Synthesis and Evaluation of Self-Assembling Properties of 3-(3,5-Difluoro-3,5-bis((alkoxy)carbonyl)-2,6-dioxoheptan-4-yl)-1-methylpyridin-1-ium Iodides
Abstract
:1. Introduction
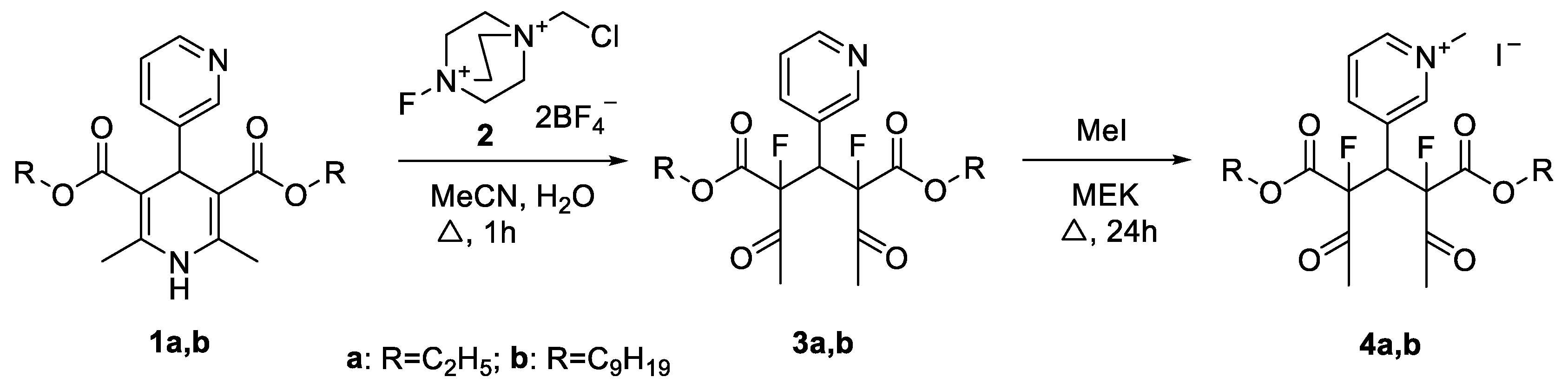
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for the Synthesis of Dialkyl 2,4-Diacetyl-2,4-difluoro-3-(pyridin-3-yl)pentanedioates 3a,b
3.2. Diethyl 2,4-Diacetyl-2,4-difluoro-3-(pyridin-3-yl)pentanedioate (3a)
3.3. Dinonyl 2,4-Diacetyl-2,4-difluoro-3-(pyridin-3-yl)pentanedioate (3b)
3.4. General Procedure for the Synthesis of 3-(3,5-Difluoro-3,5-bis((alkoxy)carbonyl)-2,6-dioxoheptan-4-yl)-1-methylpyridin-1-ium Iodide 4a,b
3.5. 3-(3,5-Difluoro-3,5-bis((ethoxy)carbonyl)-2,6-dioxoheptan-4-yl)-1-methylpyridin-1-ium Iodide (4a)
3.6. 3-(3,5-Difluoro-3,5-bis((nonyloxy)carbonyl)-2,6-dioxoheptan-4-yl)-1-methylpyridin-1-ium Iodide (4b)
3.7. Self-Assembling Properties by Dynamic Light Scattering Measurements
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamada, Y. Role of Pyridines in Medicinal Chemistry and Design of BACE1 Inhibitors Possessing a Pyridine Scaffold. In Pyridine; Pratima, P., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78923-423-7. [Google Scholar]
- Ling, Y.; Hao, Z.Y.; Liang, D.; Zhang, C.L.; Liu, Y.F.; Wang, Y. The expanding role of pyridine and dihydropyridine scaffolds in drug design. Drug Des. Devel. Ther. 2021, 15, 4289–4338. [Google Scholar] [CrossRef] [PubMed]
- Sowmiah, S.; Esperança, J.M.S.S.; Rebelo, L.P.N.; Afonso, C.A.M. Pyridinium salts: From synthesis to reactivity and applications. Org. Chem. Front. 2018, 5, 453–493. [Google Scholar] [CrossRef]
- Ilies, M.A.; Satyal, U.; Sharma, V.D. Synthetic delivery systems for DNA, siRNA, and mRNA based on pyridinium amphiphiles. In Proceedings of the ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; Volume 1271, pp. 1–34. [Google Scholar]
- Ilies, M.A.; Seitz, W.A.; Johnson, B.H.; Ezell, E.L.; Miller, A.L.; Thompson, E.B.; Balaban, A.T. Lipophilic pyrylium salts in the synthesis of efficient pyridinium-based cationic lipids, gemini surfactants, and lipophilic oligomers for gene delivery. J. Med. Chem. 2006, 49, 3872–3887. [Google Scholar] [CrossRef]
- Satyal, U.; Draghici, B.; Dragic, L.L.; Zhang, Q.; Norris, K.W.; Madesh, M.; Brailoiu, E.; Ilies, M.A. Interfacially Engineered Pyridinium Pseudogemini Surfactants as Versatile and Efficient Supramolecular Delivery Systems for DNA, siRNA, and mRNA. ACS Appl. Mater. Interfaces 2017, 9, 29481–29495. [Google Scholar] [CrossRef] [PubMed]
- Aubets, E.; Griera, R.; Felix, A.J.; Rigol, G.; Sikorski, C.; Limón, D.; Mastrorosa, C.; Busquets, M.A.; Pérez-García, L.; Noé, V.; et al. Synthesis and validation of DOPY: A new gemini dioleylbispyridinium based amphiphile for nucleic acid transfection. Eur. J. Pharm. Biopharm. 2021, 165, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, Z.; Plotniece, A.; Reine, I.; Chekavichus, B.; Duburs, G.; Urtti, A. Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery. Biochim. Biophys. Acta Biomembr. 2000, 1509, 451–466. [Google Scholar] [CrossRef] [Green Version]
