2-(4-Chlorophenyl)-4-(3,4-dimethoxy-phenyl)-6-methoxy-3-methylquinoline
Abstract
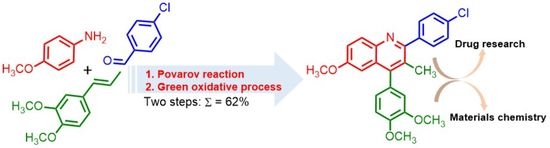
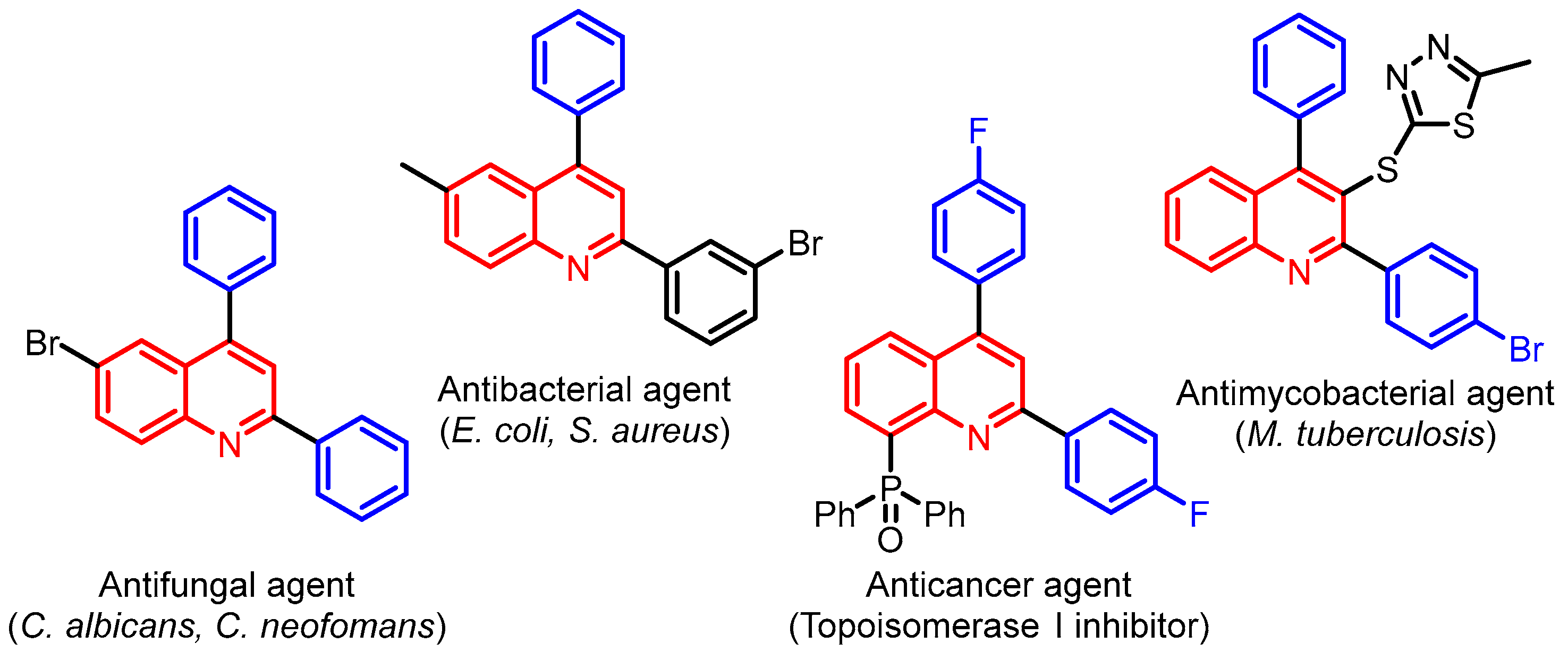
:1. Introduction
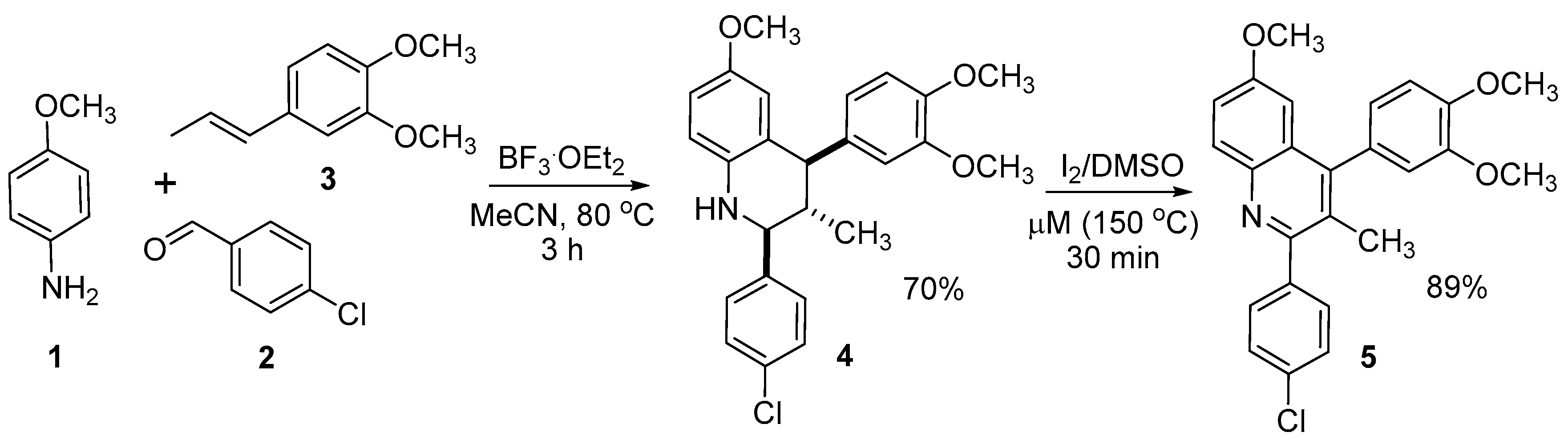
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Analysis
3.2. Synthesis of 2-(4-chlorophenyl)-4-(3,4-dimethoxyphenyl)-6-methoxy-3-methylquinoline (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jagg, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- Jain, S.; Chandra, V.; Jain, P.K.; Pathak, K.; Pathak, D.; Vaidya, A. Comprehensive review on current developments of quinoline-based anticancer agents. Arab. J. Chem. 2019, 12, 4920–4946. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.Y.; Lu, X.Q.; Ma, X.D.; Zhang, H.J.; Ji, Y.Y.; Ding, W.J.; Shen, L. Design, Synthesis and Biological Evaluation of Novel (Quinolinyl-3-pyridinyl)benzenesulfonamide-Based Hydroxamic Acids as PI3K and HDAC Dual Targeting Inhibitors. Chin. J. Org. Chem. 2020, 40, 95–107. [Google Scholar] [CrossRef]
- Tussaint, M.; Martinez, G.; Sanctis, J.D.; Monasterios, M.; Rojas, H. Antimalarial, antiproliferative, and apoptotic activity of quinoline-chalcone and quinoline-pyrazoline hybrids. A dual action. Med. Chem. Res. 2019, 28, 2050–2066. [Google Scholar]
- Kaur, K.; Jain, M.; Reddy, R.P.; Jain, R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010, 45, 3245–3264. [Google Scholar] [CrossRef]
- Elbadawi, M.M.; Eldehna, W.M.; Abd El-Hafeez, A.A.; Somaa, W.R.; Albohy, A.; Al-Rashood, S.T.; Agamah, K.K.; Elkaeedi, E.B.; Ghoshd, P.; Pommier, Y.; et al. 2-Arylquinolines as novel anticancer agents with dual EGFR/FAK kinase inhibitory activity: Synthesis, biological evaluation, and molecular modelling insights. J. Enzym. Inhib. Med. Chem. 2022, 37, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.P.; Hassan, S.; Ehlers, P.; Lecka, J.; Sévigny, J.; Rodríguez, E.T.; Iqbal, J.; Langer, P. Chemoselective Synthesis and Human Ecto-5′-nucleotidase Inhibitory Activity of 2-Trifluoromethyl-4,6-diarylquinolines. ChemistrySelect 2018, 3, 8587–8592. [Google Scholar] [CrossRef]
- Ghodsi, R.; Zarghi, A.; Daraei, B.; Hedayati, M. Design, synthesis and biological evaluation of new 2, 3-diarylquinoline derivatives as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. 2010, 18, 1029–1033. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, S.A. Quinoline: A promising antitubercular target. Biomed. Pharmacother. 2014, 68, 1161–1175. [Google Scholar] [CrossRef]
