Abstract
11H-Indeno[1,2-b]quinoxaline derivatives present an important type of nitrogen-containing heterocyclic compound that are useful intermediate products in organic synthesis and have potential pharmaceutical applications. A new 11H-indeno[1,2-b]quinoxalin-11-one-2-(4-ethylbenzylidene)hydrazone (compound 3) was synthesized. Compound 3 is the first example of an azine derivative based on the 11H-indeno[1,2-b]quinoxaline system. The Z,E-isomerism of compound 3 was investigated by DFT calculations. Bioavailability was evaluated in silico using ADME predictions. According to the ADME results, compound 3 is potentially highly bioavailable and has potential to be used for the treatment of neuroinflammation and ischemia–reperfusion injury.
1. Introduction
Compounds containing a C=N bond attached to a heterocyclic moiety exhibit various chemical reactivities and often possess pharmacological activities. Prominent representatives of these compounds are azines, which can be regarded as hydrazine derivatives of the general formula RR′C=N-N=CR′′R′′′. Azines have recently attracted attention owing to their diverse therapeutic activities [1,2]. The synthesis of azines can be performed by the condensation of hydrazine with two moles of aldehyde/ketone under reflux conditions [1]. Azines are often the major products obtained by the thermal decomposition of diazo compounds [2]. The process is bimolecular and involves the nucleophilic attack of the carbon atom of the first diazo compound (which gives carbene by the removal of N2) on the terminal nitrogen of the second compound. Zhao et al. reported the formation of symmetrical azines produced by the copper catalyzed homocoupling of oximes [3]. Nanjundaswamy and co-workers reported on the iodine catalyzed synthesis of symmetrical azines by treating NH2NH2·H2O with carbonyl compounds at 0–10 °C [4].
A prominent drug belonging to the class of azines is Guanabenz (Figure 1) [5], which has always been considered a guanylhydrazone derivative. Figure 1 shows two tautomeric forms of Guanabenz (A and B), with the azine form (B) being more stable [6,7]. Additionally, important examples are the antihypertensive agent bearing 2,6-dichlorophenyl and a 1,1-diamino moieties [5], the trypanocidal agent containing the azine unit attached to imidazopyridine [8], the antibacterial compound [9], and the anticancer agent [10] (Figure 1) 4-((E)-((E)-((4-bromophenyl)(phenyl)methylene)hydrazono)methyl)−2,6-dimethoxyphenol (BRH) that was active against MCF-7 cancerous cell lines [11].
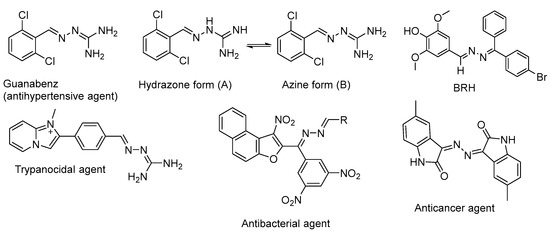
Figure 1.
Examples of biologically active azines.
At present, the variation in conjugation via the azine substructure and its modulation depending on the substituents has not been fully investigated. It is important to study redox properties and the associated characteristics of electron exchange and their influence on chemical bonds in these systems, especially in azines containing heterocyclic fragments of pharmacological importance. In this work, we have synthesized the first representative of an azine with a 11H-indeno[1,2-b]quinoxaline moiety that is contained in numerous biologically active compounds possessing anti-inflammatory [12], antimicrobial [13], anticancer [14], and JNK inhibitory [12] properties. The bioavailability and electronic structure of the synthesized compound were evaluated with the use of computational methods.
2. Results and Discussion
2.1. Synthesis
We preliminary obtained 11H-indeno[1,2-b]quinoxaline-11-one (1) and its hydrazone (2). The simplest way to synthesize compound 1 consists in the condensation of ninhydrin with o-phenylenediamine [15] (Scheme 1). The 11H-indeno[1,2-b]quinoxalin-11-one hydrazone (2) was obtained by the nucleophilic addition of hydrazine hydrate to ketone 1 [16] (Scheme 1).