- Pajuste, K.; Hyvonen, Z.; Petrichenko, O.; Kaldre, D.; Rucins, M.; Cekavicus, B.; Ose, V.; Skrivele, B.; Gosteva, M.; Morin-Picardat, E.; et al. Gene delivery agents possessing antiradical activity: Self-assembling cationic amphiphilic 1,4-dihydropyridine derivatives. New J. Chem. 2013, 37, 3062–3075. [Google Scholar] [CrossRef]
- Petrichenko, O.; Rucins, M.; Vezane, A.; Timofejeva, I.; Sobolev, A.; Cekavicus, B.; Pajuste, K.; Plotniece, M.; Gosteva, M.; Kozlovska, T.; et al. Studies of the physicochemical and structural properties of self-assembling cationic pyridine derivatives as gene delivery agents. Chem. Phys. Lipids 2015, 191, 25–37. [Google Scholar] [CrossRef]
- Petrichenko, O.; Plotniece, A.; Pajuste, K.; Rucins, M.; Dimitrijevs, P.; Sobolev, A.; Sprugis, E.; Cēbers, A. Evaluation of physicochemical properties of amphiphilic 1,4-dihydropyridines and preparation of magnetoliposomes. Nanomaterials 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Niemirowicz-Laskowska, K.; Głuszek, K.; Piktel, E.; Pajuste, K.; Durnaś, B.; Król, G.; Wilczewska, A.Z.; Janmey, P.A.; Plotniece, A.; Bucki, R. Bactericidal and immunomodulatory properties of magnetic nanoparticles functionalized by 1,4-dihydropyridines. Int. J. Nanomed. 2018, 13, 3411–3424. [Google Scholar] [CrossRef] [Green Version]
- Rucins, M.; Smits, R.; Sipola, A.; Vigante, B.; Domracheva, I.; Turovska, B.; Muhamadejev, R.; Pajuste, K.; Plotniece, M.; Sobolev, A.; et al. Pleiotropic Properties of Amphiphilic Dihydropyridines, Dihydropyridones, and Aminovinylcarbonyl Compounds. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Rucins, M.; Dimitrijevs, P.; Pajuste, K.K.; Petrichenko, O.; Jackevica, L.; Gulbe, A.; Kibilda, S.; Smits, K.; Plotniece, M.; Tirzite, D.; et al. Contribution of Molecular Structure to Self-Assembling and Biological Properties of Bifunctional Lipid-Like 4-(N-Alkylpyridinium)-1,4-Dihydropyridines. Pharmaceutics 2019, 11, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucins, M.; Kaldre, D.; Pajuste, K.; Fernandes, M.A.S.; Vicente, J.A.F.; Klimaviciusa, L.; Jaschenko, E.; Kanepe-Lapsa, I.; Shestakova, I.; Plotniece, M.; et al. Synthesis and studies of calcium channel blocking and antioxidant activities of novel 4-pyridinium and/or N-propargyl substituted 1,4-dihydropyridine derivatives. Comptes Rendus Chim. 2014, 17, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Jansone, B.; Kadish, I.; Van Groen, T.; Beitnere, U.; Moore, D.R.; Plotniece, A.; Pajuste, K.; Klusa, V. A novel 1,4-dihydropyridine derivative improves spatial learning and memory and modifies brain protein expression in wild type and transgenic APPSweDI Mice. PLoS ONE 2015, 10, e0127686. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Gaucheron, J.; Boulanger, C.; Santaella, C.; Kolbe, H.V.J.; Vierling, P. Enhanced in vitro and in vivo cationic lipid-mediated gene delivery with a fluorinated glycerophosphoethanolamine helper lipid. J. Gene Med. 2001, 3, 109–114. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Ling, J.; Yan, Y.; Hu, J.; Cheng, Y. The fluorination effect of fluoroamphiphiles in cytosolic protein delivery. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Boulanger, C.; Di Giorgio, C.; Gaucheron, J.; Vierling, P. Transfection with Fluorinated Lipoplexes Based on New Fluorinated Cationic Lipids and in the Presence of a Bile Salt Surfactant. Bioconjug. Chem. 2004, 15, 901–908. [Google Scholar] [CrossRef]
- Fisicaro, E.; Compari, C.; Bacciottini, F.; Contardi, L.; Pongiluppi, E.; Barbero, N.; Viscardi, G.; Quagliotto, P.; Donofrio, G.; Krafft, M.P. Nonviral gene-delivery by highly fluorinated gemini bispyridinium surfactant-based DNA nanoparticles. J. Colloid Interface Sci. 2017, 487, 182–191. [Google Scholar] [CrossRef]
- Massa, M.; Rivara, M.; Donofrio, G.; Cristofolini, L.; Peracchia, E.; Compari, C.; Bacciottini, F.; Orsi, D.; Franceschi, V.; Fisicaro, E. Gene-Delivery Ability of New Hydrogenated and Partially Fluorinated Gemini bispyridinium Surfactants with Six Methylene Spacers. Int. J. Mol. Sci. 2022, 23, 3062. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, L.; Zhu, Z. A fluorous biphase drug delivery system triggered by low frequency ultrasound: Controlled release from perfluorous discoidal porous silicon particles. Nanoscale Adv. 2020, 2, 3561–3569. [Google Scholar] [CrossRef]
- Makarova, N.V.; Koronova, Z.V.; Plotnietse, A.V.; Tirzite, D.Y.; Tirzit, G.D.; Duburs, G.Y. Synthesis of 1,4-dihydropyridines having an N-alkylpyridinium substituent at the 4-position and their affinity towards liposomal membranes. Chem. Heterocycl. Compd. 1995, 31, 969–973. [Google Scholar] [CrossRef]
- Pajuste, K.; Gosteva, M.; Kaldre, D.; Plotniece, M.; Cekavicus, B.; Sobolev, A.; Priksane, A.; Tirzitis, G.; Duburs, G.; Plotniece, A. Effect of the solvent nature on the course of quaternization of 3,5-diethoxycarbonyl-2,6-dimethyl-4-(3-pyridyl)-1,4-dihydropyridine. Chem. Heterocycl. Compd. 2011, 47. [Google Scholar] [CrossRef]
- Pikun, N.V.; Kolesnyk, N.P.; Rusanov, E.B.; Plotniece, A.; Rucins, M.; Sobolev, A.; Shermolovich, Y.G. Synthesis of fluorinated 2,6-heptanediones and 2-oxa-6-azabicyclo[2.2.2]octanes from 1,4-dihydropyridines. Tetrahedron 2018, 74, 2884–2890. [Google Scholar] [CrossRef]
- Pikun, N.V.; Kolesnyk, N.P.; Rusanov, E.B.; Plotniece, A.; Sobolev, A.; Domracheva, I.; Shermolovich, Y.G. Contrasting reactivity of fluorinated 2,6-heptanediones towards amines and ammonia, leading to cyclohexanediones or 2-oxa-6-azabicyclo[2.2.2]octanes and evaluation of their cytotoxicity. New J. Chem. 2019, 43, 10537–10544. [Google Scholar] [CrossRef]
