1-Phenyl-3-tosyl-1H-pyrrole
Abstract
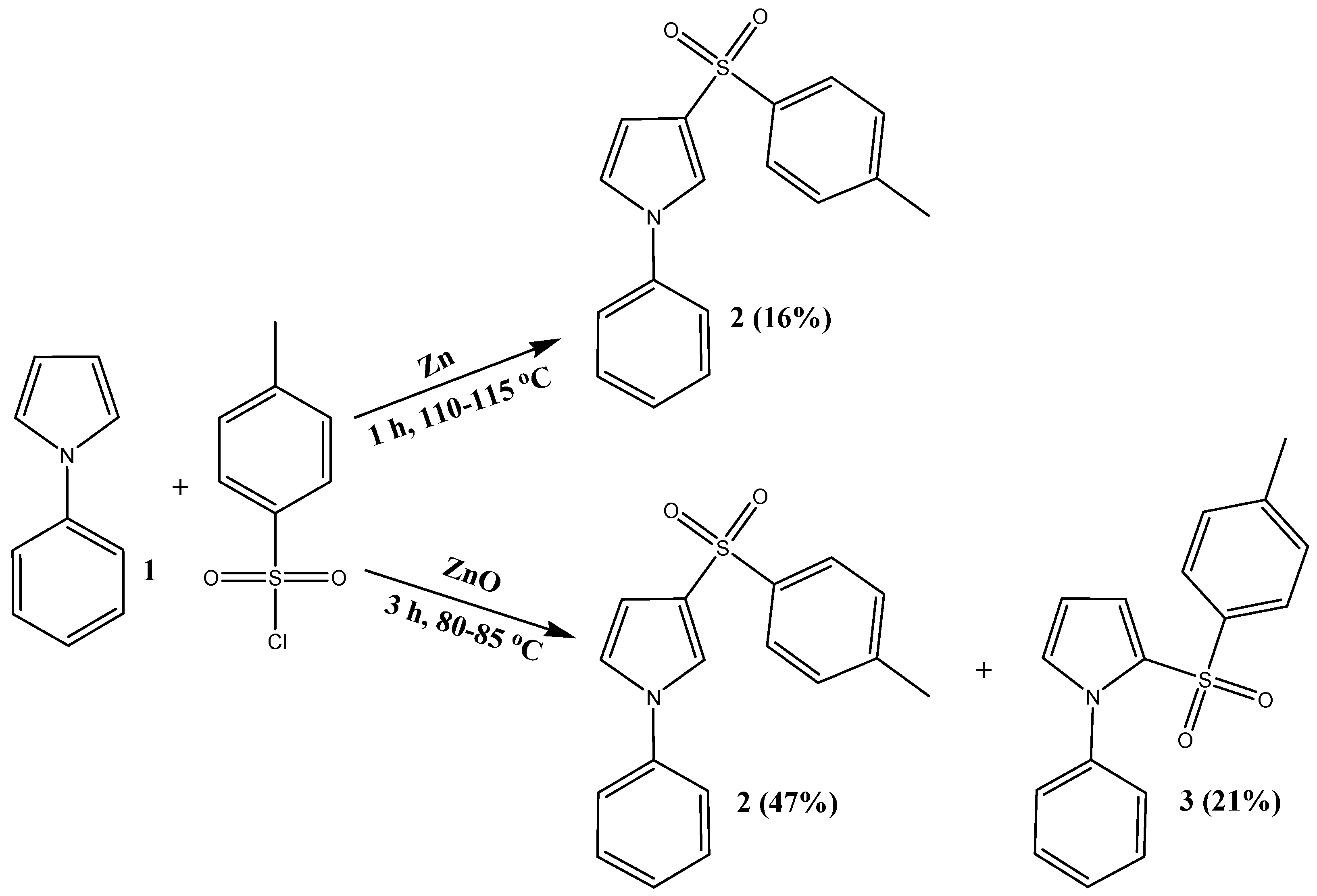
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatzopoulou, M.; Patsilinakos, A.; Vallianatou, T.; Prnova, M.S.; Žakelj, S.; Ragno, R.; Stefek, M.; Kristl, A.; Tsantili-Kakoulidou, A.; Demopoulos, V.J. Decreasing acidity in a series of aldose reductase inhibitors: 2-Fluoro-4-(1H-pyrrol-1-yl)phenol as a scaffold for improved membrane permeation. Bioorg. Med. Chem. 2014, 22, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- La Regina, G.; Bai, R.; Coluccia, A.; Famiglini, V.; Pelliccia, S.; Passacantilli, S.; Mazzoccoli, C.; Ruggieri, V.; Sisinni, L.; Bolognesi, A.; et al. New Pyrrole Derivatives with Potent Tubulin Polymerization Inhibiting Activity as Anticancer Agents Including Hedgehog-Dependent Cancer. J. Med. Chem. 2014, 57, 6531–6552. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Eva Altmann, E.; McKenna, J.M. The Most Common Functional Groups in Bioactive Molecules and How Their Popularity Has Evolved over Time. J. Med. Chem. 2020, 63, 8408–8418. [Google Scholar] [CrossRef] [PubMed]
- Preliminary Communication Presented at the IXth Joint Meeting in Medicinal Chemistry, Athens, Greece, June 2015, Abstrs. P52. Available online: https://www.researchgate.net/publication/289976579_LIPOPHILIC_LIGAND_EFFICIENCY_DRIVEN_BIOISOSTERIC_REPLACEMENT_IN_ALDOSE_REDUCTASE_INHIBITORS_THE_CASE_OF_PHENYLSULFONYL-PYRROLYL-DIFLUOROPHENOLS?channel=doi&linkId=5693c6a908aeab58a9a2ab36&showFulltext=true (accessed on 6 October 2022). [CrossRef]
- Dalla Crose, P.; Gariboldi, P.; La Rosa, C. Synthesis of Arylsulfonyl Substituted Pyrroles. J. Heterocycl. Chem. 1987, 24, 1793–1797. [Google Scholar] [CrossRef]
- Qi, Z.; Jiang, Y.; Wang, Y.; Yan, R. tert-Butyl Nitrite Promoted Oxidative Intermolecular Sulfonamination of Alkynes to Synthesize Substituted Sulfonyl Pyrroles from the Alkynylamines and Sulfinic Acids. J. Org. Chem. 2018, 83, 8636–8644. [Google Scholar] [CrossRef] [PubMed]
