Abstract
Diethyl 1-(N-acetylamino)-1-(diphenylphosphinoyl)-1-phenylmethylphosphonate was synthesized at room temperature, starting from 1-ethoxy derivative of diethyl 1-(N-acetylamino)-1-phenylmethylphosphonate via the corresponding phosphonium salt as a reactive intermediate in the Michaelis–Arbuzov-like reaction with methyl diphenylphosphinite. The structure of the compound obtained was confirmed by spectroscopic techniques (1H, 13C, 31P NMR, IR, and MS).
1. Introduction
The 1,1-Bisphosphorus derivatives are gaining more and more importance, and hence, the chemists’ interest due to their high biological activity. This feature is conditioned by the presence of the P-C-P skeleton, which is not only resistant to enzymatic hydrolysis but also provides them with an affinity for hydroxyapatite that occurs in bones [1]. One of the best-known groups of 1,1-bisphosphorus compounds are 1-amino-1,1-bisphosphonates, which can be considered phosphorus analogs of α-amino acids that are functionalized with an additional phosphonyl group [2]. These compounds are an important class of drugs currently used to treat osteoporosis and other diseases of similar etiology, such as Paget’s disease, hypercalcemia, or bone metastasis [3,4]. Their medical importance results also from other biological properties, including antibacterial, antiviral, and antiparasitic [5,6,7], as well as from their potential use as drug delivery systems to bone tissue in targeted anti-cancer therapies [8] or the possibility of the utilization in the synthesis of new diagnostic agents for imaging of bone tissue by MRI or PET [9].
Another group of 1-amino-1,1-bisphosphorus derivatives, namely their asymmetrical derivatives, including phosphonyl-phosphinoyl analogs (Figure 1), may turn out to be equally interesting, although such compounds of the P-C(N)-P backbone have been much less explored so far; hence, their potential is not yet fully discovered. Perhaps this is because most of the methods for the preparation of 1-amino-1,1-bisphosphorus derivatives commonly described in the literature concern 1-amino-1,1-bisphosphonates, and they usually consist in the simultaneous formation of both Cα-P bonds between a central carbon atom and two identical molecules of phosphorus nucleophile. It is obvious that these procedures, however, are not applicable to the synthesis of asymmetrical bisphosphorus derivatives.
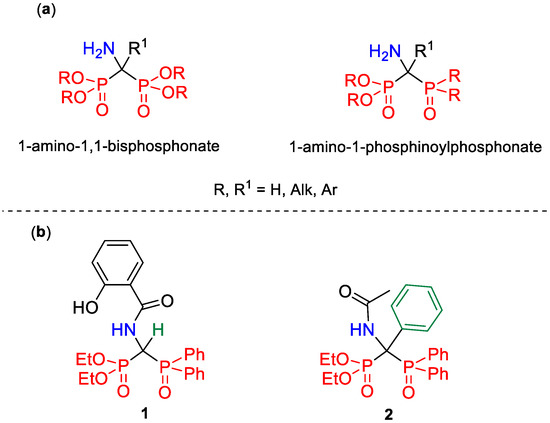
Figure 1.
Comparison of the structures of 1-amino-1,1-bisphosphonate and its asymmetrical phosphonyl-phosphinoyl analog (a). Examples of compounds with the structure of 1-amino-1-phosphinoylalkylphosphonate (b), reported in the literature source (1) [10] and described in this paper (2).
The usefulness of most of the procedures developed in the synthesis of phosphonyl-phosphinoyl analogs of 1-aminobisphosphonates has been demonstrated for a limited number of synthesized models (often single), an example of which is the first, although low-yielded (15%) synthesis of diethyl 1-(N-salicyloylamino)-1-diphenylphosphinoylmethylphosphonate 1 (Figure 1) reported by Kosta and Kotyński [10]. In addition to that, the only two known routes for the preparation of 1-amino-1-phosphinoylalkylphosphonates are either the reaction of diphenylphosphine oxide with iminophosphonates activated by the presence of a p-fluorophenyl [11] or trifluoromethyl group [12] at the α-carbon atom, or the reaction of dialkyl phosphite with N,N-dialkylamine derivatives of phosphine oxide functionalized at the α-position by a nucleofugal group, such as ethoxy [13] or chlorine [14]. Therefore, it seems justified to search for alternative methods of obtaining this type of compound, consisting in the sequential introduction of both phosphorus groups, which enables the synthesis of phosphonyl-phosphinoyl analogs as well.
