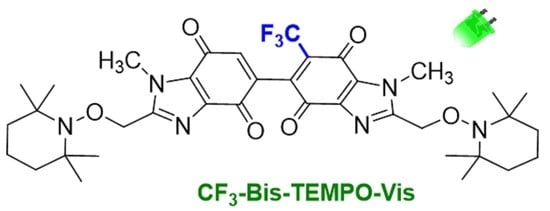
CF3-Bis-TEMPO-Vis: New Visible Light Active Bis-Benzimidazolequinone Alkoxyamine
Abstract
:1. Introduction
2. Results and Discussion
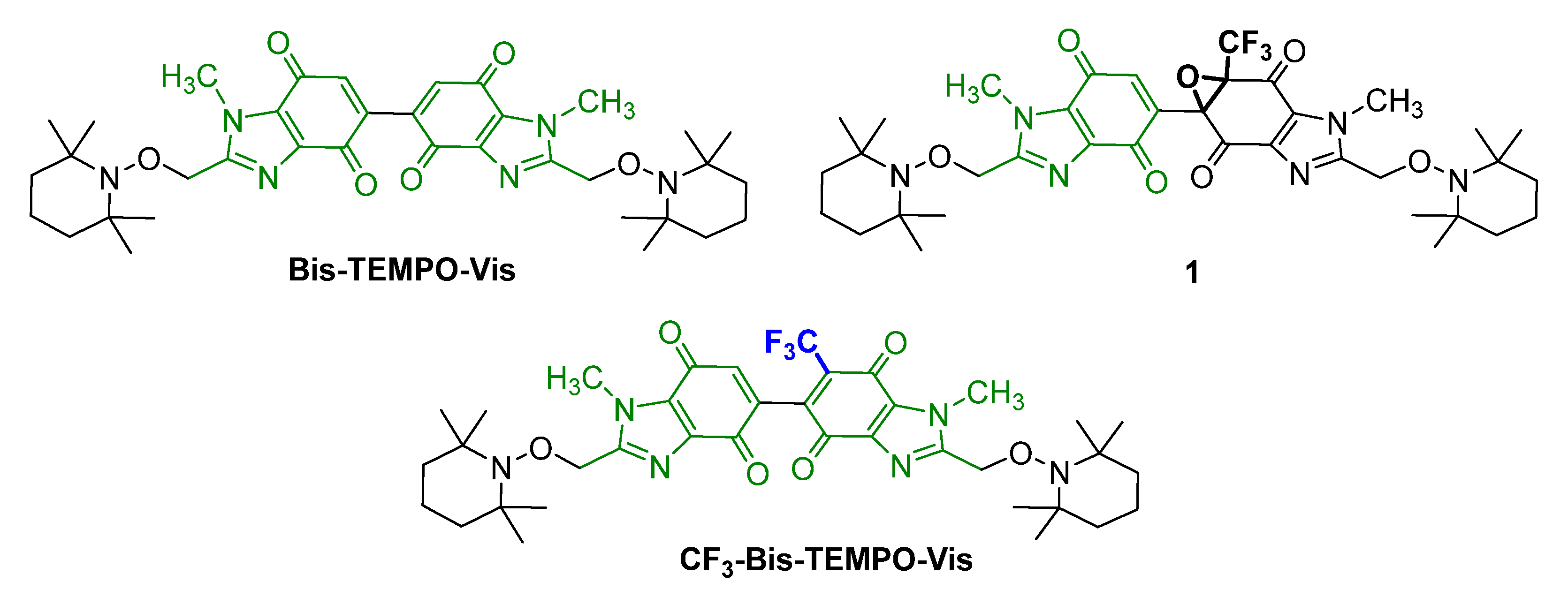
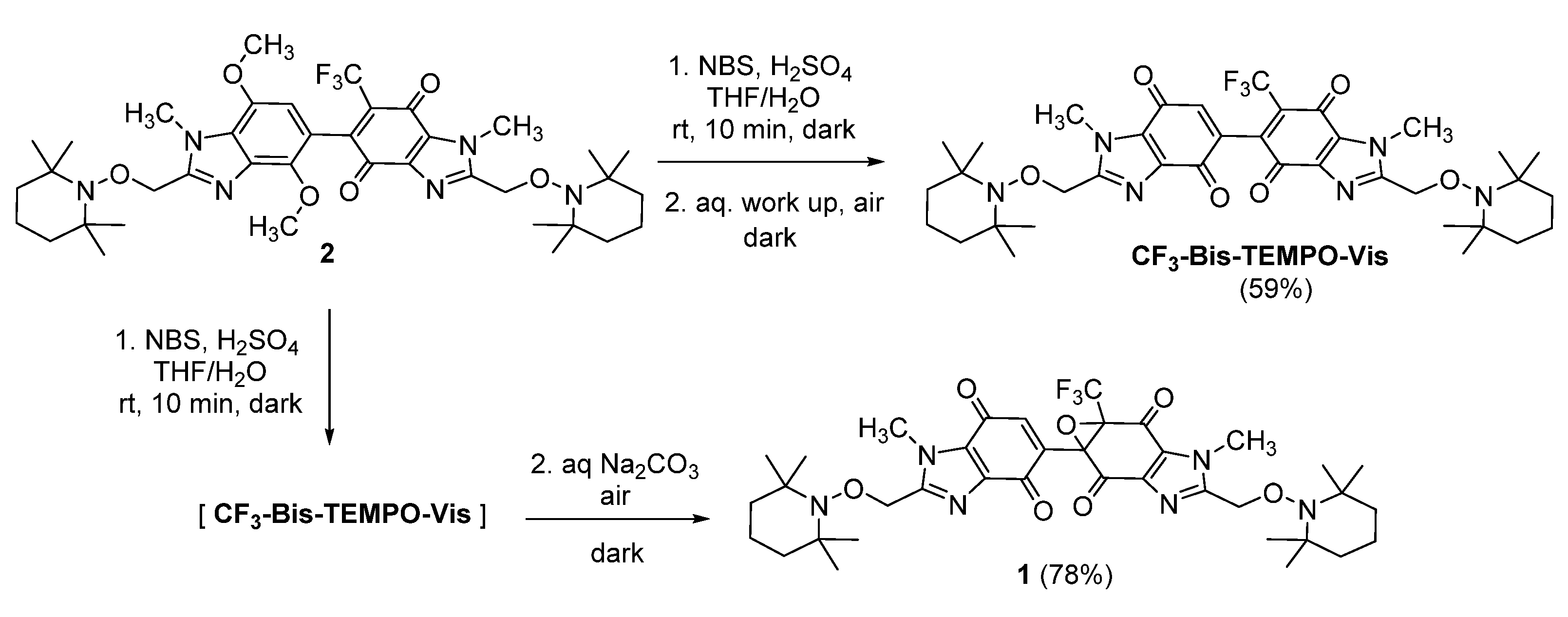
2.1. Synthesis
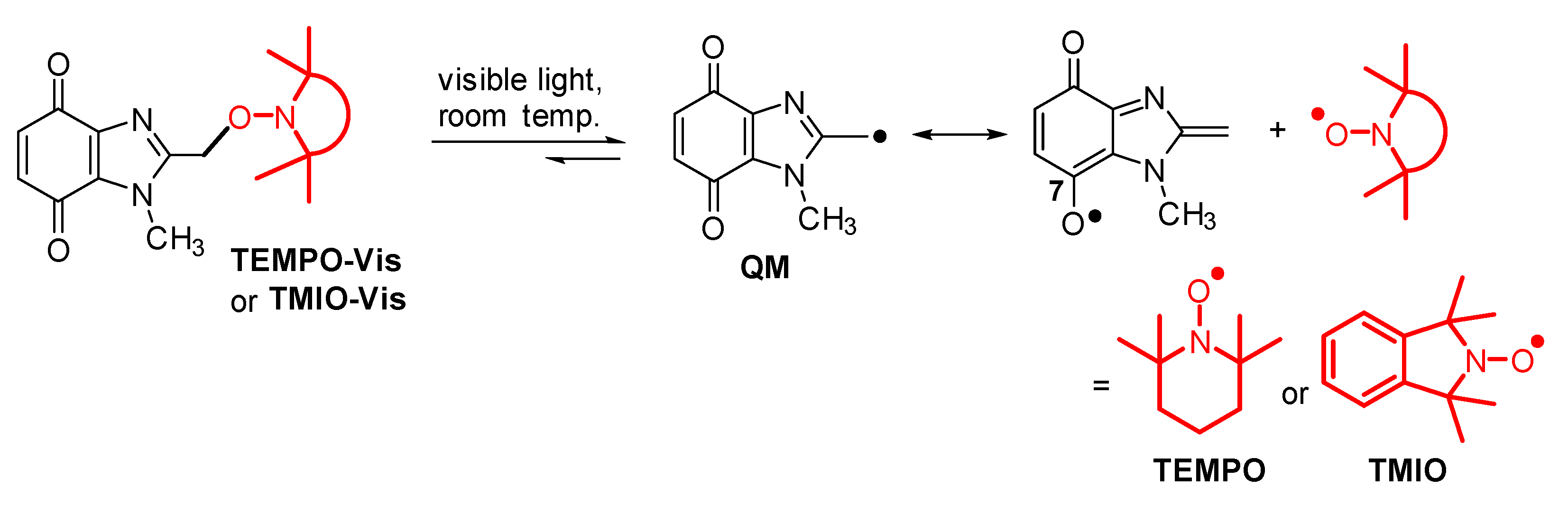
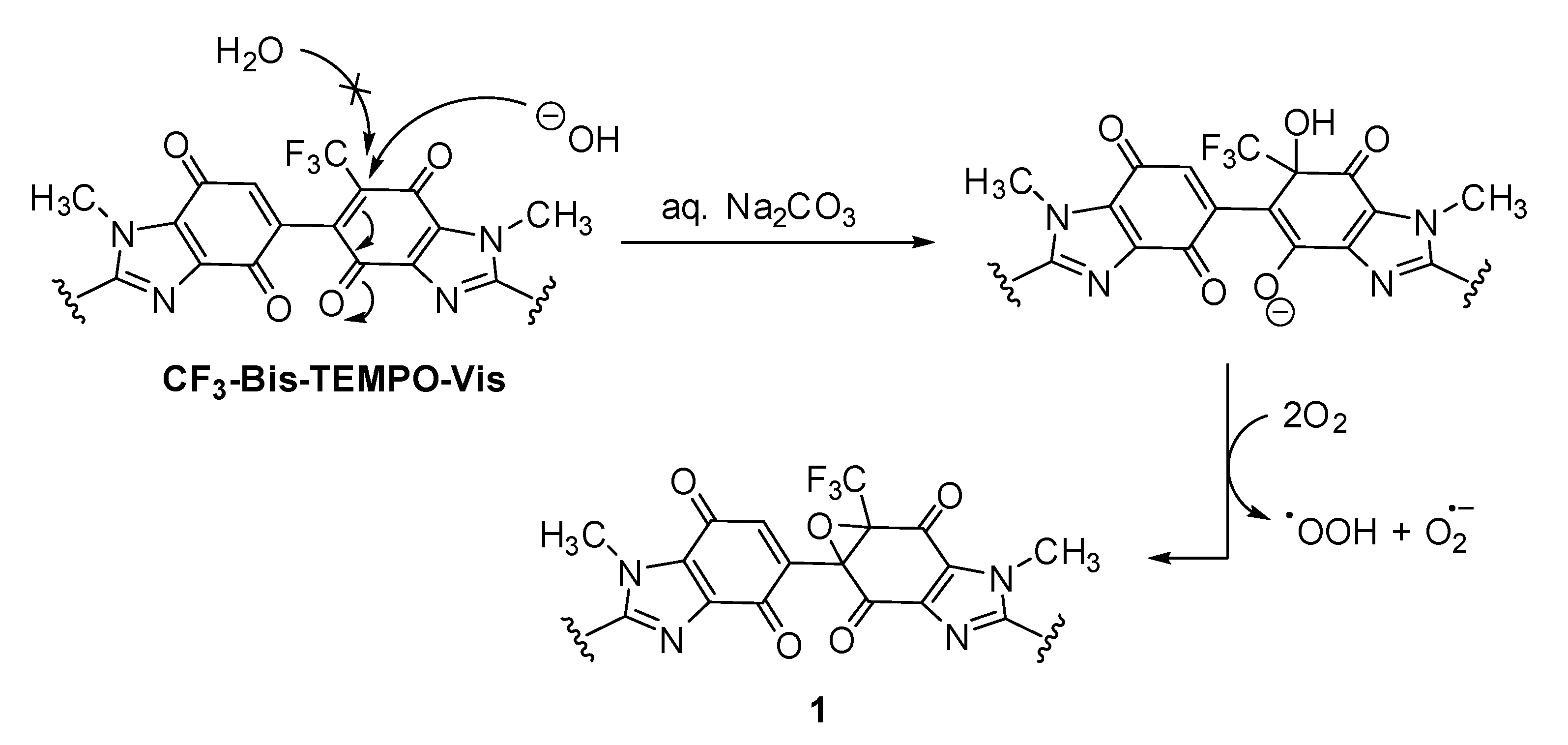
2.2. Kinetics of Homolysis
3. Experimental
3.1. Materials
3.2. Measurements
3.3. The Photoreactor
3.4. Analytical HPLC
3.5. Homolysis Rates (kd)
3.6. Synthesis of 1,1′-Dimethyl-2,2′-bis{[(2,2,6,6-tetramethylpiperidin-1-yl)oxy]methyl}-6-(trifluoromethyl)-1H,1′H-[5,5′-bibenzimidazole]-4,4′,7,7′-tetrone (CF3-Bis-Tempo-Vis)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Bagryanskaya, E.G.; Marque, S.R.A. Scavenging of organic C-centered radicals by nitroxides. Chem. Rev. 2014, 114, 5011–5056. [Google Scholar] [CrossRef] [PubMed]
- Kielty, P.; Farràs, P.; McArdle, P.; Smith, D.A.; Aldabbagh, F. Visible-light unmasking of heterocyclic quinone methide radicals from alkoxyamines. Chem. Commun. 