- Graybil, B.M. The Synthesis of Aryl Sulfones. J. Org. Chem. 1967, 32, 2931–2933. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Kondaji, G.; Srinivasa Rao, R.; Praveen Kumar, S. Zinc-mediated acylation and sulfonation of pyrrole and its derivatives. Tetrahedron Lett. 2002, 43, 8133–8135. [Google Scholar] [CrossRef]
- Katrun, P.; Mueangkaew, C.; Pohmakotr, M.; Reutrakul, V.; Jaipetch, T.; Soorukram, D.; Kuhakarn, C. Regioselective C2 Sulfonylation of Indoles Mediated by Molecular Iodine. J. Org. Chem. 2014, 79, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Kasture, S.P. Zinc-mediated fast sulfonylation of aromatics. Synth. Commun. 2001, 31, 1065–1068. [Google Scholar] [CrossRef]
- Tocco, G.; Begala, M.; Esposito, F.; Caboni, P.; Cannas, V.; Tramontano, E. ZnO-mediated regioselective C-arylsulfonylation of indoles: A facile solvent-free synthesis of 2- and 3-sulfonylindoles and preliminary evaluation of their activity against drug-resistant mutant HIV-1 reverse transcriptases (RTs). Tetrahedron Lett. 2013, 54, 6237–6241. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M. Synthesis of Bis(indolyl)methanes using a Catalytic Amount of ZnO under Solvent-Free Conditions. Synth. Commun. 2008, 38, 832–840. [Google Scholar] [CrossRef]
- Pagire, S.K.; Hossain, A.; Reiser, O. Temperature Controlled Selective C−S or C−C Bond Formation: Photocatalytic Sulfonylation versus Arylation of Unactivated Heterocycles Utilizing Aryl Sulfonyl Chlorides. Org. Lett. 2018, 20, 648–651. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kechagioglou, Z.; Demopoulos, V.J. 1-Phenyl-3-tosyl-1H-pyrrole. Molbank 2022, 2022, M1471. https://doi.org/10.3390/M1471
Kechagioglou Z, Demopoulos VJ. 1-Phenyl-3-tosyl-1H-pyrrole. Molbank. 2022; 2022(4):M1471. https://doi.org/10.3390/M1471
Chicago/Turabian StyleKechagioglou, Zoumpoulia, and Vassilis J. Demopoulos. 2022. "1-Phenyl-3-tosyl-1H-pyrrole" Molbank 2022, no. 4: M1471. https://doi.org/10.3390/M1471
APA StyleKechagioglou, Z., & Demopoulos, V. J. (2022). 1-Phenyl-3-tosyl-1H-pyrrole. Molbank, 2022(4), M1471. https://doi.org/10.3390/M1471





