Dimethyl 3,7-diamino-4,8-bis((2-methoxy-2-oxoethyl)thio)benzo[1,2-b:4,5-b’]dithiophene-2,6-dicarboxylate
Abstract
1. Introduction
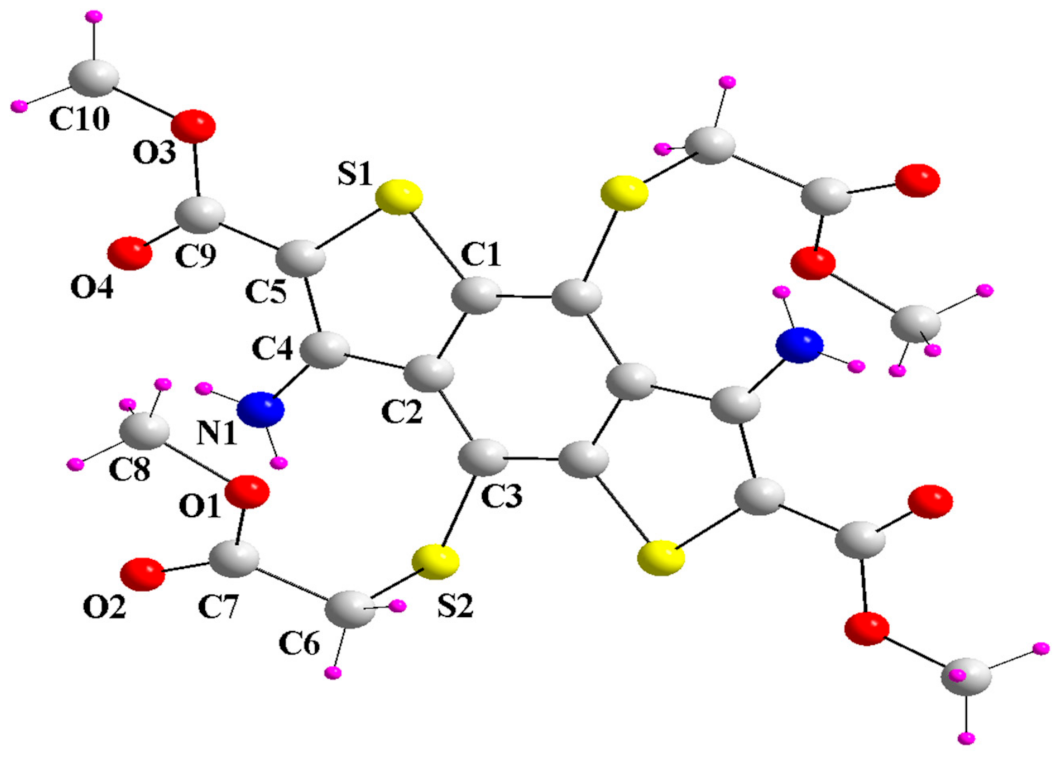
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Dimethyl 3,7-diamino-4,8-bis((2-methoxy-2-oxoethyl)thio)benzo[1,2-b:4,5-b’]dithiophene-2,6-dicarboxylate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perepichka, I.F.; Perepichka, D.F. Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics; John Wiley and Sons: Hoboken, NJ, USA, 2009; ISBN 9780470057322. [Google Scholar]
- Rasool, A.; Zahid, S.; Shehzad, R.A.; Salim Akhter, M.; Iqbal, J. Designing of Benzodithiophene (BDT) Based Non-Fullerene Small Molecules with Favorable Optoelectronic Properties for Proficient Organic Solar Cells. Comput. Theor. Chem. 2021, 1203, 113359. [Google Scholar] [CrossRef]
- Végh, D.; Milata, V. Synthesis of Highly Fluorinated 3,4-Substituted-1-Benzo[b]Thiophene and Benzo[l,2- b;4,5-b’]Dithiophene Derivatives as Potential Biologically Active Compounds and Novel Opto-Electronic Materials. In Proceedings of the FloHet-2015 Florida Heterocyclic And Synthetic Conference, Gainesville, FL, USA, 1–4 March 2015; ARKAT USA, Inc.: Gainesville, FL, USA, 2015; p. 90. [Google Scholar]
- Beugelmans, R.; Bois-Choussy, M.; Boudet, B. Etude Des Reactions de Srn1—Partie 10: Action de Sulfanions Sur Les Halogenures d’aryle Fonctionnalises. Synthese Directe de Benzothiophenes et Thienopyridines. Tetrahedron 1983, 39, 4153–4161. [Google Scholar] [CrossRef]
- Oxford Diffraction. CrysAlis PRO and CrysAlisRed; Oxford Diffraction Ltd.: Abingdon, UK, 2016. [Google Scholar]
- Palatinus, L. The Charge-Flipping Algorithm in Crystallography. Acta Crystallogr. B 2013, 69, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. IUCr A Short History of SHELX. Acta Cryst. 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Berndt, M. DIAMOND; Crystal Impact GbR: Bonn, Germany, 1999. [Google Scholar]

| Compound | BDT |
|---|---|
| Empirical formula (g/mol) | C10H10NO4S2C14 |
| Temperature (K) | 100(1) |