2019, 55, 14665–14668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielty, P.; Chalmers, B.A.; Farràs, P.; Smith, D.A.; Aldabbagh, F. Visible light activated benzimidazolequinone alkoxyamines of 1,1,3,3-tetramethyl-2,3-dihydroisoindol-2-yloxyl (TMIO). Eur. J. Org. Chem. 2021. Accepted Article. [Google Scholar] [CrossRef]
- Bass, P.D.; Gubler, D.A.; Judd, T.C.; Williams, R.M. Mitomycinoid alkaloids: Mechanism of action, biosynthesis, total syntheses, and synthetic approaches. Chem. Rev. 2013, 113, 6816–6863. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, M.; Conboy, D.; Mirallai, S.I.; Aldabbagh, F. Advances in the synthesis of ring-fused benzimidazoles and imidazobenzimidazoles. Molecules 2021, 26, 2684. [Google Scholar] [CrossRef]
- Wellington, K.M. Understanding cancer and the anticancer activities of naphthoquinones—A review. RSC Adv. 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, D.; Ye, S.; Liu, Z.; Zhu, G. Synthesis of trifluoromethylated naphthoquinones via copper-catalyzed cascade trifluoromethylation/cyclization of 2-(3-arylpropioloyl)benzaldehydes. Org. Lett. 