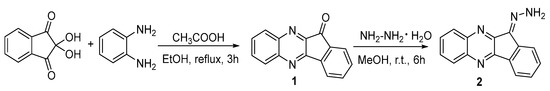
Scheme 1.
Synthesis of compounds 1 and 2.
For the first time, we have obtained a new functional compound, 11H-indeno[1,2-b]quinoxalin-11-one 2-(4-ethylbenzylidene)hydrazone (3, Scheme 2), which contains an azine group and an indenoquinoxaline system. This compound is of interest as a potential biologically active compound that could find application in medicinal, organic, and material chemistry.
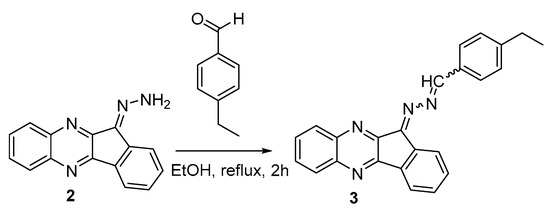
Scheme 2.
Synthesis of title compound 3.
Further modification of compound 2 to the target product 3 was performed by the action of p-ethylbenzaldehyde (Scheme 2). The reaction proceeds for 2 h under reflux in ethanol in the absence of alkalis or acids. At the end of the process, complete conversion was observed (control by TLC, eluent hexane: ethyl acetate (2:1, v/v)). The expected crude azine was isolated by filtration with 89% yield. The compound was purified by recrystallization from ethanol.
According to the NMR data (CDCl3), the recrystallized compound 3 was obtained as a Z,E-isomer mixture. The 1H NMR spectrum (Figure S1) contains two distinct low-field signals of the imine proton N=CH at 8.91 and 8.85 ppm with relative integral intensities 0.35/0.65, as well as typical signals of the ethyl group at 1.30 (two overlaid triplets from different isomers) and 2.75 ppm (multiplet). Signals of the indenoquinoxaline heterocycle were observed between 7.5 and 8.5 ppm. Separation of the isomers was impossible probably due to a relatively low energy barrier for the isomerization of the carbon–nitrogen double bond (see, e.g., [17] and our DFT results described below).
The main characteristics of the title compound 3: yellow crystals, M.p. 195–196 °C, soluble in acetone and chloroform. The NMR data are presented in Section 3.1, Figures S1 and S2.
2.2. DFT Study of Aldazine Isomerism
Azines can exhibit Z,E-isomerism due to the presence of two C=N bonds. We studied the relative stability of four possible geometric isomers of compound 3 in chloroform using the DFT method. The lowest-energy conformations of the isomers were found with B3LYP/G functional implemented in ORCA 5.0 software. The ma-def2-SVP basis set [18] was used for final geometry optimizations. This basis set includes diffuse functions pertinent for an adequate treatment of azine lone pair interactions. The optimized structures of the geometric isomers are presented in Figure S3. The E,E-isomer was found to be the most thermodynamically stable (here and below, the first symbol in isomer notation describes the configuration of the azine C=N bond attached to the indenoquinoxaline moiety while the second symbol refers to the C=N bond near the p-ethylphenyl fragment). The Z,E-, E,Z- and Z,Z-isomers have the calculated Gibbs free energies 3.16, 4.91 and 7.13 kJ/mol, respectively, higher than the E,E-isomer. Based on these results, we propose that the synthesized compound 3 consists of relatively more stable E,E- and Z,E-isomers, as the other two isomers with Z-orientation of the p-ethylphenyl substituent are characterized by higher Gibbs free energies. It should be noted that only the E,E-isomer has the optimized structure with a fully coplanar arrangement of molecular moieties.
