6-Amino-3-(prop-2-en-1-yl)-9H-purin-3-ium Tetracopper(I) Hexabromide: Synthesis and X-ray Structure Determination
Abstract
:1. Introduction
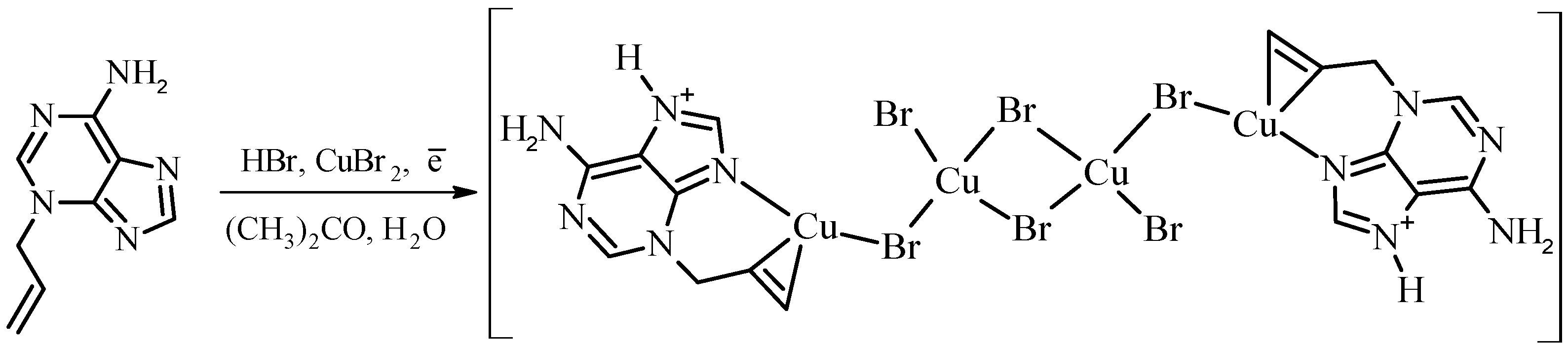
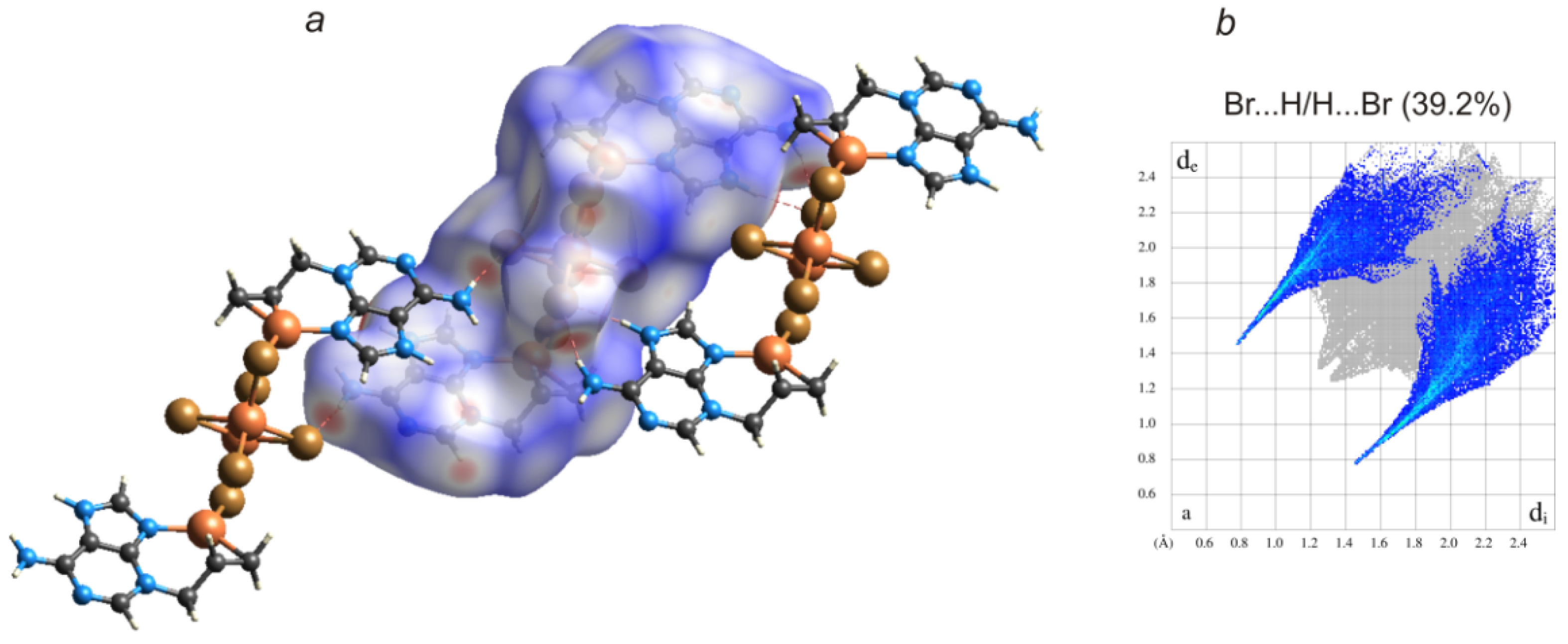
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bellussi, G.; Bohnet, M.; Bus, J.; Drauz, K.; Greim, H.; Jackel, K.-P.; Karst, U.; Kleemann, A.; Kreysa, G.; Laird, T.; et al. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Chichester, UK, 2011. [Google Scholar]
- Wang, X.-S.; Zhao, H.; Li, Y.-H.; Xiong, R.-G.; You, X.-Z. Olefin-Copper(I) Complexes and their Properties. Top. Catal. 2005, 35, 43–61. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Zhu, B.; Fu, J.; Deng, Y.; Tian, L.; Guan, W.; Bi, X. Directed Copper-Catalyzed Intermolecular Heck-Type Reaction of Unactivated Olefins and Alkyl Halides. J. Am. Chem. Soc. 2018, 140, 16929–16935. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armaroli, N.; Accorsi, G.; Cardinali, F.; Listorti, A. Photochemistry and photophysics of coordination compounds: Copper. In Photochemistry and Photophysics of Coordination Compounds I; Springer: Berlin/Heidelberg, Germany, 2007; pp. 69–115. [Google Scholar]
- Rickerby, J.; Steinke, J.H.G. Current Trends in Patterning with Copper. Chem. Rev. 2002, 102, 1525–1550. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, R.-G.; Chen, X.-T.; Xue, Z.; Peng, A.S.-M.; You, X.-Z. Two-Dimensional Luminescent Rectangular Molecular Grids Containing Copper(I)−Olefin Bonds as Bridging Spacers. Organometallics 2002, 21, 235–238. [Google Scholar] [CrossRef]
- Slyvka, Y.; Goreshnik, E.; Pavlyuk, A.; Mys’Kiv, M. Copper(I) π-complexes with allyl derivatives of heterocyclic compounds: Structural survey of their crystal engineering. Open Chem. 2013, 11, 1875–1901. [Google Scholar] [CrossRef] [Green Version]
- Fedorchuk, A.; Slyvka, Y.; Kinzhybalo, V.; Lis, T.; Mys’Kiv, M. An unusual diverse coordination of silver(I) with N-allylthiohydantoin ligand in the presence of benzene- and p-toluenesulfonate anions. Inorg. Chim. Acta 2019, 484, 79–86. [Google Scholar] [CrossRef]