2017, 19, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Scaiano, J.C.; Connolly, T.J.; Mohtat, N.; Pliva, C.N. Exploratory study of the quenching of photosensitizers by initiators of free radical “living” polymerization. Can. J. Chem. 1997, 75, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Audran, G.; Brémond, P.; Joly, J.-P.; Marque, S.R.A.; Yamasaki, T. C–ON bond homolysis in alkoxyamines. Part 12: The effect of the para-substituent in the 1-phenylethyl fragment. Org. Biomol. Chem. 2016, 14, 3574–3583. [Google Scholar] [CrossRef] [PubMed]
- Kielty, P. Heterocyclic Chemistry: Controlled Unmasking of Nitric Oxide and Nitroxides. Ph.D. Thesis, National University of Ireland Galway, Galway City, Ireland, 2019. [Google Scholar]
- Harwood, L.M. Dry-Column Flash Chromatography. Aldrichim. Acta 1985, 18, 25. [Google Scholar]



| Bis-Alkoxyamine | [Bis-Alkoxyamine]0 (mM) | mol% TEMPO Released (time, min) c,d | kd (min−1) b |
|---|---|---|---|
| CF3-Bis-TEMPO-Vis | 5.00 | 130 (348) | - |
| Bis-TEMPO-Vis | 5.00 | 152 (348) | - |