To evaluate the energy barriers for Z,E isomerization of both azine C=N bonds, we applied the climbing image nudged elastic band (CI-NEB) methodology, which is efficient at finding minimum energy paths and saddle points [19]. The climbing images (CIs) obtained for E,E ⇄ Z,E and E,E ⇄ E,Z isomerization of compound 3 can be considered good approximations of the corresponding transition states. The CIs and other intermediate images on the minimum energy paths are shown in Figure S4, where the interpolated energy diagrams are presented. Based on the calculated DFT energies of CIs, we have estimated the barriers as 89 and 84 kJ/mol for E,E ⇄ Z,E and E,E ⇄ E,Z isomerization, respectively. The obtained results suggest that the interconversion in both pairs of isomers occurs via in-plane inversion of the nitrogen atom like in other similar compounds [17,20], i.e., without rotation around C=N double bonds. Thus, the values of N-N=C valence angles in CIs are close to 160° (Figure S4).
The calculated isomerization barriers are high enough to explain the distinct signals of isomers in the NMR spectra of compound 3. However, they are close in magnitude, for example, to the rotational barrier about the C–N bond in acetamide [21]. These data agree with the observed difficulties in the isolation of individual isomers of the title compound.
2.3. In Silico ADME Predictions
We evaluated the ADME characteristics of the most potent JNK and cell-based systems of compound 3 using the SwissADME online tool [22]. We obtained bioavailability radar plots that display an assessment of the drug-likeness of azine 3. Six important physicochemical properties, including lipophilicity, size, polarity, solubility, flexibility, and insaturation, were considered. It was found that the investigated heterocyclic azine in general has satisfactory ADME properties as can be seen from a radar representation of bioavailability shown in Figure S5. The only unfavorable property is a high insaturation score of compound 3, which is true of most 11H-indeno[1,2-b]quinoxalin-11-one derivatives. Noticeably, the known JNK inhibitor SP600125 of the anthrapyrazolone series [23] also has enhanced insaturation. Compared to SP600125, compound 3 has a higher predicted lipophilicity, which usually correlates with decreased water solubility, increased metabolism, and slower excretion. Additionally, higher lipophilicity makes it more likely to penetrate the skin. According to the calculated ADME parameters (Table 1) and bioavailability radars for compound 3 and SP600125 (Figure S5), the synthesized azine 3 is expected to be bioavailable.

Table 1.
Physicochemical ADME properties of compound 3.
3. Materials and Methods
3.1. General Information and Compound 3 Synthesis
LC/MS analysis was performed on an Agilent Infinity chromatograph (Santa Clara, CA, USA) with an AccurateMass QTOF 6530 mass detector (Santa Clara, CA, USA). Chromatographic conditions: column Zorbax EclipsePlusC18 1.8 μm, 2.1 × 50 mm; eluent H2O: ACN (85%); flow 0.2 mL/min. Ionization source: ESI in positive mode. The 1H and 13C NMR spectra were recorded on a Bruker AVANCE III HD instrument (Billerica, MA, USA) (operating frequency 1H—400 MHz; 13C—100 MHz). The melting point of the obtained compound was measured using a Melting Point Apparatus SMP30 (Cole-Parmer Instrument Company, Vernon Hills, IL, USA), heating rate 3.0 °C/min. IR spectra were recorded on an FT-IR spectrometer Nicolet 5700 (Thermo Fisher Scientific Inc., Waltham, MA, USA) with KBr pellets. The reaction was monitored by thin layer chromatography (TLC) on Silufol UV-254 and Merck plates, silica gel 60, F254.
Known compounds 11H-indeno[1,2-b]quinoxaline-11-one (1) and its hydrazone (2) were prepared according to methods described in the literature [15,16].
11H-indeno[1,2-b]quinoxalin-11-one 2-(4-ethylbenzylidene)hydrazone (3). p-Ethylbenzaldehyde (0.3 mmol, 0.04 mL) was added to a solution of compound 2 (0.3 mmol, 0.074 g) in 25 mL EtOH under permanent stirring. Then, the reaction mixture was refluxed under stirring for 2 h. The reaction was monitored by TLC (eluent: chloroform). After cooling, the precipitate was filtered out and washed with EtOH. The title compound 3 was obtained as yellow crystals (yield 89%); M.p. 195–196 °C (from ethanol).