- Hordiichuk, O.; Slyvka, Y.I.; Kinzhybalo, V.V.; Goreshnik, E.A.; Bednarchuk, T.; Bednarchuk, O.; Jędryka, J.; Kityk, I.; Mys’Kiv, M. Construction of heterometallic and mixed-valence copper(I/II) chloride π-complexes with 1,2,4-triazole allyl-derivative. Inorg. Chim. Acta 2019, 495, 119012. [Google Scholar] [CrossRef]
- Ryu, S.-M.; Koo, T.; Kim, K.; Lim, K.; Baek, G.; Kim, S.-T.; Kim, H.S.; Kim, D.-E.; Lee, H.; Chung, E.; et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol. 2018, 36, 536–539. [Google Scholar] [CrossRef]
- Heithoff, D.M.; Sinsheimer, R.L.; Low, D.A.; Mahan, M.J. An Essential Role for DNA Adenine Methylation in Bacterial Virulence. Science 1999, 284, 967–970. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Lam, D.K.; Rees, H.A.; Solá-Esteves, N.M.; Barrera, L.A.; Born, D.A.; Edwards, A.; Gehrke, J.M.; Lee, S.-J.; Liquori, A.J.; et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nat. Biotechnol. 2020, 38, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, T.; Epps, L.A.; Kistenmacher, T.J.; Marzilli, L.G. Transition metal compounds of N(3)-alkylated 6-aminopurines. Synthesis and unusually high stability of bis(acetylacetonato)(nitro)(N(3)-alkylated 6-aminopurine)cobalt(III) complexes. Crystal and molecular structure of the triacanthine complex. J. Am. Chem. Soc. 1978, 100, 5756–5763. [Google Scholar] [CrossRef]
- Pérez-Yáñez, S.; Castillo, O.; Cepeda, J.; García-Terán, J.P.; Luque, A.; Román, P. Supramolecular architectures of metal–oxalato complexes containing purine nucleobases. Inorg. Chim. Acta 2011, 365, 211–219. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(i) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Willett, R.D. Crystal structure of bis(N-methylethylenediammonium) hexabromodicuprate(I). Inorg. Chim. Acta 2004, 357, 1579–1582. [Google Scholar] [CrossRef]
- Pavlyuk, A.; Davydov, V.N.; Mys’Kiv, M. Copper(I) Complexes with N-Allylquinolinium Bromide [C9H7N(C3H5)]CuBr2 and [C9H7N(C3H5)]Cu2Br3: Synthesis and Crystal Structure. Russ. J. Coord. Chem. 2003, 29, 199–202. [Google Scholar] [CrossRef]