Over the last few years, in our research team, we have developed an effective procedure for the transformation of α-alkoxyphosphonates into target 1-amino-1,1-bisphosphorus derivatives in the Michaelis–Arbuzov-like reaction through 1-(diethoxyphosphoryl)phosphonium salts as reactive intermediates [15,16]. This method has been tested for both symmetrical and asymmetrical bisphosphorus derivatives and seems to have the potential to become a general procedure. The α-alkoxy derivatives of phosphorous analogs of α-amino acids, used as starting compounds in this transformation, were synthesized by electrochemical oxidation of diethyl 1-(N-acylamino)alkylphosphonates [15] or by addition of diethyl phosphite to ethyl N-acylimidates [16], respectively. In this paper, we present the synthesis and full spectroscopic characteristics of diethyl 1-(N-acetylamino)-1-(diphenylphosphinoyl)-1-phenylmethylphosphonate 2 (Figure 1) as an extension of the library of models that are possible to obtain according to our previously reported procedure, which thus contributes to increasing its applicability.
2. Results and Discussion
As mentioned above, the synthesis of the target diethyl 1-(N-acetylamino)-1-(diphenylphosphinoyl)-1-phenylmethylphosphonate 2 was carried out using 1-(N-acetylamino)-1-ethoxy-1-phenylmethylphosphonate 3 as a starting compound according to a previously described two-step protocol [16], with some modifications in the purification stage (Scheme 1).
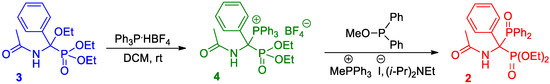
Scheme 1.
Two-step synthesis of diethyl 1-(N-acetylamino)-1-(diphenylphosphinoyl)-1-phenylmethylphosphonate 2 from α-ethoxy derivative of diethyl 1-(N-acetylamino)-1-phenylmethylphosphonate 3 [16].
In the first step, diethyl 1-(N-acetylamino)-1-triphenylphosphonio-1-phenylmethylphosphonate tetrafluoroborate 4 was synthesized by mixing diethyl 1-(N-acetylamino)-1-ethoxy-1-phenylmethylphosphonate with triphenylphosphonium tetrafluoroborate in a dichloromethane solution at room temperature for 1 h. The crude phosphonium salt, obtained after evaporation of the volatiles from the reaction mixture, was then crystallized by dissolving it in dichloromethane and precipitating with diethyl ether. After this purification process, the expected phosphonium salt 4 was provided with a purity of 96%. It was further used in the next step consisting in the Michaelis–Arbuzov-like reaction with methyl diphenylphosphinite in a double catalytic system in the presence of methyltriphenylphosphonium iodide and Hünig’s base. The reaction proceeds efficiently at room temperature in dichloromethane, so the reaction mixture was left overnight to complete it. Then, the residue obtained after evaporation of the solvent from the reaction mixture was subjected to extraction with toluene to isolate the product 2. It turned out that the purity of the resulting extract was quite enough to omit the known procedure of further purification by column chromatography, and thus only recrystallization of the crude product was performed. According to this procedure, the pure product was furnished with a yield of 97% (the melting point of the crystalline compound was 125–127 °C).