| 1 | 5.00 | 73 (180) | 0.0162 ± 0.0010 |
| CF3-Bis-TEMPO-Vis | 0.25 | 125 (80) | 0.0472 ± 0.0018 |
| Bis-TEMPO-Vis | 0.25 | 162 (15) e | 0.1480 ± 0.0020 |
| 1 | 0.25 | 43 (65) e | 0.0321 ± 0.0070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kielty, P.; Farràs, P.; Smith, D.A.; Aldabbagh, F. CF3-Bis-TEMPO-Vis: New Visible Light Active Bis-Benzimidazolequinone Alkoxyamine. Molbank 2021, 2021, M1300. https://doi.org/10.3390/M1300
Kielty P, Farràs P, Smith DA, Aldabbagh F. CF3-Bis-TEMPO-Vis: New Visible Light Active Bis-Benzimidazolequinone Alkoxyamine. Molbank. 2021; 2021(4):M1300. https://doi.org/10.3390/M1300
Chicago/Turabian StyleKielty, Patrick, Pau Farràs, Dennis A. Smith, and Fawaz Aldabbagh. 2021. "CF3-Bis-TEMPO-Vis: New Visible Light Active Bis-Benzimidazolequinone Alkoxyamine" Molbank 2021, no. 4: M1300. https://doi.org/10.3390/M1300
APA StyleKielty, P., Farràs, P., Smith, D. A., & Aldabbagh, F. (2021). CF3-Bis-TEMPO-Vis: New Visible Light Active Bis-Benzimidazolequinone Alkoxyamine. Molbank, 2021(4), M1300. https://doi.org/10.3390/M1300