- Peng, R.; Li, M.; Li, D. Copper(I) halides: A versatile family in coordination chemistry and crystal engineering. Coord. Chem. Rev. 2010, 254, 1–18. [Google Scholar] [CrossRef]
- Martin, J.D.; Dattelbaum, A.M.; Thornton, T.A.; Sullivan, R.M.; Yang, J.; Peachey, M.T. Metal Halide Analogues of Chalcogenides: A Building Block Approach to the Rational Synthesis of Solid-State Materials. Chem. Mater. 1998, 10, 2699–2713. [Google Scholar] [CrossRef]
- Xue, J.-Y.; Li, J.-C.; Li, H.-X.; Li, H.-Y.; Lang, J.-P. Chan–Lam cross-coupling reactions promoted by anionic copper(I)/iodide species with cationic methyl-((pyridinyl)-pyrazolyl)pyridin-1-ium. Tetrahedron 2016, 72, 7014–7020. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Crystallogr. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Shkurenko, A.A.; Davydov, V.N.; Mys’Kiv, M. Synthesis and Crystal Structure of Copper(I) Chloride π-Complexes with N-Allyl- and N,N′-Diallylpiperazinium Dichlorides: [C3H5NH(CH2)4NH2]Cu2Cl4 and [C3H5NH(CH2)4NHC3H5]0.5CuCl2. Russ. J. Coord. Chem. 2003, 29, 445–450. [Google Scholar] [CrossRef]
- Noshtchenko, G.; Kinzhybalo, V.; Lis, T.; Mykhalitchko, B. π-Complexes of Copper(I) with Terminal Alkynes. Synthesis and Crystal Structure of [(HC≡CCH2NH3)(Cu2Br3)] π-Complex. Z. Anorg. Allg. Chem. 2007, 633, 306–309. [Google Scholar] [CrossRef]
- Eder, E.; Lutz, D.; Jörns, M. Allylic compounds bind directly to DNA: Investigation of the binding mechanisms in vitro. Chem. Interact. 1987, 61, 97–108. [Google Scholar] [CrossRef]
- Slyvka, Y.; Pokhodylo, N.T.; Goreshnik, E.; Pavlyuk, O.; Mys’Kiv, M. Syntheses and crystal structures of two copper(I)–halide π,σ-coordination compounds based on 2-[(prop-2-en-1-yl)sulfanyl]pyridine. Acta Crystallogr. 2021, 77, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Rigaku CrysAlisPro Software System; Version 1.171.40.57a; Rigaku Oxford Diffraction (Tokyo, Japan): 2019. Available online: http://www.rigaku.com (accessed on 1 September 2019).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON, an integrated tool for the analysis of the results of a single crystal structure determination. Acta Crystallogr. 1990, 46, 34. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]

