1,3-Bis(5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)imidazolidine-4,5-dione
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
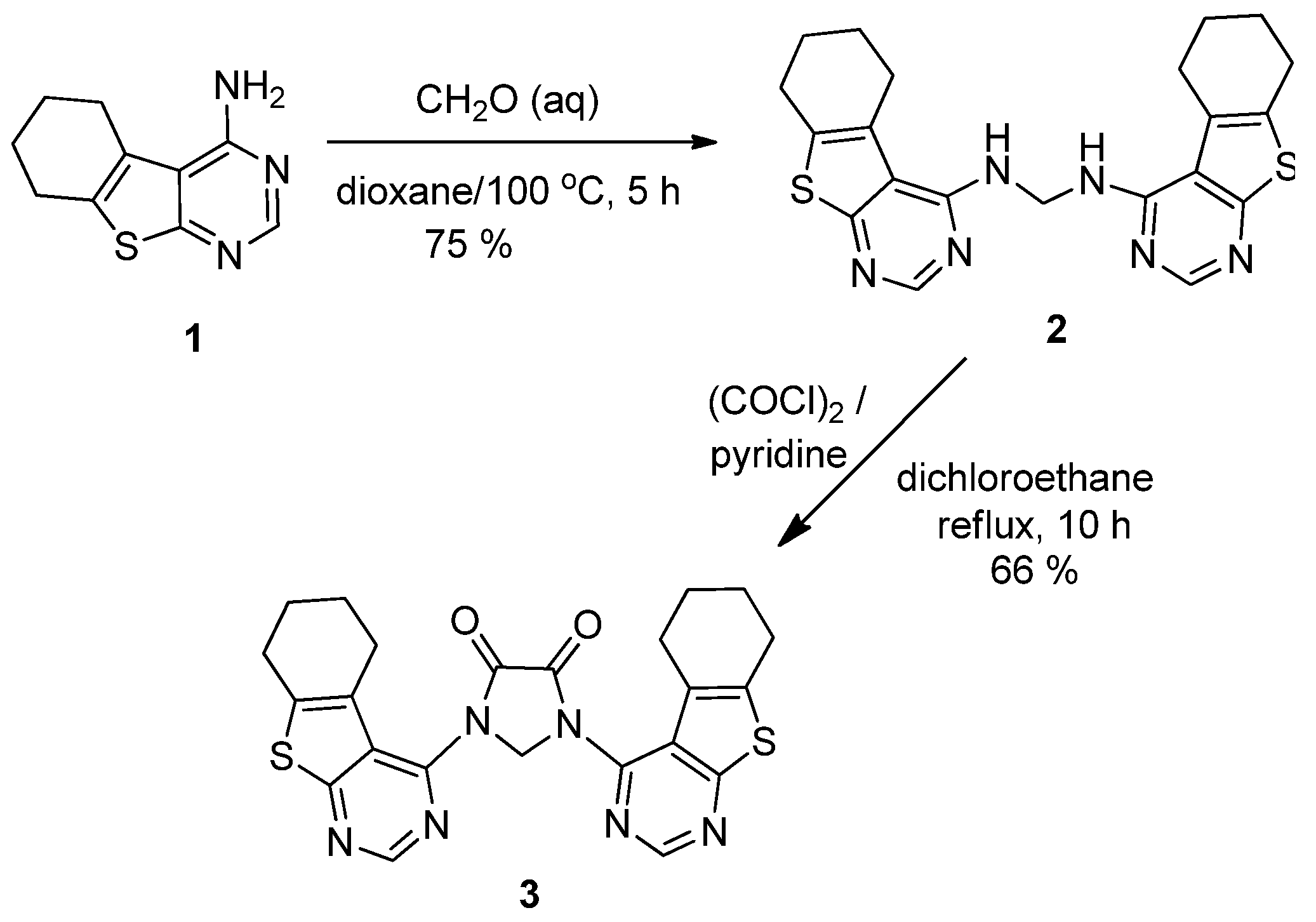
3.2. Synthesis of N,N′-Bis(5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)methanediamine (2)
3.3. Synthesis of 1,3-Bis(5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)imidazolidine-4,5-dione (3)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lagardèrè, P.; Fersing, C.; Masurier, N.; Lisowski, V. Thienopyrimidine: A promising Scaffold to access anti-infective agents. Pharmaceuticals 2022, 15, 35. [Google Scholar]
- Ali, E.M.H.; Abdel-Maksoud, M.S.; Oh, C.-H. Thieno[2,3-d]pyrimidines are a promising scaffold in medicinal chemistry: Recent advances. Bioorg. Med. Chem. 2019, 27, 1159–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Yang, J.; Zaho, H.; Deng, B.; Ren, Y.; Mai, R.; Huang, J.; Chen, J. Discovery of thieno[2,3-d]pyrimidine-based KRAS G12D inhibitors as potential anticancer agents via combinatorial virtual screening. Eur. J. Med. Chem. 2022, 233, 114243. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Singh, H.; Raj, T.; Sharma, A.; Singh, G.; Bariwal, J. 4-Substituted thieno[2,3-d]pyrimidines as potent antibacterial agents: Rational dssign, microwave-assisted synthesis, biological evaluation and molecular docking studies. Chem. Biol. Drug Des. 2017, 90, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, L.; Han, C.; Lv, H.; Chen, D.; Shen, G.; Wu, K.; Pan, S.; Ye, F. Design, synthesis, and biological activity tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine derivatives an anit-inflammatory agents. Molecules 2017, 22, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.-J.; Kim, S.M.; Rho, M.C.; Lee, S.W.; Song, Y.-H. Synthesis of thienopyrimidine derivatives as inhibitors of STAT3 activation induced by IL-6. J. Microbiol. Biotechnol. 2019, 29, 856–862. [Google Scholar] [CrossRef]
- Bassetto, M.; Leyssen, P.; Neyts, J.; Yerukhimovich, M.M.; Frick, D.N.; Brancale, A. Computer-aided identification, synthesis and evaluation of substituted thienopyrimidines as novel inhibitors of HCV replication. Eur. J. Med. Chem. 2016, 123, 31–47. [Google Scholar] [CrossRef]
- Barrows, R.D.; Hammill, J.T.; Tran, M.C.; Falade, M.O.; Rice, A.L.; Davis, C.W.; Emge, T.J.; Rablen, P.R.; Kiplin Guy, R.; Knapp, S. Evaluation of 1,1-cyclopropylidene as a thioether isostere in the 4-thio-thienopyrimidine (TTP) series of antimalarials. Bioorg. Med. Chem. 2020, 28, 115758. [Google Scholar] [CrossRef]
- Ali, O.M.; El-Sayed, W.A.; Eid, S.A.; Abdelwahed, N.A.M.; Abdel-Rahman, A.A.-H. Antimicrobial activity of new synthesized [(oxadiazolyl)methyl]phenytoin derivatives. Acta Polon. Pharm. 2012, 69, 657–667. [Google Scholar]