The progress of the entire transformation can be monitored by NMR spectroscopy, especially 31P NMR (Figure 2), and the structure of the obtained compound was confirmed by spectroscopic methods (1H, 13C, 31P NMR, MS, and IR; see also Supplementary Materials). During the conversion of diethyl 1-(N-acetylamino)-1-ethoxy-1-phenylmethylphosphonate 3 into diethyl 1-(N-acetylamino)-1-triphenylphosphonio-1-phenylmethylphosphonate tetrafluoroborate 4, in the 31P NMR spectrum, the disappearance of the singlet at 15.7 ppm and the appearance of two doublets at 36.9 and 12.8 ppm with the same coupling constant (J = 16.2 Hz) was observed. In turn, when the phosphonium salt 4 was treated with methyl diphenylphosphinite, the 31P NMR spectrum revealed the disappearance of these two mentioned above doublets and the appearance of a new doublet at 18.1 ppm (J = 6.5 Hz) and a broadened singlet at 41.7 ppm corresponding to the expected product of the Michaelis–Arbuzov reaction.
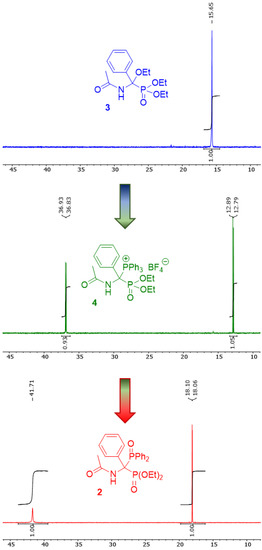
Figure 2.
Transformation of 1-(N-acetylamino)-1-ethoxy-1-phenylmethylphosphonate 3 into diethyl 1-(N-acetylamino)-1-(diphenylphosphinoyl)-1-phenylmethylphosphonate 2 via the corresponding diethyl 1-(N-acetylamino)-1-triphenylphosphonio-1-phenylmethylphosphonate tetrafluoroborate 4; changes in the characteristics of the 31P NMR spectra.
The obtained product 2 will be subjected to a hydrolysis reaction and then to biological tests to evaluate its cytotoxicity on selected tumor cell lines.
3. Materials and Methods
3.1. General
All commercially available reagents and solvents were used without further purification. Diethyl 1-(N-acetylamino)-1-ethoxy-1-phenylmethylphosphonate 3 was prepared according to our previously described procedure [16]. The melting point was determined in a glass capillary and was uncorrected. 1H and 13C NMR spectra were recorded at operating frequencies of 400 and 100 MHz, respectively, using tetramethylsilane (TMS) as the chemical shift standard. 31P NMR spectra were recorded at an operating frequency of 161.9 MHz without the chemical shift standard, with respect to H3PO4 set as 0 ppm. All chemical shifts (δ) are reported in ppm and coupling constants (J) in Hz. IR spectrum was recorded using an FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA; ATR method).
3.2. Diethyl 1-(N-acetylamino)-1-triphenylphosphonio-1-phenylmethylphosphonate tetrafluoroborate (4)
Triphenylphosphonium tetrafluoroborate (0.46 mmol, 161 mg) and diethyl 1-(N-acetylamino)-1-ethoxy-1-phenylmethylphosphonate 3 (0.5 mmol, 165 mg) were dissolved in CH2Cl2 (3 mL). The homogeneous mixture was stirred for 1 h at room temperature; then, the solvent was evaporated to dryness. The residue obtained was recrystallized by dissolution in dichloromethane (0.8 mL) and precipitation with diethyl ether (2.5 mL). The resulting crystalline phosphonium salt 4 was isolated by decanting the solvent with a Pasteur pipette followed by drying under vacuum to give the expected product 4 in 63% yield. The spectroscopic data of the resulting compound were consistent with the literature data [16].