| Wavelength (Å) | 1.54184 |
| Crystal system | triclinic |
| Space group | P-1 |
| Unit cell dimensions | a = 6.3851(3) Å, α = 73.558(3)° b = 9.5602(3) Å, β = 79.571(3)° c = 10.0541(4) Å, γ = 81.722(3)° |
| Formula weight | 272.31 |
| Volume (Å3) | 576.11 |
| Z/calculated density (Mg/m3) | 2/1.570 |
| Absorption coefficient (mm−1) | 4.247 |
| F(000) | 282 |
| Crystal size (mm) | 0.267 × 0.038 × 0.026 |
| Theta range for data collection | 4.637 to 73.894° |
| Index ranges | −7 ≤ h ≤ 7 −11 ≤ k ≤ 11 −12 ≤ l ≤ 11 |
| Reflections collected | 8848 |
| Independent reflections | 2270 (Rint = 0.0310, Rsigma = 0.0231) |
| Completeness to 2Θ = 25.000° | 99.95 % |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2270/0/194 |
| Goodness-of-fit on F2 | 1.024 |
| Final R indices (I > 2σ(I)) | R1 = 0.0325 wR2 = 0.0876 |
| R indices (all data) | R1 = 0.0375 wR2 = 0.0917 |
| Extinction coefficient | n/a |
| Largest diff. peak and hole (e.Å-3) | 0.31 and −0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavilek, B.; Végh, D.; Bortňák, D.; Šmejkalová, V.; Kožíšek, J.; Milata, V. Dimethyl 3,7-diamino-4,8-bis((2-methoxy-2-oxoethyl)thio)benzo[1,2-b:4,5-b’]dithiophene-2,6-dicarboxylate. Molbank 2022, 2022, M1474. https://doi.org/10.3390/M1474
Pavilek B, Végh D, Bortňák D, Šmejkalová V, Kožíšek J, Milata V. Dimethyl 3,7-diamino-4,8-bis((2-methoxy-2-oxoethyl)thio)benzo[1,2-b:4,5-b’]dithiophene-2,6-dicarboxylate. Molbank. 2022; 2022(4):M1474. https://doi.org/10.3390/M1474
Chicago/Turabian StylePavilek, Branislav, Daniel Végh, Dušan Bortňák, Veronika Šmejkalová, Jozef Kožíšek, and Viktor Milata. 2022. "Dimethyl 3,7-diamino-4,8-bis((2-methoxy-2-oxoethyl)thio)benzo[1,2-b:4,5-b’]dithiophene-2,6-dicarboxylate" Molbank 2022, no. 4: M1474. https://doi.org/10.3390/M1474
APA StylePavilek, B., Végh, D., Bortňák, D., Šmejkalová, V., Kožíšek, J., & Milata, V. (2022). Dimethyl 3,7-diamino-4,8-bis((2-methoxy-2-oxoethyl)thio)benzo[1,2-b:4,5-b’]dithiophene-2,6-dicarboxylate. Molbank, 2022(4), M1474. https://doi.org/10.3390/M1474