- Muscia, G.C.; Carnevale, J.P.; Luczywo, A.; Peláez, M.V.; Toole, A.R.Ó.; Buldain, G.Y.; Casal, J.J.; Asís, S.E. Synthesis, anti-tuberculosis activity and QSAR study of 2,4-diarylquinolines and analogous polycyclic derivatives. Arab. J. Chem. 2019, 12, 932–945. [Google Scholar] [CrossRef]
- Paul, N.; Murugavel, M.; Muthusubramanian, S.; Sriram, D. Camphorsulfonic acid catalysed facile tandem double Friedlander annulation protocol for the synthesis of phenoxy linked bisquinoline derivatives and discovery of antitubercular agents. Bioorg. Med. Chem. Lett. 2012, 22, 1643–1648. [Google Scholar] [CrossRef]
- Praveen, C.; DheenKumar, P.; Muralidharan, D.; Perumal, P.T. Synthesis, antimicrobial and antioxidant evaluation of quinolines and bis (indolyl) methanes. Bioorg. Med. Chem. Lett. 2010, 20, 7292–7296. [Google Scholar] [CrossRef]
- Chitra, S.; Paul, N.; Muthusubramanian, S.; Manisankar, P.; Yogeeswari, P.; Sriram, D. Synthesis of 3-heteroarylthioquinoline derivatives and their in vitro antituberculosis and cytotoxicity studies. Eur. J. Med. Chem. 2011, 46, 4897–4903. [Google Scholar] [CrossRef]
- Liberto, N.A.; Simões, J.B.; de Paiva Silva, S.; da Silva, C.J.; Modolo, L.V.; de Fátima, Â.; Silva, L.M.; Derita, M.; Zacchino, S.; Zuñiga, O.M.P.; et al. Quinolines: Microwave-assisted synthesis and their antifungal, anticancer and radical scavenger properties. Bioorg. Med. Chem. 2017, 25, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Alonso, C.; Fuertes, M.; Martín-Encinas, E.; Selas, A.; Rubiales, G.; Tesauro, C.; Knudssen, B.K.; Palacios, F. Novel topoisomerase I inhibitors. Syntheses and biological evaluation of phosphorus substituted quinoline derivates with antiproliferative activity. Eur. J. Med. Chem. 2018, 149, 225–237. [Google Scholar] [CrossRef]
- Nosova, E.V.; Achelle, S.; Lipunova, G.N.; Charushin, V.N.; Chupakhin, O.N. Functionalized benzazines as luminescent materials and components for optoelectronics. Russ. Chem. Rev. 2019, 88, 1128–1178. [Google Scholar] [CrossRef]
- Lee, H.J.; Xin, H.; Park, S.M.; Park, S.I.; Ahn, T.; Park, D.K.; Jenekhe, S.A.; Kwon, T.W. Synthesis and properties of diarylamino-substituted linear and dendritic oligoquinolines for organic light-emitting diodes. Bull. Korean Chem. Soc. 2012, 33, 1627–1637. [Google Scholar] [CrossRef] [Green Version]