1H NMR (400 MHz, CDCl3), δ, ppm: 1.23–1.34 (m, 3H, CH2CH3), 2.69–2.80 (m, 2H, CH2CH3), 7.49–7.68 (m, 2H, H-2, H-3), 7.73–7.86 (m, 2H, H-7, H-8), 8.09 (d, 1H, J 4 Hz, H-9), 8.18 (d, 1H, J 6 Hz, H-6), 8.25–8.34 (m, C6H4), 8.32 (d, 1H, J 4 Hz, H-4), 8.74 (d, 1H, J 4 Hz, H-1), 8.85 (s, 0.15 H, H-12), 8.91 (s, 0.85 H, H-12) (atom numbering is shown in Scheme 3). 13C NMR (100 MHz, CDCl3), δ, ppm: 15.34, 15.50, 29.16, 29.29, 122.59, 126.93, 128.14, 128.67, 128.79, 129.30, 129.49, 129.74, 129.77, 130.11, 130.46, 130.85, 130.93, 131.70, 132.18, 132.80, 134.47, 134.65, 138.32, 142.50, 142.70, 149.20, 150.83, 151.85, 151.96, 154.41, 155.77. IR (KBr), cm−1: ν(C=N) 1546, 1587. LC/MS (ESI+); m/z: 363.1605 experimental ([C24H18N4 + H]+ = 363.1604 theor.); m/z: 385.1425 exp. ([C24H18N4 + Na]+ = 385.1424 theor.); exit time 140–170 s. The ratio of isotopic peaks corresponds to the theoretical m/z: 363.16 (100%), 364.16 (27%), 365.16 (4%).
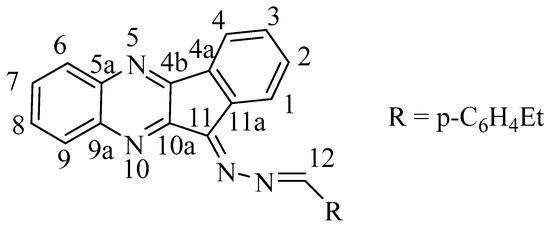
Scheme 3.
Atom numbering for NMR assignments in molecule 3.
The 1H NMR and 13C NMR spectrum are shown in Figures S1 and S2.
3.2. DFT Calculations
The ORCA 5.0 computational chemistry software [24] was used for DFT calculations of E,E-, E,Z-, Z,E- and Z,Z-isomers of compound 3. Before the calculations, conformation searches were performed for geometric isomers using the VConf 2.0 program of the VeraChem suite software (VeraChem LLC, Germantown, MD, USA). For the best ten conformations found for each isomer, singlet state geometry optimizations were carried out with ORCA 5.0 employing the BLYP functional and def2-SVP basis set. Afterwards, the lowest-energy conformation of each isomer was re-optimized with the use of the B3LYP/G functional, ma-def2-SVP basis set, and D3BJ dispersion correction. Solvation effects were taken into account using the CPCM model with chloroform as a solvent. Frequency calculations were performed for the optimized geometries in order to establish the nature of the stationary points. For CI-NEB calculations of the isomerization paths and barriers, the DFT approximation indicated above with B3LYP/G functional was applied using 10 intermediate images for each isomerization. Analysis and visualization of the DFT results were made with Chemcraft 1.8 program. The ORCA 5.0 output files for the lowest-energy conformations, CIs, and minimum energy path trajectories are available in the Supplementary Materials.
3.3. ADME Predictions
The physicochemical properties of selected compounds were computed using SwissADME (http://www.swissadme.ch) (accessed on 20 September 2021).
4. Conclusions
In this work, we presented the synthesis of the previously unknown compound 3 (11H-indeno[1,2-b]quinoxalin-11-one 2-(4-ethylbenzylidene)hydrazone). The compound structure was confirmed by NMR, IR, and LC/MS methods. According to the DFT results, compound 3 has a thermodynamically favorable E,E-configuration. The calculated isomerization paths suggest that the E,Z-isomerization occurs via in-plane inversion of the azine nitrogen atoms. An in silico estimation of ADME characteristics indicates that compound 3 can cross the blood–brain barrier and thus has perspectives to be used for the treatment of neuroinflammation and ischemia–reperfusion injury like other indenoquinoxaline analogues [12]. Despite using classic methods to obtain azine derivatives, we have discovered a new class of azines based on the heterocyclic system of 11H-indeno[1,2-b]quinoxalin-11-one, which is of great interest for further study of possible biologically active compounds.