3.3. Diethyl 1-(N-acetylamino)-1-(diphenylphosphinoyl)-1-phenylmethylphosphonate (2)
Methyltriphenylphosphonium iodide (0.038 mmol, 16 mg), methyl diphenylphosphinite (0.23 mmol, 49 mg, 45 μL), and (i-Pr)2EtN (0.017 mmol, 2.2 mg, 3 μL) were added to a solution of diethyl 1-(N-acetylamino)-1-triphenylphosphonio-1-phenylmethylphosphonate tetrafluoroborate 4 (0.16 mmol, 100 mg) in CH2Cl2 (0.6 mL). The reaction mixture was left at room temperature overnight. Next, the solvent was evaporated under reduced pressure, and the residue was extracted with toluene (4 times, 1 mL), followed by subsequent evaporation of toluene from the extract obtained. The crude product, provided in 97% yield, was recrystallized by dissolving in toluene (0.3 mL) and precipitating with hexane (0.3 mL). After isolation by decantation, the obtained white solid was dried under vacuum to give the final product 2, m.p. 125–127 °C. 1H NMR (400 MHz, CDCl3): δ 1.10 (t, J = 7.0 Hz, 3H, CH3), 1.18 (t, J = 7.2 Hz, 3H, CH3), 1.94 (s, 3H, CH3), 3.85–3.94 (m, 2H, CH2), 4.04–4.14 (m, 1H, CH), 4.18–4.28 (m, 1H, CH), 7.04–7.13 (m, 4H, Ph), 7.24–7.32 (m, 4H, Ph + NH), 7.36–7.50 (m, 4H, Ph), 7.72–7.78 (m, 2H, Ph), 8.00–8.05 (m, 2H, Ph); 13C NMR (100 MHz, CDCl3): δ 16.1 (d, J = 6.1 Hz), 16.2 (d, J = 5.5 Hz), 23.8, 63.7 (d, J = 7.7 Hz), 64.3 (d, J = 7.3 Hz), 68.2 (dd, J1 = 146.0 Hz, J2 = 56.4 Hz, Cα), 126.9 (t, J = 2.0 Hz), 127.3 (t, J = 2.5 Hz), 127.6 (d, J = 12.3 Hz), 127.8 (d, J = 12.3 Hz), 128.4 (t, J = 4.8 Hz), 129.5 (d, J = 102.9 Hz), 130.6 (d, J = 97.2 Hz), 131.3 (t, J = 2.2 Hz), 131.9 (t, J = 3.3 Hz), 133.2 (d, J = 9.2 Hz), 133.5 (d, J = 9.1 Hz), 168.9 (t, J = 6.7 Hz); 31P NMR (161.9 MHz, CDCl3): δ 18.1 (d, J = 6.5 Hz), 41.7 (br s). IR (ATR): 3185, 2983, 1683, 1522, 1438, 1254, 1051, 1020, 753, 688 cm−1. HRMS (ESI) m/z: calcd for C25H30NO5P2 [M + H]+ 486.1599, found 486.1603.
4. Conclusions
The synthesis of diethyl 1-(N-acetylamino)-1-(diphenylphosphinoyl)-1-phenylmethylphosphonate from 1-ethoxy derivative of diethyl 1-(N-acetylamino)-1-phenylmethylphosphonate in two reaction stages was described. It proceeds smoothly at room temperature in CH2Cl2; however, the second step requires a double catalytic system. No chromatography is needed to purify the product.
Supplementary Materials
1H NMR, 13C NMR, 31P NMR, IR, and MS spectra of diethyl 1-(N-acetylamino)-1-(diphenylphosphinoyl)-1-phenylmethylphosphonate 2.