| Bond | d, Å | Angle | ω, ° |
|---|---|---|---|
| Cu(1)–N(1) | 1.978(4) | N(1)–Cu(1)–Br(1) | 113.3(1) |
| Cu(1)–C(7) | 2.039(4) | N(1)–Cu(1)–C(7) | 93.7(2) |
| Cu(1)–C(8) | 2.059(5) | N(1)–Cu(1)–C(8) | 131.5(2) |
| Cu(1)–Br(1) | 2.321(1) | C(7)–Cu(1)–C)8) | 38.9(2) |
| Cu(1)–m | 1.932(1) | Br(1)–Cu(2)–Br(3) i 1 | 111.03(3) |
| Cu(2)–Br(1) | 2.484(1) | Br(2)–Cu(2)–Br(1) | 102.54(3) |
| Cu(2)–Br(2) | 2.462(1) | Br(2)–Cu(2)–Br(3) i | 106.31(3) |
| Cu(2)–Br(3) | 2.478(1) | Br(2)–Cu(2)–Br(3) | 109.25(3) |
| Cu(2)–Br(3) i | 2.499(1) | Br(3)–Cu(2)–Br(1) | 111.27(3) |
| Br(3)–Cu(2)–Br(3) i | 115.50(2) |
| Bond | d(D–H), Å | d(H...A), Å | d(D...A), Å | ω (D–H...A), ° |
|---|---|---|---|---|
| N(2)–H(2)...Br(2) ii 1 | 0.89(7) | 2.34(7) | 3.215(4) | 168(6) |
| N(5)–H(5A)...Br(2) ii | 0.88(6) | 2.60(6) | 3.460(5) | 166(5) |
| N(5)–H(5B)...Br(3) iii | 0.73(7) | 2.79(7) | 3.521(5) | 174(6) |
| C(4)–H(4)...Br(1) iv | 0.99(5) | 2.76(6) | 3.624(5) | 146(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlyuk, O.V.; Slyvka, Y.I.; Goreshnik, E.A.; Mys’kiv, M.G. 6-Amino-3-(prop-2-en-1-yl)-9H-purin-3-ium Tetracopper(I) Hexabromide: Synthesis and X-ray Structure Determination. Molbank 2022, 2022, M1401. https://doi.org/10.3390/M1401
Pavlyuk OV, Slyvka YI, Goreshnik EA, Mys’kiv MG. 6-Amino-3-(prop-2-en-1-yl)-9H-purin-3-ium Tetracopper(I) Hexabromide: Synthesis and X-ray Structure Determination. Molbank. 2022; 2022(3):M1401. https://doi.org/10.3390/M1401
Chicago/Turabian StylePavlyuk, Oleksiy V., Yurii I. Slyvka, Evgeny A. Goreshnik, and Marian G. Mys’kiv. 2022. "6-Amino-3-(prop-2-en-1-yl)-9H-purin-3-ium Tetracopper(I) Hexabromide: Synthesis and X-ray Structure Determination" Molbank 2022, no. 3: M1401. https://doi.org/10.3390/M1401
APA StylePavlyuk, O. V., Slyvka, Y. I., Goreshnik, E. A., & Mys’kiv, M. G. (2022). 6-Amino-3-(prop-2-en-1-yl)-9H-purin-3-ium Tetracopper(I) Hexabromide: Synthesis and X-ray Structure Determination. Molbank, 2022(3), M1401. https://doi.org/10.3390/M1401