- Pikun, N.V.; Sobolev, A.; Plotniece, A.; Rucins, M.; Vigante, B.; Petrova, M.; Muhamadejev, R.; Pajuste, K.; Shermolovich, Y.G. Synthesis of Fluorinated 3,6-Dihydropyridines and 2-(Fluoromethyl)pyridines by Electrophilic Fluorination of 1,2-Dihydropyridines with Selectfluor®. Molecules 2020, 25, 3143. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Vessally, E. N-Fluorobenzenesulfonimide: A useful and versatile reagent for the direct fluorination and amination of (hetero)aromatic C–H bonds. RSC Adv. 2020, 10, 16756–16768. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Tarui, T.; Shibata, N. A novel and efficient synthesis of 3-fluorooxindoles from indoles mediated by selectfluor. Org. Lett. 2000, 2, 639–642. [Google Scholar] [CrossRef]
- Yang, Q.; Dai, G.L.; Yang, Y.M.; Luo, Z.; Tang, Z.Y. Solvent Effects: Syntheses of 3,3-Difluorooxindoles and 3-Fluorooxindoles from Hydrazonoindolin-2-one by Selectfluor. J. Org. Chem. 2018, 83, 6762–6768. [Google Scholar] [CrossRef]
- Putri, D.C.A.; Dwiastuti, R.; Marchaban, M.; Nugroho, A.K. Optimization of mixing temperature and sonication duration in liposome preparation. J. Pharm. Sci. Community 2017, 14, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Tirzite, D.; Koronova, J.; Plotniece, A. Influence of some quaternised 1,4-dihydropyridine derivatives on liposomes and erythrocyte membranes. Biochem. Mol. Biol. Int. 1998, 45, 849–856. [Google Scholar] [CrossRef] [PubMed]
| Comp. | PDI | Zav DH, nm | ||||
|---|---|---|---|---|---|---|
| Fresh * | 1 Day ** | 5 Days *** | Fresh * | 1 Day ** | 5 Days *** | |
| 4a | 1.00 ± 0.01 | - | - | 736 ± 84 | - | - |
| 4b | 0.31 ± 0.05 | 0.42 ± 0.04 | 0.40 ± 0.06 | 371 ± 49 | 335 ± 66 | 427 ± 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikun, N.; Lacis, D.; Sobolev, A.; Rucins, M.; Plotniece, M.; Pajuste, K.; Plotniece, A. Synthesis and Evaluation of Self-Assembling Properties of 3-(3,5-Difluoro-3,5-bis((alkoxy)carbonyl)-2,6-dioxoheptan-4-yl)-1-methylpyridin-1-ium Iodides. Molbank 2022, 2022, M1402. https://doi.org/10.3390/M1402
Pikun N, Lacis D, Sobolev A, Rucins M, Plotniece M, Pajuste K, Plotniece A. Synthesis and Evaluation of Self-Assembling Properties of 3-(3,5-Difluoro-3,5-bis((alkoxy)carbonyl)-2,6-dioxoheptan-4-yl)-1-methylpyridin-1-ium Iodides. Molbank. 2022; 2022(3):M1402. https://doi.org/10.3390/M1402
Chicago/Turabian StylePikun, Nadiia, Davis Lacis, Arkadij Sobolev, Martins Rucins, Mara Plotniece, Karlis Pajuste, and Aiva Plotniece. 2022. "Synthesis and Evaluation of Self-Assembling Properties of 3-(3,5-Difluoro-3,5-bis((alkoxy)carbonyl)-2,6-dioxoheptan-4-yl)-1-methylpyridin-1-ium Iodides" Molbank 2022, no. 3: M1402. https://doi.org/10.3390/M1402
APA StylePikun, N., Lacis, D., Sobolev, A., Rucins, M., Plotniece, M., Pajuste, K., & Plotniece, A. (2022). Synthesis and Evaluation of Self-Assembling Properties of 3-(3,5-Difluoro-3,5-bis((alkoxy)carbonyl)-2,6-dioxoheptan-4-yl)-1-methylpyridin-1-ium Iodides. Molbank, 2022(3), M1402. https://doi.org/10.3390/M1402