Author Contributions
Conceptualization, A.K. and J.A.; methodology, A.K.; formal analysis, D.K. investigation, A.K. and D.K. writing—original draft preparation, A.K. and J.A.; writing—review and editing, A.K. and J.A.; supervision, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Russell, R.G.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, V.D.; Kukhar, V.P. 1-amino-1,1-bisphosphonates. Fundamental syntheses and new developments. Arkivoc 2012, 2012, 127–166. [Google Scholar] [CrossRef]
- Zhang, S.; Gangal, G.; Uludağ, H. ‘Magic bullets’ for bone diseases: Progress in rational design of bone-seeking medicinal agents. Chem. Soc. Rev. 2006, 36, 507–531. [Google Scholar] [CrossRef]
- Hiraga, T.; Tanaka, S.; Yamamoto, M.; Nakajima, T.; Ozawa, H. Inhibitory effects of bisphosphonate (YM175) on bone resorption induced by a metastatic bone tumor. Bone 1996, 18, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Kamath, A.; Das, H.; Li, L.; Bukowski, J.F. Antibacterial effect of human Vγ2Vδ2 T cells in vivo. J. Clin. Investig. 2001, 108, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, E.; Kafarski, P. Physiologic activity of bisphosphonates—Recent advances. Open Pharm. Sci. J. 2016, 3, 56–78. [Google Scholar] [CrossRef]
- Szajnman, S.H.; Ravaschino, E.L.; Docampo, R.; Rodriguez, J.B. Synthesis and biological evaluation of 1-amino-1,1-bisphosphonates derived from fatty acids against Trypanosoma cruzi targeting farnesyl pyrophosphate synthase. Bioorganic Med. Chem. Lett. 2005, 15, 4685–4690. [Google Scholar] [CrossRef] [PubMed]
- Farrell, K.B.; Karpeisky, A.; Thamm, D.H.; Zinnen, S. Bisphosphonate conjugation for bone specific drug targeting. Bone Rep. 2018, 9, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Kuźnik, A.; Październiok-Holewa, A.; Jewula, P.; Kuźnik, N. Bisphosphonates—Much more than only drugs for bone diseases. Eur. J. Pharmacol. 2019, 866, 172773. [Google Scholar] [CrossRef] [PubMed]
- Kosta, K.; Kotyński, A. Diethyl 2,3-dihydro-4H-1,3-benzoxazin-4-one-2-phosphonate and its reactions. Phosphorus Sulfur Silicon Relat. Elem. 1990, 47, 261–265. [Google Scholar] [CrossRef]
- Rassukana, Y.V.; Sinitsa, A.A.; Onys’Ko, P.P. O,O-diphenyl N-sulfonylbenzimidoylphosphonates, a novel type of C-phosphorylated imines. Russ. Chem. Bull. Int. Ed. 2005, 54, 2652–2655. [Google Scholar] [CrossRef]
- Rassukana, Y.V.; Kolotylo, M.V.; Sinitsa, O.A.; Pirozhenko, V.V.; Onys’Ko, P.P. α-iminotrifluoroethylphosphonates: The first representatives of N-H imidoyl phosphonates. Synthesis 2007, 17, 2627–2630. [Google Scholar] [CrossRef]
- Prishchenko, A.A.; Livantsov, M.V.; Novikova, O.P.; Livantsova, L.I.; Petrosyan, V.S. Synthesis of the new types of N-substituted aminomethylenebisorganophosphorus acids and their derivatives. Heteroat. Chem. 2009, 20, 319–324. [Google Scholar] [CrossRef]
- Morgalyuk, V.P.; Strelkova, T.V.; Nifant’Ev, E.E. Ambident reactivity of chloro(dialkylamino)(diphenylphosphinoyl)methanes. Bull. Chem. Soc. Jpn. 2012, 85, 93–100. [Google Scholar] [CrossRef]
- Kuźnik, A.; Mazurkiewicz, R.; Grymel, M.; Zielińska, K.; Adamek, J.; Chmielewska, E.; Bochno, M.; Kubica, S. A new method for the synthesis of α-aminoalkylidenebisphosphonates and their asymmetric phosphonyl-phosphinyl and phosphonyl-phosphinoyl analogues. Beilstein J. Org. Chem. 2015, 11, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Kuźnik, A.; Mazurkiewicz, R.; Zięba, M.; Erfurt, K. 1-(N-Acylamino)-1-triphenylphosphoniumalkylphosphonates: General synthesis and prospects for further synthetic applications. Tetrahedron Lett. 2018, 59, 3307–3310. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).