; Khatyr, A.; Guyon, F.; Karsenti, P.L.; Strohmann, C.; Kubicki, M.M.; Rousselin, Y.; Harvey, P.D.; et al. 2-Azabutadiene complexes of rhenium(I): S,N-chelated species with photophysical properties heavily governed by the ligand hidden traits. Dalton Trans. 2021, 50, 2945–2963. [Google Scholar] [CrossRef]
- Mansour, A.M.; Radacki, K.; Phukan, H.J.; Roy, M.; Kumar, S.; Purkayastha, S.; Guha, A.K.; Srimani, D. Phototriggered cytotoxic properties of tricarbonyl manganese(I) complexes bearing α-diimine ligands towards HepG2. J. Biolog. Inorg. Chem. 2021, 26, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 5574–5584. [Google Scholar] [CrossRef]
- Amorim, A.L.; Peterle, M.M.; Guerreiro, A.; Coimbra, D.F.; Heying, R.S.; Caramori, G.F.; Braga, A.L.; Bortoluzzi, A.J.; Neves, A.; Bernardes, G.J.L.; et al. Synthesis, characterization and biological evaluation of new manganese metal carbonyl compounds that contain sulfur and selenium ligands as a promising new class of CORMs. Dalton Trans. 2019, 48, 5574–5584. [Google Scholar] [CrossRef] [PubMed]
- Skelton, B.W.; Tolhurst, V.-A.; White, A.H.; Williams, A.M.; Wilson, A.J. Synthesis and spectroscopic studies of organometallic Mn(I) complexes containing the novel mixed donor ligands 2 {MeSeCH(2-n)(SiMe3)n}C5H4N(n = 0-2). J. Organomet. Chem. 2003, 674, 38–44. [Google Scholar] [CrossRef]
- Mondal, A.; Pal, D.; Phukan, H.J.; Roy, M.; Kumar, S.; Purkayastha, S.; Guha, A.K.; Srimani, D. Manganese Complex Catalyzed Sequential Multi-component Reaction: Enroute to a Quinoline-Derived Azafluorenes. ChemSusChem 2024, 17, e202301138. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, S.E.A.; Durgaprasad, G.; Muthiah, K.A.T.; Rose, M.J. Tuning coordination modes of pyridine/thioether Schiff base (NNS) ligands to mononuclear manganese carbonyls. Dalton Trans. 2014, 43, 10725–10738. [Google Scholar] [CrossRef]
- Al-Masri, H.T.; Almejled, A.A.; Moussa, Z. Synthesis, characterization, X-ray structures, and catalytic activity of new Mn(I) and Re(I) metal complexes of chelating phosphinopyridylamine and its sulfide ligands. J. Organomet. Chem. 2025, 1032, 123620. [Google Scholar] [CrossRef]
- Gerber, T.I.A.; Betz, R.; Booysen, I.N.; Potgieter, K.C.; Mayer, P. Coordination of bidentate aniline derivatives to the fac-[Re(CO)3] + core. Polyhedron 2011, 30, 1739–1745. [Google Scholar] [CrossRef]
- Kinghat, R.; Khatyr, A.; Schmitt, G.; Knorr, M.; Kubicki, M.M.; Vigier, E.; Villafañe, F. Mono- and di-nuclear 2,3-diazabutadiene and 2-azabutadiene complexes of Rhenium(I): Syntheses, luminescence spectra and X-ray structures. Inorg. Chem. Commun. 2008, 11, 1060–1063. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules, A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Popelier, P. Atoms in Molecules, An Introduction; Prentice Hall: London, UK, 2000. [Google Scholar]
- King, R.B. Organometallic Syntheses. Vol.1 Transition-Metal Compounds; Academic Press: New York, NY, USA, 1965; ISBN 0-444-42607-8. [Google Scholar]
- Hooft, R.W.W. COLLECT; Nonius BV: Delft, The Netherlands, 1998. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Cryst. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Jorge, F.E.; Canal Neto, A.; Camiletti, G.G.; Machado, S.F. Contracted Gaussian basis sets for Douglas-Kroll-Hess calculations: Estimating scalar relativistic effects of some atomic and molecular properties. J. Chem. Phys. 2009, 130, 064108. [Google Scholar] [CrossRef] [PubMed]
- Keith, T.A. AIMAll (Version 17.11.14), TK Gristmill Software; AIMAll: Overland Park, KS, USA, 2017; Available online: http://aim.tkgristmill.com (accessed on 28 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).