Supplementary Materials
The following are available online. Figure S1: The 1H NMR spectrum of recrystallized compound 3; Figure S2: The 13C NMR spectrum of compound 3; Figure S3: The structures of E,E-, Z,E-, E,Z-, and Z,Z-isomers of compound 3 optimized by the DFT method; Figure S4: The energy diagrams and the climbing image conformations for E,E⇄Z,E and E,E⇄E,Z isomerization of compound 3; Figure S5: Bioavailability radar plots of compound 3 and SP600125. Files: ORCA 5.0 output files for four geometric isomers of the title compound, XYZ files for CI conformations and isomerization trajectories.
Author Contributions
Conceptualization was conducted by A.R.K. and A.I.K.; methodology and experimental work were conducted by A.R.K. and E.I.S.; data analysis, writing and editing of the paper were conducted by A.R.K. and A.I.K.; project administration and supervision was conducted by A.I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Higher Education of the Russian Federation (project no. Nauka FSWW-2020-0011) and by the Tomsk Polytechnic University development program. The synthesis and DFT study of compound 3 were funded by the Russian Science Foundation (grant No. 17-15-01111).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors wish to thank Alexander A. Bondarev for the MS analysis of compound 3.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Outirite, M.; Lebrini, M.; Lagrenée, M.; Bentiss, F. New one step synthesis of 3,5-disubstituted pyrazoles under microwave irradiation and classical heating. J. Heterocycl. Chem. 2008, 4, 503–505. [Google Scholar] [CrossRef]
- Regitz, M.; Weise, G.; Lenz, B.; Förster, U.; Urgast, K.; Maas, G. Diazo Compounds. Part 67. Electrophilic Diazoalkane Substitution with Donor‐substituted Cations. Bull. Soc. Chim. Belg. 1985, 94, 499–520. [Google Scholar] [CrossRef]
- Zhao, M.-N.; Liang, H.; Ren, Z.-H.; Guan, Z.-H. Copper-Catalyzed N–N Bond Formation by Homocoupling of Ketoximes via N–O Bond Cleavage: Facile, Mild, and Efficient Synthesis of Azines. Synthesis 2012, 1501–1506. [Google Scholar] [CrossRef]
- Nanjundaswamy, H.; Pasha, M. Rapid, Chemoselective and Facile Synthesis of Azines by Hydrazine/I2. Synth. Commun. 2007, 37, 3417–3420. [Google Scholar] [CrossRef]
- Diamant, S.; Agranat, I.; Goldblum, A.; Cohen, S.; Atlas, D. β-adrenergic activity and conformation of the antihypertensive specific α2-agonist drug, guanabenz. Biochem. Pharmacol. 1985, 34, 491–498. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Chourasiya, S.S.; Bharatam, P.V. Azine or hydrazone? The dilemma in amidinohydrazones. RSC Adv. 2015, 5, 55938–55947. [Google Scholar] [CrossRef]
- Chourasiya, S.S.; Kathuria, D.; Nikam, S.S.; Ramakrishnan, A.; Khullar, S.; Mandal, S.K.; Chakraborti, A.K.; Bharatam, P.V. Azine-Hydrazone Tautomerism of Guanylhydrazones: Evidence for the Preference Toward the Azine Tautomer. J. Org. Chem. 2016, 81, 7574–7583. [Google Scholar] [CrossRef]
- Sundberg, R.J.; Dahlhausen, D.J.; Manikumar, G.; Mavunkel, B.; Biswas, A.; Srinivasan, V.; Musallam, H.; Reid, W.A., Jr.; Ager, A.L. Cationic antiprotozoal drugs. Trypanocidal activity of 2-(4′-formylphenyl)imidazo[1,2-a]pyridinium guanylhydrazones and related derivatives of quaternary heteroaromatic compounds. J. Med. Chem. 1990, 33, 298–307. [Google Scholar] [CrossRef]