- Adeloye, A.O.; Mphahlele, M.J. 2,4-Diarylquinolines: Synthesis, absorption and emission properties. J. Chem. Res. 2014, 38, 254–259. [Google Scholar] [CrossRef]
- Ghate, M.; Dahule, H.K.; Thejo Kalyani, N.; Dhoble, S.J. Synthesis and characterization of high quantum yield and oscillator strength 6-chloro-2-(4-cynophenyl)-4-phenyl quinoline (cl-CN-DPQ) organic phosphor for solid-state lighting. Luminescence 2018, 33, 297–304. [Google Scholar] [CrossRef]
- Karmakar, S.; Dey, S.; Upadhyay, M.; Ray, D. Phenoxazine—Quinoline Conjugates: Impact of Halogenation on Charge Transfer Triplet Energy Harvesting via Aggregate Induced Phosphorescence. ACS Omega 2022, 7, 16827–16836. [Google Scholar] [CrossRef]
- Bharate, J.B.; Vishwakarma, R.A.; Bharate, S.B. Metal-free domino one-pot protocols for quinoline synthesis. RSC Adv. 2015, 5, 42020–42053. [Google Scholar] [CrossRef]
- Ramann, G.A.; Cowen, B.J. Recent advances in metal-free quinoline synthesis. Molecules 2016, 21, 986. [Google Scholar] [CrossRef]
- Naidoo, S.; Jeena, V. Synthesis of 2,4-disubstituted quinoline derivatives via A3-coupling: An ecoscale evaluation. Synthesis 2017, 49, 2621–2631. [Google Scholar] [CrossRef]
- Devarajan, N.; Suresh, P. Iron-MOF-Catalyzed Domino Cyclization and Aromatization Strategy for the Synthesis of 2,4-Diarylquinolines. Asian J. Org. Chem. 2020, 9, 437–444. [Google Scholar] [CrossRef]
- Cheng, D.; Yan, X.; Shen, J.; Pu, Y.; Xu, X.; Yan, J. Synthesis of 2,4-Diarylquinoline Derivatives via Chloranil-Promoted Oxidative Annulation and One-Pot Reaction. Synthesis 2020, 52, 1833–1840. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Rojas Ruiz, F.A.; Vargas Mendez, L.Y.; Gupta, M.P. Simple C-2-substituted quinolines and their anticancer activity. Lett. Drug Des. Discov. 2012, 9, 680–686. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Gómez Meléndez, C.M.; Derita, M.G.; Svetaz, L.; del Olmo, E.; Zacchino, S.A. Synthesis and antifungal activity of diverse C-2 pyridinyl and pyridinylvinyl substituted quinolines. Bioorg. Med. Chem. 2012, 20, 6506–6512. [Google Scholar] [CrossRef]
- Villamizar-Mogotocoro, A.-F.; Vargas-Méndez, L.Y.; Kouznetsov, V.V. Pyridine and quinoline molecules as crucial protagonists in the never-stopping discovery of new agents against tuberculosis. Eur. J. Pharm. Sci. 2020, 151, 105374. [Google Scholar] [CrossRef]