- Thenmozhiyal, J.C.; Wong, P.T.-H.; Chui, W.-K. Anticonvulsant activity of phenylmethylenehydantoins: A structure-activity relationship study. J. Med. Chem. 2004, 47, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Struck, R.F.; Kirk, M.C.; Rice, L.S.; Suling, W.J. Isolation, synthesis and antitumor evaluation of spirohydantoin aziridine, a mutagenic metabolite of spirohydantoin mustard. J. Med. Chem. 1986, 29, 1319–1321. [Google Scholar] [CrossRef]
- Kim, D.; Wang, L.; Caldwell, C.G.; Chen, P.; Finke, P.E.; Oates, B.; MacCoss, M.; Mills, S.G.; Malkowitz, L.; Gould, S.L.; et al. Discovery of human CCR5 antagonists containing hydantoins for the treatment of HIV-1 infection. Bioorg. Med. Chem. Lett. 2001, 11, 3099–3102. [Google Scholar] [CrossRef]
- Liang, T.; Xue, J.; Yao, Z.; Ye, Y.; Yang, X.; Hou, X.; Fang, H. Design, synthesis and biological evaluation of 3,4-disubstituted imidazolidine-2,5-dione derivatives as HDAC6 selective inhibitors. Eur. J. Med. Chem. 2021, 221, 113526. [Google Scholar] [CrossRef]
- Verardo, G.; Giumanini, A.G.; Gorassini, F.; Tolazzi, M.; Strazzolini, P. Heterocycles from heterocycles. 1,3-Diaryl-4,5-imidazolinediones from 1,3,5-triarylhexahydro-1,3,5-triazines and oxalyl chloride. Tetrahedron 1993, 46, 10609–10628. [Google Scholar] [CrossRef]
- Verardo, G.; Giumanini, A.G.; Gorassini, F.; Strazzolini, P. Heterocycles from heterocycles. 1,3-Dialkyl-4,5-imidazolinediones from 1,3,5-trialkylhexahydro-1,3,5-triazines and oxalyl chloride. Monatsh. Chem. 1995, 126, 103–105. [Google Scholar] [CrossRef]
- Ghandi, M.; Salimi, F. Synthesis of new 1,3-bis(heteroaryl)-4,5-imidazolinediones through reaction of N,N-bis(heteroaryl)methanediamines with oxalyl chloride. Synth. Commun. 2007, 37, 637–644. [Google Scholar] [CrossRef]
- Shi, T.; Zerio, C.J.; Sivinski, J.; Ambrose, A.J.; Moore, K.T.; Buckley, T.; Kaneko, L.; Zhang, M.; Zhang, D.D.; Chapman, E. A one-step, atom economical synthesis of thieno[2,3-d]pyrimidin-4-amine derivatives a four-component reaction. Eur. J. Org. Chem. 2019, 2019, 3269–3272. [Google Scholar] [CrossRef] [PubMed]
- Marzona, M.; Carpignano, R. Condensation of heterocyclic amines with formaldehyde. IV. Ann. Chim. 1965, 55, 1007–1013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Kim, S.M.; Song, Y.-H. 1,3-Bis(5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)imidazolidine-4,5-dione. Molbank 2022, 2022, M1403. https://doi.org/10.3390/M1403
Yin X, Kim SM, Song Y-H. 1,3-Bis(5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)imidazolidine-4,5-dione. Molbank. 2022; 2022(3):M1403. https://doi.org/10.3390/M1403
Chicago/Turabian StyleYin, Xuelian, Sung Min Kim, and Yang-Heon Song. 2022. "1,3-Bis(5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)imidazolidine-4,5-dione" Molbank 2022, no. 3: M1403. https://doi.org/10.3390/M1403
APA StyleYin, X., Kim, S. M., & Song, Y.-H. (2022). 1,3-Bis(5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)imidazolidine-4,5-dione. Molbank, 2022(3), M1403. https://doi.org/10.3390/M1403