- Davydov, V.; Sokol, V.; Polyanskaya, N.; Linko, R.; Ryabov, M.; Sergienko, V. Synthesis, crystal structure, and spectral studies of 10-(2-Benzothiazolylazo)-9-phenanthrol. Crystallogr. Rep. 2012, 57, 227–234. [Google Scholar] [CrossRef]
- Giorgi, G.; Ponticelli, F.; Savini, L.; Chiasserini, L.; Pellerano, C. On the isomerism/tautomerism of hydrazones. Crystal structures, study in solution and theoretical calculations of new series of α-N-heterocyclic hydrazones. J. Chem. Soc. Perkin Trans. 2 2000, 11, 2259–2264. [Google Scholar] [CrossRef]
- Ganga, M.; Sankaran, K.R. Synthesis, spectral characterization, DFT, molecular docking and biological evaluation of some newly synthesized asymmetrical azines of 3,5-dimethoxy-4–hydroxy benzaldehyde. Chem. Data Collect. 2020, 28, 100475–100489. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Khlebnikov, A.I.; Potapov, A.S.; Kovrizhina, A.R.; Matveevskaya, V.V.; Belyanin, M.L.; Quinn, M.T. Synthesis, biological evaluation, and molecular modeling of 11H-indeno[1,2-b]quinoxalin-11-one derivatives and tryptanthrin-6-oxime as c-Jun N-terminal kinase inhibitors. Eur. J. Med. Chem. 2019, 161, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Kotharkar, S.A.; Shinde, D.B. Synthesis of antimicrobial 2,9,10-trisubstituted-6-oxo-7,12-dihydrochromeno[3,4-b]quinoxalines. Bioinorg. Med. Chem. Let. 2006, 16, 6181–6184. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Chen, Y.-R.; Tzeng, C.-C.; Liu, W.; Chou, C.-K.; Chiu, C.-C.; Chen, Y.-L. Discovery of indeno[1,2-b]quinoxaline derivatives as potential anticancer agents. Eur. J. Med. Chem. 2016, 108, 258–273. [Google Scholar] [CrossRef]
- Pearson, B.D. Indenoquinolines. III. Derivatives of 11H-Indeno-[1,2-b]quinoxaline and related indenoquinolines. J. Org. Chem. 1962, 27, 1674–1678. [Google Scholar] [CrossRef]
- Balamurugan, G.; Velmathi, S. Quinoxaline based redox relay receptor for iodide ions and its application towards real sample analysis and logic gate function. Sens. Actuators B 2018, 256, 126–134. [Google Scholar]
- Warren, C.H.; Wettermark, G.; Weiss, K. Cis-trans isomerization about the carbon-nitrogen double bond. Structures of the isomers of N-benzylideneaniline. J. Am. Chem. Soc. 1971, 93, 4658–4663. [Google Scholar]
- Zheng, J.; Xu, X.; Truhlar, D.G. Minimally augmented Karlsruhe basis sets. Theor. Chem. Acc. 2011, 128, 295–305. [Google Scholar] [CrossRef]
- Ásgeirsson, V.; Birgisson, B.O.; Bjornsson, R.; Becker, U.; Neese, F.; Riplinger, C.; Jónsson, H. Nudged Elastic Band Method for Molecular Reactions Using Energy-Weighted Springs Combined with Eigenvector Following. J. Chem. Theory Comput. 2021, 17, 4929–4945. [Google Scholar] [CrossRef]
- Blanco, F.; Alkorta, I.; Elguero, J. Barriers about Double Carbon-Nitrogen Bond in Imine Derivatives (Aldimines, Oximes, Hydrazones, Azines). Croat. Chem. Acta 2009, 82, 173–183. [Google Scholar]
- Gasparro, F.P.; Kolodny, N.H. NMR Determination of the Rotational Barrier in N,N-dimethylacetamide. J. Chem. Educ. 1977, 54, 258–261. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).