- Echeverry-Gonzalez, C.A.; Ortiz Villamizar, M.C.; Kouznetsov, V.V. The remarkable selectivity of the 2-arylquinoline-based acyl hydrazones toward copper salts: Exploration of their catalytic applications in the copper catalysed N-arylation of indole derivatives and C1-alkynylation of tetrahydroisoquinolines via the A 3 reaction. New J. Chem. 2021, 45, 243–250. [Google Scholar]
- Povarov, L.S. α,β-Unsaturated ethers and their analogues in reactions of diene synthesis. Russ. Chem. Rev. 1967, 36, 656–670. [Google Scholar] [CrossRef]
- Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels-Alder reaction (Povarov reaction): Application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 2009, 14, 2721–2750. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Bohorquez, A.R.R.; Stashenko, E.E. Three-component imino Diels–Alder reaction with essential oil and seeds of anise: Generation of new tetrahydroquinolines. Tetrahedron Lett. 2007, 48, 8855–8860. [Google Scholar] [CrossRef]
- Reddy, P.N.; Reddy, B.V.S.; Padmaja, P. Emerging Role of Green Oxidant I2/DMSO in Organic Synthesis. Curr. Org. Synth. 2018, 15, 815–838. [Google Scholar] [CrossRef]
- Peñaranda Gómez, A.; Puerto Galvis, C.E.; Macías, M.A.; Ochoa-Puentes, C.; Kouznetsov, V.V. I2/DMSO-Promoted Synthesis of Chromeno [4,3-b] quinolines through an Imine Formation/Aza-Diels–Alder/Aromatization Tandem Reaction under Metal-Catalyst-and Photosensitizer-Free Conditions. Synthesis 2022, 54, 1857–1869. [Google Scholar]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.molinspiration.com (accessed on 12 April 2022).
- Veber, D.F.; Jhonson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Enciso, D.A.; Puerto Galvis, C.E.; Kouznetsov, V.V. 2-(4-Chlorophenyl)-4-(3,4-dimethoxy-phenyl)-6-methoxy-3-methylquinoline. Molbank 2022, 2022, M1383. https://doi.org/10.3390/M1383
Rodríguez Enciso DA, Puerto Galvis CE, Kouznetsov VV. 2-(4-Chlorophenyl)-4-(3,4-dimethoxy-phenyl)-6-methoxy-3-methylquinoline. Molbank. 2022; 2022(2):M1383. https://doi.org/10.3390/M1383
Chicago/Turabian StyleRodríguez Enciso, Duván A., Carlos E. Puerto Galvis, and Vladimir V. Kouznetsov. 2022. "2-(4-Chlorophenyl)-4-(3,4-dimethoxy-phenyl)-6-methoxy-3-methylquinoline" Molbank 2022, no. 2: M1383. https://doi.org/10.3390/M1383
APA StyleRodríguez Enciso, D. A., Puerto Galvis, C. E., & Kouznetsov, V. V. (2022). 2-(4-Chlorophenyl)-4-(3,4-dimethoxy-phenyl)-6-methoxy-3-methylquinoline. Molbank, 2022(2), M1383. https://doi.org/10.3390/M1383